Long-Term Burden of Increased Body Mass Index from Childhood on Adult Dyslipidemia: The i3C Consortium Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Cohort
2.2. Examinations
2.3. Statistical Methods
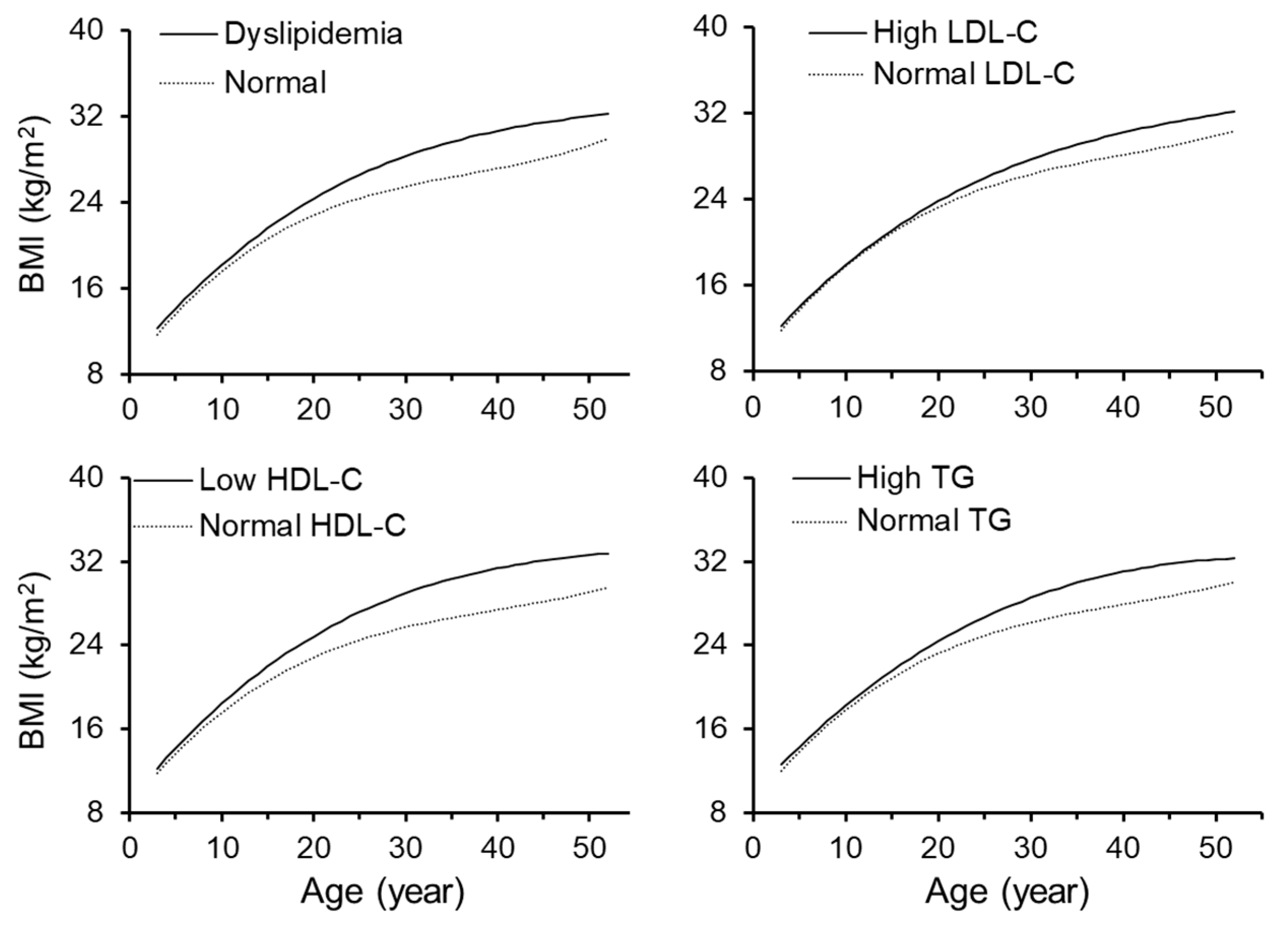
3. Results
4. Discussion
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults. Executive Summary of the Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III). JAMA 2001, 285, 2486–2497. [Google Scholar]
- GBD 2017 Risk Factor Collaborators. Global, regional, and national comparative risk assessment of 84 behavioural, environmental and occupational, and metabolic risks or clusters of risks for 195 countries and territories, 1990–2017: A systematic analysis for the Global Burden of Disease Study 2017. Lancet 2018, 392, 1923–1994. [Google Scholar]
- Wilson, P.W.; D’Agostino, R.B.; Levy, D.; Belanger, A.M.; Silbershatz, H.; Kannel, W.B. Prediction of coronary heart disease using risk factor categories. Circulation 1998, 97, 1837–1847. [Google Scholar] [CrossRef] [PubMed]
- NCD Risk Factor Collaboration (NCD-RisC). Worldwide trends in body-mass index, underweight, overweight, and obesity from 1975 to 2016: A pooled analysis of 2416 population-based measurement studies in 128.9 million children, adolescents, and adults. Lancet 2017, 390, 2627–2642. [Google Scholar] [CrossRef]
- Bays, H.E.; Toth, P.P.; Kris-Etherton, P.M.; Abate, N.; Aronne, L.J.; Brown, W.V.; Gonzalez-Campoy, J.M.; Jones, S.R.; Kumar, R.; La Forge, R.; et al. Obesity, adiposity, and dyslipidemia: A consensus statement from the National Lipid Association. J. Clin. Lipidol. 2013, 7, 304–383. [Google Scholar] [CrossRef] [Green Version]
- Kotsis, V.; Jordan, J.; Micic, D.; Finer, N.; Leitner, D.R.; Toplak, H.; Tokgozoglu, L.; Athyros, V.; Elisaf, M.; Filippatos, T.D.; et al. Obesity and cardiovascular risk: A call for action from the European Society of Hypertension Working Group of Obesity, Diabetes and the High-risk Patient and European Association for the Study of Obesity: Part A: Mechanisms of obesity induced hypertension, diabetes and dyslipidemia and practice guidelines for treatment. J. Hypertens. 2018, 36, 1427–1440. [Google Scholar]
- Stępień, A.; Stępień, M.; Wlazeł, R.N.; Paradowski, M.; Banach, M.; Rysz, J. Assessment of the relationship between lipid parameters and obesity indices in non-diabetic obese patients: A preliminary report. Med. Sci. Monit. 2014, 20, 2683–2688. [Google Scholar]
