Characterization of Purified Red Cabbage Anthocyanins: Improvement in HPLC Separation and Protective Effect against H2O2-Induced Oxidative Stress in HepG2 Cells
Abstract
:1. Introduction
2. Results and Discussions
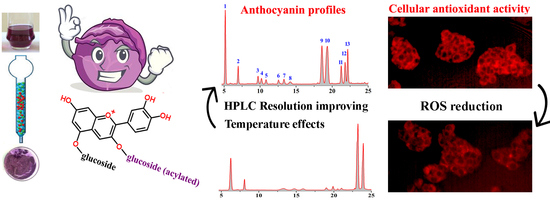
2.1. Effect of Temperature on the HPLC Resolution of RCAs
2.2. HPLC-ESI-MS Analysis and Quantification of RCAs
2.3. HPLC Method Validation and Quantification
2.4. Effect of RCAs against H2O2-Induced Oxidative Stress in HepG2 Cells
3. Materials and Methods
3.1. Materials
3.2. Purification of RCAs by Resin Adsorption
3.3. HPLC/ESI-MS Characterization
3.4. HPLC Method Validation and Quantification
3.5. Cell Culture-Based Assays for Antioxidant Activity
3.5.1. Cell Culture
3.5.2. Cell Count and ROS Assays by HCA
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Dangles, O.; Fenger, J.A. The chemical reactivity of anthocyanins and its consequences in food science and nutrition. Molecules 2018, 23, 1970. [Google Scholar] [CrossRef]
- Rose, P.M.; Cantrill, V.; Benohoud, M.; Tidder, A.; Rayner, C.M.; Blackburn, R.S. Application of anthocyanins from blackcurrant (Ribes nigrum L.) fruit waste as renewable hair dyes. J. Agric. Food Chem. 2018, 66, 6790–6798. [Google Scholar] [CrossRef] [PubMed]
- Wiczkowski, W.; Szawara-Nowak, D.; Topolska, J. Red cabbage anthocyanins: Profile, isolation, identification, and antioxidant activity. Food Res. Int. 2013, 51, 303–309. [Google Scholar] [CrossRef]
- Wiczkowski, W.; Szawara-Nowak, D.; Topolska, J. Changes in the content and composition of anthocyanins in red cabbage and its antioxidant capacity during fermentation storage and stewing. Food Chem. 2015, 167, 115–123. [Google Scholar] [CrossRef] [PubMed]
- Moloney, M.; Robbins, R.J.; Collins, T.M.; Kondo, T.; Yoshida, K.; Dangles, O. Red cabbage anthocyanins: The influence of D-glucose acylation by hydroxycinnamic acids on their structural transformations in acidic to mildly alkaline conditions and on the resulting color. Dyes Pigments 2018, 158, 342–352. [Google Scholar] [CrossRef]
- Scalzo, R.L.; Genna, A.; Branca, F.; Chedin, M.; Chassaigne, H. Anthocyanin composition of cauliflower (Brassica oleracea L. var. botrytis) and cabbage (B. oleracea L. var. capitata) and its stability in relation to thermal treatments. Food Chem. 2008, 107, 136–144. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, Z.; Liu, X. Characterization of acylated anthocyanins in red cabbage via comprehensive two-dimensional high performance liquid chromatography and HPLC-MS. J. Food Process. Pres. 2016, 41, e13129. [Google Scholar] [CrossRef]
- Willemse, C.M.; Stander, M.A.; Tredoux, A.G.J.; Villiers, A.D. Comprehensive two-dimensional liquid chromatographic analysis of anthocyanins. J. Chromatogr. A 2014, 1359, 189–201. [Google Scholar] [CrossRef] [Green Version]
- Buko, V.; Zavodnik, I.; Kanuka, O.; Belonovskaya, E.; Naruta, E.; Lukivskaya, O.; Kirko, S.; Budryn, G.; Żyżelewicz, D.; Oracz, J.; et al. Antidiabetic effects and erythrocyte stabilization by red cabbage extract in streptozotocin-treated rats. Food Funct. 2018, 9, 1850–1863. [Google Scholar] [CrossRef]
