Self-Assembling Peptides and Their Application in the Treatment of Diseases
Abstract
:1. Introduction
2. Self-Assembling Peptides: Structure and Characteristics
2.1. Building Blocks
2.1.1. Dipeptides
2.1.2. Surfactant-Like Peptides
2.1.3. Peptide Amphiphiles with an Alkyl Group
2.1.4. Bolaamphiphilic Peptides
2.1.5. Ionic-Complementary Self-Assembling Peptides
2.1.6. Cyclic Peptides
2.2. Formation of Nanostructures
2.2.1. Nanofibers
2.2.2. Nanotubes
2.2.3. Nanoparticles
2.2.4. Nanotapes
2.2.5. Hydrogels
3. Factors for Peptide Self-Assembly
3.1. pH
3.2. Temperature
3.3. Other Stimuli
4. Application of Self-Assembling Peptide in Disease Treatment
4.1. Application in Cancer Treatment
4.1.1. Targeting
4.1.2. Drug Delivery
4.2. Application in Regenerative Medicine
4.2.1. Self-Assembling Peptides for Hepatocyte Regeneration
4.2.2. Self-Assembling Peptides for Neuronal Regeneration
4.2.3. Self-Assembling Peptides for Cartilage Regeneration
4.2.4. Self-Assembling Peptides for Vascular Regeneration
4.3. Other Applications
4.4. A New Paradigm of Nanostructure Formation with Reverse Self-Assembly
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- US Food and Drugs Administration. Drug Approval Package. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/nda/2005/21660_AbraxaneTOC.cfm (accessed on 14 November 2019).
- Cui, H.; Webber, M.J.; Stupp, S.I. Self-assembly of peptide amphiphiles: From molecules to nanostructures to biomaterials. Biopolymers 2010, 94, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Dreis, S.; Rothweiler, F.; Michaelis, M.; Cinatl, J., Jr.; Kreuter, J.; Langer, K. Preparation, characterisation and maintenance of drug efficacy of doxorubicin-loaded human serum albumin (HSA) nanoparticles. Int. J. Pharm. 2007, 341, 207–214. [Google Scholar] [CrossRef] [PubMed]
- Maghsoudi, A.; Shojaosadati, S.A.; Farahani, E.V. 5-Fluorouracil-loaded BSA nanoparticles: Formulation optimization and in vitro release study. AAPS PharmSciTech 2008, 9, 1092–1096. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Cui, F.; Cun, D.; Tao, A.; Shi, K.; Lin, W. Preparation, characterization and biodistribution of the lactone form of 10-hydroxycamptothecin (HCPT)-loaded bovine serum albumin (BSA) nanoparticles. Int. J. Pharm. 2007, 340, 163–172. [Google Scholar] [CrossRef]
- Burger, A.M.; Hartung, G.; Stehle, G.; Sinn, H.; Fiebig, H.H. Pre-clinical evaluation of a methotrexate-albumin conjugate (MTX-HSA) in human tumor xenografts in vivo. Int. J. Cancer 2001, 92, 718–724. [Google Scholar] [CrossRef]
- Liu, J.; Zhang, L.; Yang, Z.; Zhao, X. Controlled release of paclitaxel from a self-assembling peptide hydrogel formed in situ and antitumor study in vitro. Int. J. Nanomedicine 2011, 6, 2143–2153. [Google Scholar] [CrossRef]
- Edwards-Gayle, C.J.C.; Hamley, I.W. Self-assembly of bioactive peptides, peptide conjugates, and peptide mimetic materials. Org. Biomol. Chem. 2017, 15, 5867–5876. [Google Scholar] [CrossRef]
- Whitesides, G.M.; Boncheva, M. Beyond molecules: Self-assembly of mesoscopic and macroscopic components. Proc. Natl. Acad. Sci. USA 2002, 99, 4769–4774. [Google Scholar] [CrossRef]