- Freedman, D.S.; Khan, L.K.; Dietz, W.H.; Srinivasan, S.R.; Berenson, G.S. Relationship of childhood obesity to coronary heart disease risk factors in adulthood: The Bogalusa Heart Study. Pediatrics 2001, 108, 712–718. [Google Scholar] [CrossRef]
- Skidmore, P.M.; Hardy, R.J.; Kuh, D.J.; Langenberg, C.; Wadsworth, M.E. Life course body size and lipid levels at 53 years in a British birth cohort. J. Epidemiol. Community Health 2007, 61, 215–220. [Google Scholar] [CrossRef] [Green Version]
- Lyngdoh, T.; Viswanathan, B.; van Wijngaarden, E.; Myers, G.J.; Bovet, P. Cross-sectional and longitudinal associations between body mass index and cardiometabolic risk factors in adolescents in a country of the African region. Int. J. Endocrinol. 2013, 2013, 801832. [Google Scholar] [CrossRef]
- Sundaram, M.E.; Berg, R.L.; Economos, C.; Coleman, L.A. The relationship between childhood BMI and adult serum cholesterol, LDL, and ankle brachial index. Clin. Med. Res. 2014, 12, 33–39. [Google Scholar] [CrossRef] [PubMed]
- Umer, A.; Kelley, G.A.; Cottrell, L.E.; Giacobbi, P.; Innes, K.E.; Lilly, C.L. Childhood obesity and adult cardiovascular disease risk factors: A systematic review with meta-analysis. BMC Public Health 2017, 17, 683. [Google Scholar] [CrossRef] [PubMed]
- Bayer, O.; Krüger, H.; von Kries, R.; Toschke, A.M. Factors associated with tracking of BMI: A meta-regression analysis on BMI tracking. Obesity (Silver Spring) 2011, 19, 1069–1076. [Google Scholar] [CrossRef] [PubMed]
- Simmonds, M.; Llewellyn, A.; Owen, C.G.; Woolacott, N. Predicting adult obesity from childhood obesity: A systematic review and meta-analysis. Obes. Rev. 2016, 17, 95–107. [Google Scholar] [CrossRef]
- Zhang, T.; Xu, J.; Li, S.; Bazzano, L.A.; He, J.; Whelton, P.K.; Chen, W. Trajectories of childhood BMI and adult diabetes: The Bogalusa Heart Study. Diabetologia 2019, 62, 70–77. [Google Scholar] [CrossRef]
- Buscot, M.J.; Thomson, R.J.; Juonala, M.; Sabin, M.A.; Burgner, D.P.; Lehtimäki, T.; Hutri-Kähönen, N.; Viikari, J.; Raitakari, O.T.; Magnussen, C.G. Distinct child-to-adult body mass index trajectories are associated with different levels of adult cardiometabolic risk. Eur. Heart J. 2018, 39, 2263–2270. [Google Scholar] [CrossRef]
- Huang, R.C.; de Klerk, N.H.; Smith, A.; Kendall, G.E.; Landau, L.I.; Mori, T.A.; Newnham, J.P.; Stanley, F.J.; Oddy, W.H.; Hands, B.; et al. Lifecourse childhood adiposity trajectories associated with adolescent insulin resistance. Diabetes Care 2011, 34, 1019–1025. [Google Scholar] [CrossRef]