- Degenhardt, A.; Knapp, H.; Winterhalter, P. Separation and purification of anthocyanins by high-speed countercurrent chromatography and screening for antioxidant activity. J. Agric. Food Chem. 2000, 48, 338–343. [Google Scholar] [CrossRef]
- Nordberg, J.; Arnér, E.S. Reactive oxygen species, antioxidants, and the mammalian thioredoxin system. Free Radicals Biol. Med. 2001, 31, 1287–1312. [Google Scholar] [CrossRef]
- Chaudhary, A.; Bag, S.; Banerjee, P.; Chatterjee, J. Honey extracted polyphenolics reduce experimental hypoxia in human keratinocytes culture. J. Agric. Food. Chem. 2017, 65, 3460–3473. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Zhang, X.; Zheng, S.; Lu, K.; Zhao, G.; Ming, J. The composition.; antioxidant and antiproliferative capacities of phenolic compounds extracted from tartary buckwheat bran [Fagopyrum tartaricum, (L.) Gaerth]. J. Funct. Foods 2016, 22, 145–155. [Google Scholar] [CrossRef]
- Omar, S.H.; Kerr, P.G.; Scott, C.J.; Hamlin, A.S.; Obied, H.K. Olive (Olea europaea L.) Biophenols: A Nutriceutical against Oxidative Stress in SH-SY5Y Cells. Molecules 2017, 22, 1858. [Google Scholar] [CrossRef]
- Guo, Y.; Sun, L.; Yu, B.; Qi, J. An integrated antioxidant activity fingerprint for commercial teas based on their capacities to scavenge reactive oxygen species. Food Chem. 2017, 237, 645–653. [Google Scholar] [CrossRef] [PubMed]
- Salla, S.; Sunkara, R.; Ogutu, S.; Walker, L.T.; Verghese, M. Antioxidant activity of papaya seed extracts against H2O2 induced oxidative stress in HepG2 cells. LWT-Food Sci. Technol. 2016, 66, 293–297. [Google Scholar] [CrossRef]
- Sankhari, J.M.; Thounaojam, M.C.; Jadeja, R.N.; Devkar, R.V.; Ramachandran, A.V. Anthocyanin-rich red cabbage (Brassica oleracea L.) extract attenuates cardiac and hepatic oxidative stress in rats fed an atherogenic diet. J. Sci. Food Agr. 2012, 92, 1688–1693. [Google Scholar] [CrossRef]
- Saluk, J.; Bijak, M.; Posmyk, M.M.; Zbikowska, H.M. Red cabbage anthocyanins as inhibitors of lipopolysaccharide-induced oxidative stress in blood platelets. Int. J. Biol. Macromol. 2015, 80, 702–709. [Google Scholar] [CrossRef]
- Qu, D.; Gu, Y.; Feng, L.; Han, J. High content analysis technology for evaluating the joint toxicity of sunset yellow and sodium sulfite in vitro. Food Chem. 2017, 233, 135–143. [Google Scholar] [CrossRef]
- Dolan, J.W. Temperature selectivity in reversed-phase high performance liquid chromatography. J. Chromatogr. A 2002, 965, 195–205. [Google Scholar] [CrossRef]
- Gant, J.R.; Dolan, J.W.; Snyder, L.R. Systematic approach to optimizing resolution in reversed-phase liquid chromatography, with emphasis on the role of temperature. J. Chromatogr. A 1979, 185, 153–177. [Google Scholar] [CrossRef]
- Khalaf, R.; Baur, D.; Pfister, D. Optimization of reversed-phase chromatography methods for peptide analytics. J. Chromatogr. A 2015, 1425, 198–203. [Google Scholar] [CrossRef]