- Ariga, K.; Hill, J.P.; Lee, M.V.; Vinu, A.; Charvet, R.; Acharya, S. Challenges and breakthroughs in recent research on self-assembly. Sci. Tech. Adv. Mat. 2008, 9, 014109. [Google Scholar] [CrossRef]
- Stendahl, J.C.; Rao, M.S.; Guler, M.O.; Stupp, S.I. Intermolecular forces in the self-assembly of peptide amphiphile nanofibers. Adv. Functi. Mat. 2006, 16, 499–508. [Google Scholar] [CrossRef]
- Dong, X.; Guo, X.; Liu, G.; Fan, A.; Wang, Z.; Zhao, Y. When self-assembly meets topology: An enhanced micelle stability. Chem. Commun. 2017, 53, 3822–3825. [Google Scholar] [CrossRef] [PubMed]
- Sevink, G.J.A.; Zvelindovsky, A.V. Self-assembly of complex vesicles. Macromolecules 2005, 38, 7502–7513. [Google Scholar] [CrossRef]
- Blau, W.J.; Fleming, A.J. Designer nanotubes by molecular self-assembly. Science 2004, 304, 1457. [Google Scholar] [CrossRef] [PubMed]
- Israelachvili, J. Dynamic Biointeraction. In Intermolecular and Surface Forces, 3rd ed.; Israelachvili, J., Ed.; Elsevier Inc.: Waltham, MA, USA, 2011; pp. 617–633. [Google Scholar]
- Reches, M.; Gazit, E. Casting metal nanowires within discrete self-assembled peptide nanotubes. Science 2003, 300, 625. [Google Scholar] [CrossRef] [PubMed]
- Mahler, A.; Reches, M.; Rechter, M.; Cohen, S.; Gazit, E. Rigid, self-assembled hydrogel composed of a modified aromatic dipeptide. Adv. Mat. 2006, 18, 1365–1370. [Google Scholar] [CrossRef]
- Yang, Z.; Liang, G.; Xu, B. Supramolecular hydrogels based on β-amino acid derivatives. Chem. Commun. 2006, 738–740. [Google Scholar] [CrossRef]
- Argudo, P.G.; Contreras-Montoya, R.; Álvarez de Cienfuegos, L.; Cuerva, J.M.; Cano, M.; Alba-Molina, D.; Martín-Romero, M.T.; Camacho, L.; Giner-Casares, J.J. Unravelling the 2D self-assembly of Fmoc-dipeptides at fluid interfaces. Soft Matter 2018, 14, 9343–9350. [Google Scholar] [CrossRef]
- Zhao, X. Design of self-assembling surfactant-like peptides and their applications. Curr. Opin. Colloid Interface Sci. 2009, 14, 340–348. [Google Scholar] [CrossRef]
- Vauthey, S.; Santoso, S.; Gong, H.; Watson, N.; Zhang, S. Molecular self-assembly of surfactant-like peptides to form nanotubes and nanovesicles. Proc. Natl. Acad. Sci. USA 2002, 99, 5355. [Google Scholar] [CrossRef]
- Wang, J.; Han, S.; Meng, G.; Xu, H.; Xia, D.; Zhao, X.; Schweins, R.; Lu, J.R. Dynamic self-assembly of surfactant-like peptides A6K and A9K. Soft Matter 2009, 5, 3870–3878. [Google Scholar] [CrossRef]
- Hartgerink, J.D.; Beniash, E.; Stupp, S.I. Self-assembly and mineralization of peptide-amphiphile nanofibers. Science 2001, 294, 1684. [Google Scholar] [CrossRef] [PubMed]
- Hartgerink, J.D.; Beniash, E.; Stupp, S.I. Peptide-amphiphile nanofibers: A versatile scaffold for the preparation of self-assembling materials. Proc. Natl. Acad. Sci. USA 2002, 99, 5133. [Google Scholar] [CrossRef] [PubMed]
- Claussen, R.C.; Rabatic, B.M.; Stupp, S.I. Aqueous self-assembly of unsymmetric peptide bolaamphiphiles into nanofibers with hydrophilic cores and surfaces. J. Am. Chem. Soc. 2003, 125, 12680–12681. [Google Scholar] [CrossRef] [PubMed]