- Khan, S.S.; Shah, S.J.; Colangelo, L.A.; Panjwani, A.; Liu, K.; Lewis, C.E.; Shay, C.M.; Goff, D.C.; Reis, J.; Vasconcellos, H.D.; et al. Association of patterns of change in adiposity with diastolic function and systolic myocardial mechanics from early adulthood to middle age: The Coronary Artery Risk Development in Young Adults Study. J. Am. Soc. Echocardiogr. 2018, 31, 1261–1269. [Google Scholar] [CrossRef]
- Zheng, Y.; Song, M.; Manson, J.E.; Giovannucci, E.L.; Hu, F.B. Group-based trajectory of body shape from ages 5 to 55 years and cardiometabolic disease risk in 2 US cohorts. Am. J. Epidemiol. 2017, 186, 1246–1255. [Google Scholar] [CrossRef]
- Dwyer, T.; Sun, C.; Magnussen, C.G.; Raitakari, O.T.; Schork, N.J.; Venn, A.; Burns, T.L.; Juonala, M.; Steinberger, J.; Sinaiko, A.R.; et al. Cohort Profile: The international childhood cardiovascular cohort (i3C) consortium. Int. J. Epidemiol. 2013, 42, 86–96. [Google Scholar] [CrossRef]
- Sinaiko, A.R.; Jacobs, D.R.; Woo, J.G.; Bazzano, L.; Burns, T.; Hu, T.; Juonala, M.; Prineas, R.; Raitakari, O.; Steinberger, J.; et al. The International Childhood Cardiovascular Cohort (i3C) consortium outcomes study of childhood cardiovascular risk factors and adult cardiovascular morbidity and mortality: Design and recruitment. Contemp. Clin. Trials 2018, 69, 55–64. [Google Scholar] [CrossRef] [PubMed]
- Berenson, G.S. Bogalusa Heart Study: A long-term community study of a rural biracial (Black/White) population. Am. J. Med. Sci. 2001, 322, 293–300. [Google Scholar] [CrossRef] [PubMed]
- Raitakari, O.T.; Juonala, M.; Kähönen, M.; Taittonen, L.; Laitinen, T.; Mäki-Torkko, N.; Järvisalo, M.J.; Uhari, M.; Jokinen, E.; Rönnemaa, T.; et al. Cardiovascular risk factors in childhood and carotid artery intima-media thickness in adulthood: The Cardiovascular Risk in Young Finns Study. JAMA 2003, 290, 2277–2283. [Google Scholar] [CrossRef] [PubMed]
- Lauer, R.M.; Lee, J.; Clarke, W.R. Factors affecting the relationship between childhood and adult cholesterol levels: The Muscatine Study. Pediatrics 1988, 82, 309–318. [Google Scholar] [PubMed]
- Thompson, D.R.; Obarzanek, E.; Franko, D.L.; Barton, B.A.; Morrison, J.; Biro, F.M.; Daniels, S.R.; Striegel-Moore, R.H. Childhood overweight and cardiovascular disease risk factors: The National Heart, Lung, and Blood Institute Growth and Health Study. J. Pediatr. 2007, 150, 18–25. [Google Scholar] [CrossRef]
