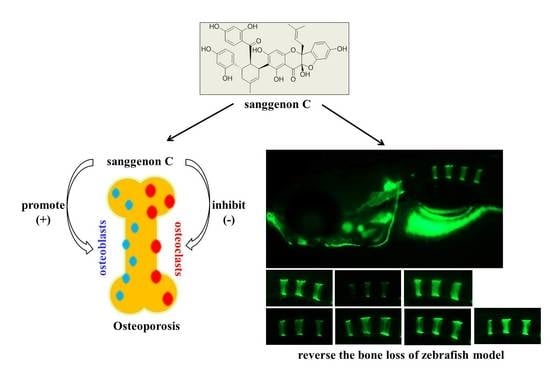
Sanggenon C Stimulates Osteoblastic Proliferation and Differentiation, Inhibits Osteoclastic Resorption, and Ameliorates Prednisone-Induced Osteoporosis in Zebrafish Model
Abstract
:1. Introduction
2. Results
2.1. Effect of SC on MC3T3-E1 Cell Proliferation
2.2. Effect of SC on MC3T3-E1 Cell Differentiation
2.3. Effect of SC on Osteoclast Formation
2.4. Effect of SC on Bone Resorption Function of Osteoclasts
2.5. Effect of SC on the Expression of Osteoclast-Specific Genes
2.6. Prevention Effect of SC on Zebrafish Osteoporosis Induced by Prednisone
3. Discussion
4. Materials and Methods
4.1. Chemicals and Reagents
4.2. MC3T3-E1 Cell Proliferation Assay
4.3. MC3T3-E1 Cell Differentiation
4.4. Culture of Rabbit Osteoclasts
4.5. Tartrate-Resistant Acid Phosphatase Staining
4.6. Bone Resorption Assays
4.7. Relative Expression of Osteoclast-Specific Gene
4.8. Quantitative Real-Time PCR
4.9. Animals and Treatments
4.10. Bone Matrix Calcein Labeling
4.11. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Che, C.T.; Wong, M.S.; Lam, C.W. Natural products from Chinese medicines with potential benefits to bone health. Molecules 2016, 21, 239. [Google Scholar] [CrossRef] [PubMed]
- Eriksen, E.F. Cellular mechanisms of bone remodeling. Rev. Endocr. Metab. Disord. 2010, 11, 219–227. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boyle, W.J.; Simonet, W.S.; Lacey, D.L. Osteoclast differentiation and activation. Nature 2003, 423, 337–342. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lam, K.S. New aspects of natural products in drug discovery. Trends Microbiol. 2007, 15, 279–289. [Google Scholar] [CrossRef] [PubMed]
- Harvey, A.L. Natural products in drug discovery. Drug Discov. Today 2008, 13, 894–901. [Google Scholar] [CrossRef] [PubMed]
- An, J.; Hao, D.; Zhang, Q.; Chen, B.; Zhang, R.; Wang, Y.; Yang, H. Natural products for treatment of bone erosive diseases: The effects and mechanisms on inhibiting osteoclastogenesis and bone resorption. Int. Immunopharmacol. 2016, 36, 118–131. [Google Scholar] [CrossRef] [PubMed]
- Xiao, L.; Gu, Y.; Gao, L.; Shangguan, J.; Chen, Y.; Zhang, Y.; Li, L. Sanggenon C protects against pressure overload-induced cardiac hypertrophy via the calcineurin/NFAT2 pathway. Mol. Med. Rep. 2017, 16, 5338–5346. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, H.; Liu, N.; Zhao, K.; Zhu, C.; Lu, X.; Li, S.; Lian, W.; Zhou, P.; Dong, X.; Zhao, C.; et al. Sanggenon C decreases tumor cell viability associated with proteasome inhibition. Front. Biosci. 2011, 3, 1315–1325. [Google Scholar] [CrossRef]
- Chen, L.D.; Liu, Z.H.; Zhang, L.F.; Yao, J.N.; Wang, C.F. Sanggenon C induces apoptosis of colon cancer cells via inhibition of NO production, iNOS expression and ROS activation of the mitochondrial pathway. Oncol. Rep. 2017, 38, 2123–2131. [Google Scholar] [CrossRef] [PubMed]