- Qiu, F.; Tang, C.; Chen, Y. Amyloid-like aggregation of designer bolaamphiphilic peptides: Effect of hydrophobic section and hydrophilic heads. J. Peptide. Sci. 2018, 24, e3062. [Google Scholar] [CrossRef] [PubMed]
- Yao, L.; He, M.; Li, D.; Liu, H.; Wu, J.; Xiao, J. Self-assembling bolaamphiphile-like collagen mimetic peptides. New J. Chem. 2018, 42, 7439–7444. [Google Scholar] [CrossRef]
- Da Silva, E.R.; Alves, W.A.; Castelletto, V.; Reza, M.; Ruokolainen, J.; Hussain, R.; Hamley, I.W. Self-assembly pathway of peptide nanotubes formed by a glutamatic acid-based bolaamphiphile. Chem. Commun. 2015, 51, 11634–11637. [Google Scholar]
- Da Silva, E.R.; Walter, M.N.; Reza, M.; Castelletto, V.; Ruokolainen, J.; Connon, C.J.; Alves, W.A.; Hamley, I.W. Self-assembled arginine-capped peptide bolaamphiphile nanosheets for cell culture and controlled wettability surfaces. Biomacromolecules 2015, 16, 3180–3190. [Google Scholar] [CrossRef]
- Dhasaiyan, P.; Prasad, B.L.V. Self-assembly of bolaamphiphilic molecules. Chem. Rec. 2017, 17, 597–610. [Google Scholar] [CrossRef]
- Chen, P. Self-assembly of ionic-complementary peptides: A physicochemical viewpoint. Colloids. Surf. 2005, 261, 3–24. [Google Scholar] [CrossRef]
- D’Auria, G.; Vacatello, M.; Falcigno, L.; Paduano, L.; Mangiapia, G.; Calvanese, L.; Gambaretto, R.; Dettin, M.; Paolillo, L. Self-assembling properties of ionic-complementary peptides. J. Pept. Sci. 2009, 15, 210–219. [Google Scholar] [CrossRef]
- Zhang, H.; Park, J.; Jiang, Y.; Woodrow, K.A. Rational design of charged peptides that self-assemble into robust nanofibers as immune-functional scaffolds. Acta. Biomater. 2017, 55, 183–193. [Google Scholar] [CrossRef] [PubMed]
- Kabiri, M.; Bushnak, I.; McDermot, M.T.; Unsworth, L.D. Toward a mechanistic understanding of ionic self-complementary peptide self-assembly: Role of water molecules and ions. Biomacromolecules 2013, 14, 3943–3950. [Google Scholar] [CrossRef] [PubMed]
- Hartgerink, J.D.; Clark, T.D.; Ghadiri, M.R. Peptide nanotubes and beyond. Chem. Eur. J. 1998, 4, 1367–1372. [Google Scholar] [CrossRef]
- Scott, C.P.; Abel-Santos, E.; Wall, M.; Wahnon, D.C.; Benkovic, S.J. Production of cyclic peptides and proteins in vivo. Proc. Natl. Acad. Sci. USA 1999, 96, 13638. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Choi, S.J.; Jeong, W.J.; Kang, S.K.; Lee, M.; Kim, E.; Ryu du, Y.; Lim, Y.B. Differential self-assembly behaviors of cyclic and linear peptides. Biomacromolecules 2012, 13, 1991–1995. [Google Scholar] [CrossRef]
- Jeong, W.J.; Choi, S.J.; Choi, J.S.; Lim, Y.B. Chameleon-like self-assembling peptides for adaptable biorecognition nanohybrids. ACS Nano 2013, 7, 6850–6857. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Fan, Z.; Wang, Y.; Huang, Y.; Schmidt, M.; Zhang, M. Tunable synthesis of self-assembled cyclic peptide nanotubes and nanoparticles. Soft Matter 2015, 11, 3822–3832. [Google Scholar] [CrossRef]
- Reches, M.; Gazit, E. Formation of closed-cage nanostructures by self-assembly of aromatic dipeptides. Nano Lett. 2004, 4, 581–585. [Google Scholar] [CrossRef]