- Cook, N.R.; Rosner, B.A.; Chen, W.; Srinivasan, S.R.; Berenson, G.S. Using the area under the curve to reduce measurement error in predicting young adult blood pressure from childhood measures. Stat. Med. 2004, 23, 3421–3435. [Google Scholar] [CrossRef]
- Chen, W.; Li, S.; Cook, N.R.; Rosner, B.A.; Srinivasan, S.R.; Boerwinkle, E.; Berenson, G.S. An autosomal genome scan for loci influencing longitudinal burden of body mass index from childhood to young adulthood in white sibships: The Bogalusa Heart Study. Int. J. Obes. Relat. Metab. Disord. 2004, 28, 462–469. [Google Scholar] [CrossRef] [Green Version]
- Aucott, L.; Gray, D.; Rothnie, H.; Thapa, M.; Waweru, C. Effects of lifestyle interventions and long-term weight loss on lipid outcomes—A systematic review. Obes. Rev. 2011, 12, e412–e425. [Google Scholar] [CrossRef]
- Cai, L.; Wu, Y.; Cheskin, L.J.; Wilson, R.F.; Wang, Y. Effect of childhood obesity prevention programmes on blood lipids: A systematic review and meta-analysis. Obes. Rev. 2014, 15, 933–944. [Google Scholar] [CrossRef]
- Juonala, M.; Magnussen, C.G.; Berenson, G.S.; Venn, A.; Burns, T.L.; Sabin, M.A.; Srinivasan, S.R.; Daniels, S.R.; Davis, P.H.; Chen, W.; et al. Childhood adiposity, adult adiposity, and cardiovascular risk factors. N. Engl. J. Med. 2011, 365, 1876–1885. [Google Scholar] [CrossRef]
- Klop, B.; Elte, J.W.; Cabezas, M.C. Dyslipidemia in obesity: Mechanisms and potential targets. Nutrients 2013, 5, 1218–1240. [Google Scholar] [CrossRef] [PubMed]
- Casavalle, P.L.; Lifshitz, F.; Romano, L.S.; Pandolfo, M.; Caamaño, A.; Boyer, P.M.; Rodríguez, P.N.; Friedman, S.M. Prevalence of dyslipidemia and metabolic syndrome risk factor in overweight and obese children. Pediatr. Endocrinol. Rev. 2014, 12, 213–223. [Google Scholar] [PubMed]
- Sumner, A.E. Ethnic differences in triglyceride levels and high-density lipoprotein lead to underdiagnosis of the metabolic syndrome in black children and adults. J. Pediatr. 2009, 155, e7–e11. [Google Scholar] [CrossRef] [PubMed]
- Taylor, H.A.; Coady, S.A.; Levy, D.; Walker, E.R.; Vasan, R.S.; Liu, J.; Akylbekova, E.L.; Garrison, R.J.; Fox, C. Relationships of BMI to cardiovascular risk factors differ by ethnicity. Obesity (Silver Spring) 2010, 18, 1638–1645. [Google Scholar] [CrossRef] [PubMed]