- Gu, Y.; Gao, L.; Chen, Y.; Xu, Z.; Yu, K.; Zhang, D.; Zhang, G.; Zhang, X. Sanggenon C protects against cardiomyocyte hypoxia injury by increasing autophagy. Mol. Med. Rep. 2017, 16, 8130–8136. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, L.C.; Shen, F.; Hou, Q.; Cheng, G.F. Inhibitory effect and mechanism of action of sanggenon C on human polymorphonuclear leukocyte adhesion to human synovial cells. Acta Pharmacol. Sin. 2002, 23, 138–142. [Google Scholar] [PubMed]
- Dat, N.T.; Binh, P.T.; Quynh le, T.P.; Huong, H.T.; Minh, C.V. Sanggenon C and O inhibit NO production, iNOS expression and NF-κB activation in LPS-induced RAW264.7 cells. Immunopharmacol. Immunotoxicol. 2012, 34, 84–88. [Google Scholar] [CrossRef] [PubMed]
- Lee, N.K.; Choi, Y.G.; Baik, J.Y.; Han, S.Y.; Jeong, D.W.; Bae, Y.S.; Kim, N.; Lee, S.Y. A crucial role for reactive oxygen species in RANKL-induced osteoclast differentiation. Blood 2005, 106, 852–859. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, Q.; Shi, J.; Gao, B.; Zhang, H.Y.; Fan, J.; Li, X.J.; Fan, J.Z.; Han, Y.H.; Zhang, J.K.; Yang, L.; et al. Gastrodin: An ancient Chinese herbal medicine as a source for anti-osteoporosis agents via reducing reactive oxygen species. Bone 2015, 73, 132–144. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.C.; Wang, Y.; Fang, Z.Y.; Liu, X.W.; Sheng, T.; Wang, X.X. Herbs protect against osteoporosis through anti-inflammation action. Chin. J. Tissue Eng. Res. 2018, 22, 638–643. [Google Scholar]
- Hotokezaka, H.; Sakai, E.; Ohara, N.; Hotokezaka, Y.; Gonzales, C.; Matsuo, K.; Fujimura, Y.; Yoshida, N.; Nakayama, K. Molecular analysis of RANKL-independent cell fusion of osteoclast-like cells induced by TNF-alpha, lipopolysaccharide, or peptidoglycan. J. Cell Biochem. 2007, 101, 122–134. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.R.; Lai, Y.H.; Tsai, J.J.; Hsiao, C.D. Live fluorescent staining platform for drug-screening and mechanism-analysis in zebrafish for bone mineralization. Molecules 2017, 22, 2068. [Google Scholar] [CrossRef] [PubMed]
- Carnovali, M.; Ottria, R.; Pasqualetti, S.; Banfi, G.; Ciuffreda, P.; Mariotti, M. Effects of bioactive fatty acid amide derivatives in zebrafish scale model of bone metabolism and disease. Pharmacol. Res. 2016, 104, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Luo, S.Y.; Yang, Y.J.; Chen, J.F.; Zhong, Z.G.; Huang, H.X.; Zhang, J.J.; Cui, L. Tanshinol stimulates bone formation and attenuates dexamethasone-induced inhibition of osteogenesis in larval zebrafish. J. Orthop. Transl. 2016, 4, 35–45. [Google Scholar] [CrossRef] [PubMed]