- Prior, R.L.; Wu, X.; Schaich, K. Standardized methods for the determination of antioxidant capacity and phenolics in foods and dietary supplements. J. Agric. Food Chem. 2005, 53, 4290–4302. [Google Scholar] [CrossRef]
- Ahmadiani, N.; Robbins, R.J.; Collins, T.M.; Giusti, M.M. Anthocyanins contents, profiles, and color characteristics of red cabbage extracts from different cultivars and maturity stages. J. Agric. Food Chem. 2014, 62, 7524–7531. [Google Scholar] [CrossRef]
- Wiczkowski, W.; Piskuła, M.K. Food flavonoids. Pol. J. Food Nutr. Sci. 2004, 13, 101–114. [Google Scholar]
- Yeh, C.T.; Ching, L.C.; Yen, G.C. Inducing gene expression of cardiac antioxidant enzymes by dietary phenolic acids in rats. J. Nutr. Biochem. 2009, 20, 163–171. [Google Scholar] [CrossRef]
- Yang, G.; Liao, J.; Kim, K.; Yurkow, E.J.; Yang, C. Inhibition of growth and induction of apoptosis in human cancer cell lines by tea polyphenols. Carcinogenesis 1998, 19, 611–616. [Google Scholar] [CrossRef] [Green Version]
- Huang, W.Y.; Wu, H.; Li, D.J.; Song, J.F.; Xiao, Y.D.; Liu, C.Q.; Zhou, J.Z.; Sui, Z.Q. Protective effects of blueberry anthocyanins against H2O2-induced oxidative injuries in human retinal pigment epithelial cells. J. Agric. Food Chem. 2018, 66, 1638–1648. [Google Scholar] [CrossRef]
- Shih, P.H.; Yeh, C.T.; Yen, G.C. Anthocyanins induce the activation of phase II enzymes through the antioxidant response element pathway against oxidative stress-induced apoptosis. J. Agric. Food Chem. 2007, 55, 9427–9435. [Google Scholar] [CrossRef]
- Lee, S.G.; Kim, B.; Yang, Y.; Pham, T.X.; Park, Y.K.; Manatou, J.; Koo, S.I.; Chun, O.K.; Lee, J.Y. Berry anthocyanins suppress the expression and secretion of proinflammatory mediators in macrophages by inhibiting nuclear translocation of NF-kB independent of NRF2-mediated mechanism. J. Nutr. Biochem. 2014, 25, 404–411. [Google Scholar] [CrossRef]
- Cheng, L.; Fang, S.; Ruan, M. Influence of blanching pretreatment on the drying characteristics of cherry tomato and mathematical modeling. Int. J. Food Eng. 2015, 11, 265–274. [Google Scholar] [CrossRef]
- Chen, Y.; Zhang, W.; Zhao, T.; Li, F.; Zhang, M.; Li, J.; Zou, Y.; Wang, W.; Cobbina, S.J.; Wu, X.; et al. Adsorption properties of macroporous adsorbent resins for separation of anthocyanins from mulberry. Food Chem. 2016, 194, 712–722. [Google Scholar] [CrossRef] [PubMed]
- Buran, T.J.; Sandhu, A.K.; Li, Z.; Rock, C.R.; Yang, W.; Gu, L. Adsorption/desorption characteristics and separation of anthocyanins and polyphenols from blueberries using macroporous adsorbent resins. J. Food Eng. 2014, 128, 167–173. [Google Scholar] [CrossRef]
- Liang, X.; Wu, H.; Su, W. A rapid UPLC-PAD fingerprint analysis of chrysanthemum morifolium ramat combined with chemometrics methods. Food Anal. Method. 2014, 7, 197–204. [Google Scholar] [CrossRef]
- Yan, F.; Chen, Y.; Azat, R.; Zheng, X. Mulberry Anthocyanin Extract Ameliorates Oxidative Damage in HepG2 Cells and Prolongs the Lifespan of Caenorhabditis elegans through MAPK and Nrf2 Pathways. Oxid. Med. Cell. Longev. 2017, 2017. [Google Scholar] [CrossRef] [PubMed]