- Min, J.; Wen, X.; Xue, H.; Wang, Y. Ethnic disparities in childhood BMI trajectories and obesity and potential causes among 29,250 US children: Findings from the Early Childhood Longitudinal Study-Birth and Kindergarten Cohorts. Int. J. Obes. (Lond.) 2018, 42, 1661–1670. [Google Scholar] [CrossRef]

| Variable | BHS (n = 1739) | YFS (n = 1718) | MUSC (n = 776) | NGHS (n = 575) | PHBPC (n = 387) | Total (n = 5195) |
|---|---|---|---|---|---|---|
| Male, n (%) | 765 (44.0) | 765 (44.5) | 350 (45.1) | 0 (0.0) | 189 (48.8) | 2069 (39.8) |
| White, n (%) | 1181 (67.9) | 1718 (100.0) | 776 (100.0) | 258 (44.9) | 279 (72.1) | 4212 (80.1) |
| Childhood (Fist exam) | ||||||
| Age (year) | 10.2 (3.1) | 9.7 (4.1) | 12.0 (2.2) | 10.0 (0.5) | 7.7 (0.7) | 10.1 (3.2) |
| BMI (kg/m2) | 17.8 (3.5) | 17.2 (2.6) | 19.0 (3.2) | 18.3 (3.6) | 16.5 (2.2) | 17.7 (3.2) |
| Adulthood (Last exam) | ||||||
| Age (year) | 38.5 (7.1) | 39.5 (4.4) | 39.9 (8.7) | 26.2 (0.9) | 39.1 (1.9) | 37.7 (7.2) |
| BMI (kg/m2) | 29.4 (7.6) | 26.2 (4.7) | 27.8 (6.1) | 28.4 (7.9) | 29.2 (6.9) | 28.0 (6.7) |
| Overweight, n (%) a | 526 (30.3) | 621 (36.2) | 235 (30.3) | 134 (23.3) | 118 (30.5) | 1634 (31.5) |
| Obesity, n (%) b | 673 (38.7) | 326 (19.0) | 251 (32.4) | 200 (34.8) | 150 (38.8) | 1600 (30.8) |
| LDL-C (mg/dL) | 126.1 (33.4) | 124.8 (31.6) | 117.5 (33.1) | 101.4 (30.7) | 111.5 (29.2) | 120.6 (33.2) |
| HDL-C (mg/dL) | 48.3 (14.6) | 50.8 (12.3) | 49.8 (14.4) | 51.5 (13.2) | 50.4 (13.8) | 49.9 (13.7) |
| TG (mg/dL) | 131.1 (96.0) | 110.7 (57.3) | 110.5 (66.0) | 95.6 (51.0) | 116.2 (65.8) | 116.2 (74.6) |
| High LDL-C, n (%) | 343 (19.7) | 251 (14.6) | 122 (15.7) | 26 (4.5) | 34 (8.8) | 776 (14.9) |
| Low HDL-C, n (%) | 568 (32.7) | 357 (20.8) | 232 (29.9) | 102 (17.7) | 96 (24.8) | 1355 (26.1) |
| High TG, n (%) | 337 (19.4) | 179 (10.4) | 123 (15.9) | 26 (4.5) | 54 (14.0) | 719 (13.8) |
| Dyslipidemia, n (%) | 794 (45.7) | 569 (33.1) | 300 (38.7) | 132 (22.8) | 121 (31.3) | 1916 (36.9) |
| AUC measures | ||||||
| Average age (year) | 22.5 (4.4) | 23.4 (4.4) | 26.4 (6.3) | 17.6 (0.8) | 14.9 (0.7) | 22.3 (5.3) |
| BMI AUCt (kg/m2) | 24.7 (5.1) | 22.7 (3.3) | 24.5 (4.2) | 24.5 (5.5) | 24.7 (4.7) | 24.0 (4.6) |
| BMI AUCi (kg/m2) | 6.9 (3.9) | 5.6 (2.3) | 5.3 (3.1) | 5.9 (3.2) | 8.5 (3.7) | 6.2 (3.3) |
| Curve Parameters | Dyslipidemia | High LDL-C | Low HDL-C | High TG |
|---|---|---|---|---|
| n (yes/no) | 1916/3279 | 776/4419 | 1355/3840 | 719/4476 |