- Song, Y.; Challa, S.R.; Medforth, C.J.; Qiu, Y.; Watt, R.K.; Pena, D.; Miller, J.E.; van Swol, F.; Shelnutt, J.A. Synthesis of peptide-nanotube platinum-nanoparticle composites. Chem. Commun. 2004, 7, 1044–1045. [Google Scholar] [CrossRef]
- Yemini, M.; Reches, M.; Gazit, E.; Rishpon, J. Peptide nanotube-modified electrodes for enzyme-biosensor applications. Anal. Chem. 2005, 77, 5155–5159. [Google Scholar] [CrossRef]
- Yemini, M.; Reches, M.; Rishpon, J.; Gazit, E. Novel electrochemical biosensing platform using self-assembled peptide nanotubes. Nano Lett. 2005, 5, 183–186. [Google Scholar] [CrossRef] [PubMed]
- Reches, M.; Gazit, E. Self-assembly of peptide nanotubes and amyloid-like structures by charged-termini-capped diphenylalanine peptide analogues. Isr. J. Chem. 2005, 45, 363–371. [Google Scholar] [CrossRef]
- Yang, Z.; Ho, P.-L.; Liang, G.; Chow, K.H.; Wang, Q.; Cao, Y.; Guo, Z.; Xu, B. Using β-lactamase to trigger supramolecular hydrogelation. J. Am. Chem. Soc. 2007, 129, 266–267. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Greenfield, M.A.; Mata, A.; Palmer, L.C.; Bitton, R.; Mantei, J.R.; Aparicio, C.; de la Cruz, M.O.; Stupp, S.I. A self-assembly pathway to aligned monodomain gels. Nat. Mater. 2010, 9, 594–601. [Google Scholar] [CrossRef] [PubMed]
- Fuhrhop, J.-H.; Wang, T. Bolaamphiphiles. Chem. Rev. 2004, 104, 2901–2938. [Google Scholar] [CrossRef]
- Nuraje, N.; Bai, H.; Su, K. Bolaamphiphilic molecules: Assembly and applications. Prog. Polym. Sci. 2013, 38, 302–343. [Google Scholar] [CrossRef]
- Sun, X.L.; Biswas, N.; Kai, T.; Dai, Z.; Dluhy, R.A.; Chaikof, E.L. Membrane-mimetic films of asymmetric phosphatidylcholine lipid bolaamphiphiles. Langmuir 2006, 22, 1201–1208. [Google Scholar] [CrossRef]
- Maity, I.; Parmar, H.S.; Rasale, D.B.; Das, A.K. Self-programmed nanovesicle to nanofiber transformation of a dipeptide appended bolaamphiphile and its dose dependent cytotoxic behaviour. J. Mat. Chem. B 2014, 2, 5272–5279. [Google Scholar] [CrossRef]
- Weissig, V.; Torchilin, V.P. Mitochondriotropic cationic vesicles: A strategy towards mitochondrial gene therapy. Curr. Pharm. Biotechnol. 2000, 1, 325–346. [Google Scholar] [CrossRef]
- Zhang, S.; Holmes, T.; Lockshin, C.; Rich, A. Spontaneous assembly of a self-complementary oligopeptide to form a stable macroscopic membrane. Proc. Natl. Acad. Sci. USA 1993, 90, 3334–3338. [Google Scholar] [CrossRef] [Green Version]
- Zhang, S.G.; Altman, M. Peptide self-assembly in functional polymer science and engineering. React. Funct. Polym. 1999, 41, 91–102. [Google Scholar]
- Scanlon, S.; Aggeli, A. Self-assembling peptide nanotubes. Nano Today 2008, 3, 22–30. [Google Scholar] [CrossRef]
- Chapman, R.; Danial, M.; Koh, M.L.; Jolliffe, K.A.; Perrier, S. Design and properties of functional nanotubes from the self-assembly of cyclic peptide templates. Chem. Soc. Rev. 2012, 41, 6023–6041. [Google Scholar] [CrossRef] [PubMed]
- Muraoka, T.; Cui, H.; Stupp, S.I. Quadruple helix formation of a photoresponsive peptide amphiphile and its light-triggered dissociation into single fibers. J. Am. Chem. Soc. 2008, 130, 2946–2947. [Google Scholar] [CrossRef]