- Hwang, Y.P.; Choi, J.H.; Choi, J.M.; Chung, Y.C.; Jeong, H.G. Protective mechanisms of anthocyanins from purple sweet potato against tert-butyl hydroperoxide-induced hepatotoxicity. Food Chem. Toxicol. 2011, 49, 2081–2089. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds are not available from the authors. |



| Peak No. | k25 | k30 | k35 | k40 | k45 | a |
|---|---|---|---|---|---|---|
| 1 | 1.110 | 1.074 | 0.954 | 0.869 | 0.751 | 2251 |
| 2 | 1.750 | 1.703 | 1.568 | 1.476 | 1.329 | 1552 |
| 3 | 3.426 | 3.100 | 2.803 | 2.542 | 2.244 | 2057 |
| 4 | 3.909 | 3.331 | 3.013 | 2.728 | 2.397 | 2095 |
| 5 | 4.298 | 3.591 | 3.270 | 2.963 | 2.609 | 2036 |
| 6 | 5.334 | 4.363 | 4.006 | 3.633 | 3.183 | 2011 |
| 7 | 5.630 | 4.678 | 4.307 | 3.927 | 3.437 | 1959 |
| 8 | 6.006 | 5.289 | 4.806 | 4.304 | 3.730 | 2231 |
| 9 | 6.737 | 6.518 | 6.134 | 5.712 | 5.193 | 1451 |
| 10 | 6.737 | 6.599 | 6.299 | 5.916 | 5.442 | 1235 |
| 11 | 7.076 | 6.890 | 6.726 | 6.517 | 6.286 | 590 |
| 12 | 7.076 | 6.952 | 6.783 | 6.611 | 6.453 | 482 |
| 13 | 7.076 | 6.952 | 6.824 | 6.672 | 6.488 | 443 |
| Peak | tR/min | M+ m/z | Tentative Identification |
|---|---|---|---|
| 1 | 6.14 | 773 | Cyanidin-3-diglucoside-5-glucoside |
| 2 | 8.14 | 979 | Cyanidin-3-(sinapoyl)-diglucoside-5-glucoside |
| 3 | 11.12 | 1081 | Cyanidin-3-(caffeoyl)(p-coumaroyl)-diglucosides-5-glucoside |
| 4 | 11.67 | 1111 | Cyanidin-3-(feruloyl)-triglucosides-5-glucoside |
| 5 | 12.39 | 1141 | Cyanidin-3-(sinapoyl)-triglucoside-5-glucoside |
| 6 | 14.39 | 1287 | Cyanidin-3-(feruloyl)(feruloyl)-triglucoside-5-glucoside |
| 7 | 15.29 | 1317 | Cyanidin-3-(feruloyl)(sinapoyl)-triglucoside-5-glucoside |
| 8 | 16.11 | 935 | Cyanidin-3-(caffeoyl)-diglucoside-5-glucoside |
| 9 | 19.73 | 919 | Cyanidin-3-(p-coumaroyl)-diglucoside-5-glucoside |
| 10 | 20.45 | 949 | Cyanidin-3-(feruloyl)-diglucoside-5-glucoside |
| 11 | 22.88 | 1125 | Cyanidin-3-(feruloyl)(feruloyl)-diglucoside-5-glucoside |
| 12 | 23.42 | 1155 | Cyanidin-3-(feruloyl)(sinapoyl)-diglucoside-5-glucoside |
| 13 | 23.78 | 1185 | Cyanidin-3-(sinapoyl)(sinapoyl)-diglucoside-5-glucoside |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fang, S.; Lin, F.; Qu, D.; Liang, X.; Wang, L. Characterization of Purified Red Cabbage Anthocyanins: Improvement in HPLC Separation and Protective Effect against H2O2-Induced Oxidative Stress in HepG2 Cells. Molecules 2019, 24, 124. https://doi.org/10.3390/molecules24010124
Fang S, Lin F, Qu D, Liang X, Wang L. Characterization of Purified Red Cabbage Anthocyanins: Improvement in HPLC Separation and Protective Effect against H2O2-Induced Oxidative Stress in HepG2 Cells. Molecules. 2019; 24(1):124. https://doi.org/10.3390/molecules24010124
Chicago/Turabian StyleFang, Sheng, Fubin Lin, Daofeng Qu, Xianrui Liang, and Liping Wang. 2019. "Characterization of Purified Red Cabbage Anthocyanins: Improvement in HPLC Separation and Protective Effect against H2O2-Induced Oxidative Stress in HepG2 Cells" Molecules 24, no. 1: 124. https://doi.org/10.3390/molecules24010124