| β0 + b0 (kg/m2) | ||||
| Yes | 25.3 (5.3) | 24.9 (4.8) | 26.0 (5.5) | 25.4 (5.0) |
| No | 23.5 (4.4) | 24.1 (4.8) | 23.6 (4.4) | 24.0 (4.8) |
| P a | <0.001 | <0.001 | <0.001 | <0.001 |
| β1 + b1 (kg/m2/year) | ||||
| Yes | 0.45 (0.26) | 0.43 (0.24) | 0.47 (0.27) | 0.48 (0.24) |
| No | 0.32 (0.26) | 0.35 (0.27) | 0.33 (0.26) | 0.35 (0.27) |
| pa | <0.001 | <0.001 | <0.001 | <0.001 |
| β2 + b2 (10 kg/m2/year2) b | ||||
| Yes | −0.101 (0.100) | −0.091 (0.087) | −0.108 (0.104) | −0.093 (0.095) |
| No | −0.106 (0.096) | −0.107 (0.099) | −0.103 (0.094) | −0.106 (0.098) |
| pa | 0.043 | <0.001 | 0.155 | <0.001 |
| β3 + b3 (20 kg/m2/year3) b | ||||
| Yes | 0.002 (0.010) | 0.002 (0.009) | 0.002 (0.010) | 0.001 (0.009) |
| No | 0.005 (0.011) | 0.004 (0.011) | 0.004 (0.010) | 0.004 (0.011) |
| pa | <0.001 | <0.001 | <0.001 | <0.001 |
| Independent Variable | Dependent Variable | |||
|---|---|---|---|---|
| Dyslipidemia | High LDL-C | Low HDL-C | High TG | |
| Childhood BMI a | 1.22 (1.15–1.29) | 1.10 (1.02–1.19) | 1.29 (1.22–1.37) | 1.13 (1.05–1.22) |
| Adulthood BMI | 1.85 (1.74–1.97) | 1.42 (1.32–1.53) | 1.82 (1.71–1.95) | 1.65 (1.53–1.77) |
| BMI AUCt b | 1.61 (1.52–1.71) | 1.30 (1.21–1.40) | 1.68 (1.57–1.79) | 1.48 (1.38–1.59) |
| BMI AUCi c | 1.59 (1.50–1.69) | 1.30 (1.21–1.40) | 1.62 (1.52–1.73) | 1.50 (1.40–1.62) |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yan, Y.; Bazzano, L.A.; Juonala, M.; Raitakari, O.T.; Viikari, J.S.A.; Prineas, R.; Dwyer, T.; Sinaiko, A.; Burns, T.L.; Daniels, S.R.; et al. Long-Term Burden of Increased Body Mass Index from Childhood on Adult Dyslipidemia: The i3C Consortium Study. J. Clin. Med. 2019, 8, 1725. https://doi.org/10.3390/jcm8101725
Yan Y, Bazzano LA, Juonala M, Raitakari OT, Viikari JSA, Prineas R, Dwyer T, Sinaiko A, Burns TL, Daniels SR, et al. Long-Term Burden of Increased Body Mass Index from Childhood on Adult Dyslipidemia: The i3C Consortium Study. Journal of Clinical Medicine. 2019; 8(10):1725. https://doi.org/10.3390/jcm8101725
Chicago/Turabian StyleYan, Yinkun, Lydia A. Bazzano, Markus Juonala, Olli T. Raitakari, Jorma S. A. Viikari, Ronald Prineas, Terence Dwyer, Alan Sinaiko, Trudy L. Burns, Stephen R. Daniels, and et al. 2019. "Long-Term Burden of Increased Body Mass Index from Childhood on Adult Dyslipidemia: The i3C Consortium Study" Journal of Clinical Medicine 8, no. 10: 1725. https://doi.org/10.3390/jcm8101725