- Bulut, S.; Erkal, T.S.; Toksoz, S.; Tekinay, A.B.; Tekinay, T.; Guler, M.O. Slow release and delivery of antisense oligonucleotide drug by self-assembled peptide amphiphile nanofibers. Biomacromolecules 2011, 12, 3007–3014. [Google Scholar] [CrossRef] [Green Version]
- Ceylan, H.; Kocabey, S.; Tekinay, A.B.; Guler, M.O. Surface-adhesive and osteogenic self-assembled peptide nanofibers for bioinspired functionalization of titanium surfaces. Soft Matter 2012, 8, 3929–3937. [Google Scholar] [CrossRef]
- Ghadiri, M.R.; Granja, J.R.; Milligan, R.A.; McRee, D.E.; Khazanovich, N. Self-assembling organic nanotubes based on a cyclic peptide architecture. Nature 1993, 366, 324–327. [Google Scholar] [CrossRef]
- Zhao, X.; Pan, F.; Xu, H.; Yaseen, M.; Shan, H.; Hauser, C.A.; Zhang, S.; Lu, J.R. Molecular self-assembly and applications of designer peptide amphiphiles. Chem. Soc. Rev. 2010, 39, 3480–3498. [Google Scholar] [CrossRef]
- Discher, D.E.; Eisenberg, A. Polymer vesicles. Science 2002, 297, 967. [Google Scholar] [CrossRef] [Green Version]
- Bellomo, E.G.; Wyrsta, M.D.; Pakstis, L.; Pochan, D.J.; Deming, T.J. Stimuli-responsive polypeptide vesicles by conformation-specific assembly. Nat. Mater. 2004, 3, 244–248. [Google Scholar] [CrossRef]
- Nasrolahi Shirazi, A.; Tiwari, R.K.; Oh, D.; Banerjee, A.; Yadav, A.; Parang, K. Efficient delivery of cell impermeable phosphopeptides by a cyclic peptide amphiphile containing tryptophan and arginine. Mol. Pharm. 2013, 10, 2008–2020. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aggeli, A.; Bell, M.; Boden, N.; Keen, J.N.; McLeish, T.C.B.; Nyrkova, I.; Radford, S.E.; Semenov, A. Engineering of peptide β-sheet nanotapes. J. Mat. Chem. 1997, 7, 1135–1145. [Google Scholar] [CrossRef]
- Dehsorkhi, A.; Castelletto, V.; Hamley, I.W.; Adamcik, J.; Mezzenga, R. The effect of pH on the self-assembly of a collagen derived peptide amphiphile. Soft Matter 2013, 9, 6033–6036. [Google Scholar] [CrossRef] [Green Version]
- Hamley, I.W.; Dehsorkhi, A.; Jauregi, P.; Seitsonen, J.; Ruokolainen, J.; Coutte, F.; Chataigne, G.; Jacques, P. Self-assembly of three bacterially-derived bioactive lipopeptides. Soft Matter 2013, 9, 9572–9578. [Google Scholar] [CrossRef] [Green Version]
- Kopeček, J.; Yang, J. Peptide-directed self-assembly of hydrogels. Acta Biomaterialia 2009, 5, 805–816. [Google Scholar] [CrossRef] [Green Version]
- Yu, Z.; Xu, Q.; Dong, C.; Lee, S.S.; Gao, L.; Li, Y.; D’Ortenzio, M.; Wu, J. Self-assembling peptide nanofibrous hydrogel as a versatile drug delivery platform. Curr. Pharm. Des. 2015, 21, 4342–4354. [Google Scholar] [CrossRef]
- Kisiday, J.; Jin, M.; Kurz, B.; Hung, H.; Semino, C.; Zhang, S.; Grodzinsky, A.J. Self-assembling peptide hydrogel fosters chondrocyte extracellular matrix production and cell division: Implications for cartilage tissue repair. Proc. Natl. Acad. Sci. USA 2002, 99, 9996. [Google Scholar] [CrossRef] [Green Version]