- Kitamura, K.; Takahira, K.; Inari, M.; Satoh, Y.; Hayakawa, K.; Tabuchi, Y.; Ogai, K.; Nishiuchi, T.; Kondo, T.; Mikuni-Takagaki, Y.; et al. Zebrafish scales respond differently to in vitro dynamic and static acceleration: Analysis of interaction between osteoblasts and osteoclasts. Comp. Biochem. Physiol. 2013, 166, 74–80. [Google Scholar] [CrossRef] [PubMed]
- Carnovali, M.; Luzi, L.; Banfi, G.; Mariotti, M. Chronic hyperglycemia affects bone metabolism in adult zebrafish scale model. Endocrine 2016, 54, 808–817. [Google Scholar] [CrossRef] [PubMed]
- de Vrieze, E.; Moren, M.; Metz, J.R.; Flik, G.; Lie, K.K. Arachidonic acid enhances turnover of the dermal skeleton: Studies on zebrafish scales. PLoS ONE 2014, 9, e89347. [Google Scholar] [CrossRef] [PubMed]
- Lü, X.; Zhou, Y.; Chen, K.M.; Zhao, Z.; Zhou, J.; Ma, X.N. Inhibitory effect of 8-prenylnaringenin on osteoclastogensis of bone marrow cells and boneresorption activity. Acta Pharm. Sin. 2013, 48, 347–351. [Google Scholar]
- Westerfield, M. The Zebrafish Book. A Guide for the Laboratory Use of Zebrafish (Danio Rerio); University of Oregon Press: Eugene, OR, USA, 2007. [Google Scholar]
Sample Availability: Samples of the compounds are not available from the authors. |







| Gene | Forward(5′–3′) | Reverse(5′–3′) | bp |
|---|---|---|---|
| osteoblas | |||
| Runx2 | AACTTCCTGTGCTCCGTGCT | CCTGGCTACTTGGTTTTTCA | 186 |
| Collagen I | CTCCGGCTCCTGCTCCTCTT | CATTGCATTGCACGTCATCG | 184 |
| OPG | TCTGTGAAAGCAGCGTG | GTTTTGGGAAAGTGGGA | 253 |
| RANKL | ATGAAAGGAGGGAGCA | AGGGAAGGGTTGGACA | 128 |
| β-actin | AGGCCAACCGTGAAAAGATG | GGCGTGAGGGAGAGCATAG | 185 |
| Osteoclasts | |||
| TRAP | CGCCAAGCAAATCGGCAAAG | CGGTCACTGAACACGTCCTCGA | 141 |
| Cathepsin K | GCCTCAAAGTACCCCCG | GTAGCCCCCTCCACAGC | 269 |
| TRAF6 | TGGCTGCCATGAAAAGATGC | TCACGTGGAGGTATGGGAGT | 132 |
| NFATc1 | AGCGTGAACCTGAGGAGTTG | ACCTCCAACATGCGAGCTAC | 108 |
| GAPDH | AGAGCACCAGAGGAGGACG | TGGGATGGAAACTGTGAAGAG | 105 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, H.; Feng, T.; Guo, D.; Zhang, M.; Chen, L.; Zhou, Y. Sanggenon C Stimulates Osteoblastic Proliferation and Differentiation, Inhibits Osteoclastic Resorption, and Ameliorates Prednisone-Induced Osteoporosis in Zebrafish Model. Molecules 2018, 23, 2343. https://doi.org/10.3390/molecules23092343
Wang H, Feng T, Guo D, Zhang M, Chen L, Zhou Y. Sanggenon C Stimulates Osteoblastic Proliferation and Differentiation, Inhibits Osteoclastic Resorption, and Ameliorates Prednisone-Induced Osteoporosis in Zebrafish Model. Molecules. 2018; 23(9):2343. https://doi.org/10.3390/molecules23092343
Chicago/Turabian StyleWang, Huijuan, Tingting Feng, Donggui Guo, Min Zhang, Lin Chen, and Ying Zhou. 2018. "Sanggenon C Stimulates Osteoblastic Proliferation and Differentiation, Inhibits Osteoclastic Resorption, and Ameliorates Prednisone-Induced Osteoporosis in Zebrafish Model" Molecules 23, no. 9: 2343. https://doi.org/10.3390/molecules23092343