- Xing, R.; Li, S.; Zhang, N.; Shen, G.; Möhwald, H.; Yan, X. Self-assembled injectable peptide hydrogels capable of triggering antitumor immune response. Biomacromolecules 2017, 18, 3514–3523. [Google Scholar] [CrossRef]
- Orbach, R.; Adler-Abramovich, L.; Zigerson, S.; Mironi-Harpaz, I.; Seliktar, D.; Gazit, E. Self-assembled fmoc-peptides as a platform for the formation of nanostructures and hydrogels. Biomacromolecules 2009, 10, 2646–2651. [Google Scholar] [CrossRef]
- Khandogin, J.; Chen, J.; Brooks, C.L., 3rd. Exploring atomistic details of pH-dependent peptide folding. Proc. Natl. Acad. Sci. USA 2006, 103, 18546–18550. [Google Scholar] [CrossRef] [Green Version]
- Cerpa, R.; Cohen, F.E.; Kuntz, I.D. Conformational switching in designed peptides: The helix/sheet transition. Fold. Des. 1996, 1, 91–101. [Google Scholar] [CrossRef] [Green Version]
- Furukawa, K.; Oba, M.; Toyama, K.; Opiyo, G.O.; Demizu, Y.; Kurihara, M.; Doi, M.; Tanaka, M. Low pH-triggering changes in peptide secondary structures. Org. Biomol. Chem. 2017, 15, 6302–6305. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Do, T.D.; LaPointe, N.E.; Economou, N.J.; Buratto, S.K.; Feinstein, S.C.; Shea, J.-E.; Bowers, M.T. Effects of pH and charge state on peptide assembly: The YVIFL model system. J. Phys. Chem. B. 2013, 117, 10759–10768. [Google Scholar] [CrossRef] [PubMed]
- Waqas, M.; Jeong, W.-j.; Lee, Y.-J.; Kim, D.-H.; Ryou, C.; Lim, Y.-B. pH-dependent in-cell self-assembly of peptide inhibitors increases the anti-prion activity while decreasing the cytotoxicity. Biomacromolecules 2017, 18, 943–950. [Google Scholar] [CrossRef]
- Ozkan, A.D.; Tekinay, A.B.; Guler, M.O.; Tekin, E.D. Effects of temperature, pH and counterions on the stability of peptide amphiphile nanofiber structures. RSC Adv. 2016, 6, 104201–104214. [Google Scholar] [CrossRef]
- Huang, R.; Wang, Y.; Qi, W.; Su, R.; He, Z. Temperature-induced reversible self-assembly of diphenylalanine peptide and the structural transition from organogel to crystalline nanowires. Nanoscale Res. Lett. 2014, 9, 653. [Google Scholar] [CrossRef] [Green Version]
- Weitzhandler, I.; Dzuricky, M.; Hoffmann, I.; Garcia Quiroz, F.; Gradzielski, M.; Chilkoti, A. Micellar self-assembly of recombinant resilin-/elastin-like block copolypeptides. Biomacromolecules 2017, 18, 2419–2426. [Google Scholar] [CrossRef]
- Cao, M.; Shen, Y.; Wang, Y.; Wang, X.; Li, D. Self-assembly of short elastin-like amphiphilic peptides: Effects of temperature, molecular hydrophobicity and charge distribution. Molecules 2019, 24, 202. [Google Scholar] [CrossRef] [Green Version]
- Hassouneh, W.; Fischer, K.; MacEwan, S.R.; Branscheid, R.; Fu, C.L.; Liu, R.; Schmidt, M.; Chilkoti, A. Unexpected multivalent display of proteins by temperature triggered self-assembly of elastin-like polypeptide block copolymers. Biomacromolecules 2012, 13, 1598–1605. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.; Liu, K.; Xing, R.; Yan, X. Peptide self-assembly: Thermodynamics and kinetics. Chem. Soc. Rev. 2016, 45, 5589–5604. [Google Scholar] [CrossRef]
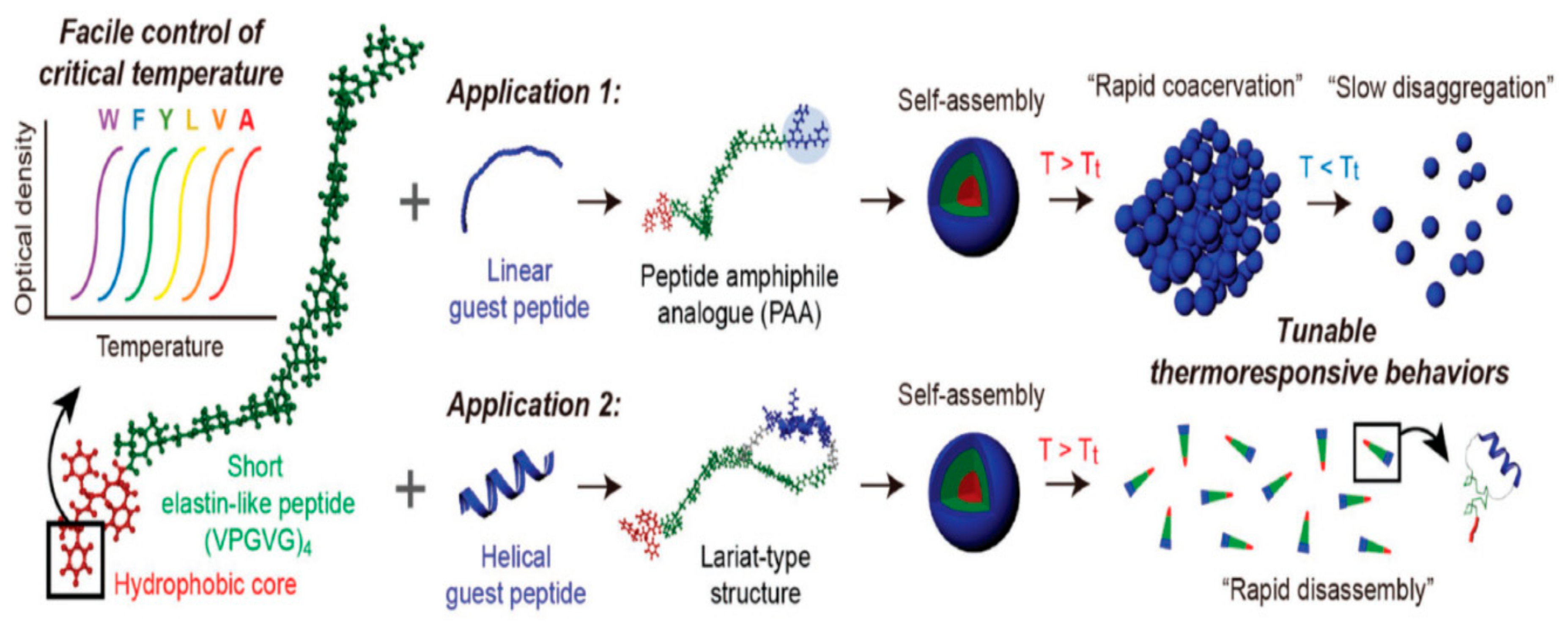
- Jeong, W.J.; Kwon, S.H.; Lim, Y.B. Modular self-assembling peptide platform with a tunable thermoresponsiveness via a single amino acid substitution. Adv. Funct. Mat. 2018, 28, 1803114. [Google Scholar] [CrossRef]
- Liyanage, W.; Rubeo Paul, W.; Nilsson Bradley, L. Redox-sensitive reversible self-assembly of amino acid–naphthalene diimide conjugates. Interface Focus 2017, 7, 20160099. [Google Scholar] [CrossRef] [PubMed]
- Piccoli, J.P.; Santos, A.; Santos-Filho, N.A.; Lorenzón, E.N.; Cilli, E.M.; Bueno, P.R. The self-assembly of redox active peptides: Synthesis and electrochemical capacitive behavior. Pept. Sci. 2016, 106, 357–367. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.W.; Yang, K.; Jeong, W.J.; Choi, S.J.; Lee, J.S.; Cho, A.N.; Chang, G.E.; Cheong, E.; Cho, S.W.; Lim, Y.B. Photoactivation of noncovalently assembled peptide ligands on carbon nanotubes enables the dynamic regulation of stem cell differentiation. ACS Appl. Mater. Interfaces 2016, 8, 26470–26481. [Google Scholar] [CrossRef] [PubMed]
- Shi, J.; Fichman, G.; Schneider, J.P. Enzymatic control of the conformational landscape of self-assembling peptides. Angew Chem. Int. Ed. Engl. 2018, 57, 11188–11192. [Google Scholar] [CrossRef] [PubMed]
- Kumada, Y.; Hammond, N.A.; Zhang, S.G. Functionalized scaffolds of shorter self-assembling peptides containing MMP-2 cleavable motif promote fibroblast proliferation and significantly accelerate 3-D cell migration independent of scaffold stiffness. Soft Matter 2010, 6, 5073–5079. [Google Scholar] [CrossRef]
- Chow, L.W.; Bitton, R.; Webber, M.J.; Carvajal, D.; Shull, K.R.; Sharma, A.K.; Stupp, S.I. A bioactive self-assembled membrane to promote angiogenesis. Biomaterials 2011, 32, 1574–1582. [Google Scholar] [CrossRef] [Green Version]
- Rajangam, K.; Behanna, H.A.; Hui, M.J.; Han, X.; Hulvat, J.F.; Lomasney, J.W.; Stupp, S.I. Heparin binding nanostructures to promote growth of blood vessels. Nano Lett. 2006, 6, 2086–2090. [Google Scholar] [CrossRef]
- Jung, J.P.; Jones, J.L.; Cronier, S.A.; Collier, J.H. Modulating the mechanical properties of self-assembled peptide hydrogels via native chemical ligation. Biomaterials 2008, 29, 2143–2151. [Google Scholar] [CrossRef] [Green Version]
- Luo, G.; Yu, X.; Jin, C.; Yang, F.; Fu, D.; Long, J.; Xu, J.; Zhan, C.; Lu, W. LyP-1-conjugated nanoparticles for targeting drug delivery to lymphatic metastatic tumors. Int. J. Pharm. 2010, 385, 150–156. [Google Scholar] [CrossRef]
- Altunbas, A.; Lee, S.J.; Rajasekaran, S.A.; Schneider, J.P.; Pochan, D.J. Encapsulation of curcumin in self-assembling peptide hydrogels as injectable drug delivery vehicles. Biomaterials 2011, 32, 5906–5914. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guo, H.D.; Cui, G.H.; Yang, J.J.; Wang, C.; Zhu, J.; Zhang, L.S.; Jiang, J.; Shao, S.J. Sustained delivery of VEGF from designer self-assembling peptides improves cardiac function after myocardial infarction. Biochem. Biophys. Res. Commun. 2012, 424, 105–111. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Venkatraman, S.S.; Yang, Y.Y.; Guo, K.; Lu, J.; He, B.; Moochhala, S.; Kan, L. Polymeric micelles anchored with TAT for delivery of antibiotics across the blood-brain barrier. Biopolymers 2008, 90, 617–623. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Jiang, X.; Gong, S.; Feng, L.; Zhong, Y.; Pang, Z. The proton permeability of self-assembled polymersomes and their neuroprotection by enhancing a neuroprotective peptide across the blood-brain barrier after modification with lactoferrin. Nanoscale 2014, 6, 3250–3258. [Google Scholar] [CrossRef]
- Murphy, E.A.; Majeti, B.K.; Barnes, L.A.; Makale, M.; Weis, S.M.; Lutu-Fuga, K.; Wrasidlo, W.; Cheresh, D.A. Nanoparticle-mediated drug delivery to tumor vasculature suppresses metastasis. Proc. Natl. Acad. Sci. USA 2008, 105, 9343–9348. [Google Scholar] [CrossRef] [Green Version]
- Matson, J.B.; Stupp, S.I. Drug release from hydrazone-containing peptide amphiphiles. Chem. Commun. 2011, 47, 7962–7964. [Google Scholar] [CrossRef] [Green Version]
- Jabbari, E.; Yang, X.; Moeinzadeh, S.; He, X. Drug release kinetics, cell uptake, and tumor toxicity of hybrid VVVVVVKK peptide-assembled polylactide nanoparticles. Eur. J. Pharm. Biopharm. 2013, 84, 49–62. [Google Scholar] [CrossRef] [Green Version]
- Fung, S.Y.; Yang, H.; Bhola, P.T.; Sadatmousavi, P.; Muzar, E.; Liu, M.; Chen, P. Self-assembling peptide as a potential carrier for dydrophobic anticancer drug ellipticine: Complexation, release and in vitro delivery. Adv. Funct. Mater. 2009, 19, 74–83. [Google Scholar] [CrossRef]
- Briuglia, M.L.; Urquhart, A.J.; Lamprou, D.A. Sustained and controlled release of lipophilic drugs from a self-assembling amphiphilic peptide hydrogel. Int. J. Pharm. 2014, 474, 103–111. [Google Scholar] [CrossRef] [Green Version]
- Nagai, Y.; Unsworth, L.D.; Koutsopoulos, S.; Zhang, S. Slow release of molecules in self-assembling peptide nanofiber scaffold. J. Control. Release 2006, 115, 18–25. [Google Scholar] [CrossRef]
- Semino, C.E.; Merok, J.R.; Crane, G.G.; Panagiotakos, G.; Zhang, S. Functional differentiation of hepatocyte-like spheroid structures from putative liver progenitor cells in three-dimensional peptide scaffolds. Differentiation 2003, 71, 262–270. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, M.; Yang, Z.; Liu, Y.; Liu, B.; Zhao, X. The 3-D culture and in vivo growth of the human hepatocellular carcinoma cell line HepG2 in a self-assembling peptide nanofiber scaffold. J. Nanomater. 2010, 2010, 1–7. [Google Scholar] [CrossRef]
- Holmes, T.C.; de Lacalle, S.; Su, X.; Liu, G.; Rich, A.; Zhang, S. Extensive neurite outgrowth and active synapse formation on self-assembling peptide scaffolds. Proc. Natl. Acad. Sci. USA 2000, 97, 6728–6733. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gelain, F.; Bottai, D.; Vescovi, A.; Zhang, S. Designer self-assembling peptide nanofiber scaffolds for adult mouse neural stem cell 3-dimensional cultures. PLoS ONE 2006, 1, e119. [Google Scholar] [CrossRef] [Green Version]
- Cunha, C.; Panseri, S.; Villa, O.; Silva, D.; Gelain, F. 3D culture of adult mouse neural stem cells within functionalized self-assembling peptide scaffolds. Int. J. Nanomed. 2011, 6, 943–955. [Google Scholar] [CrossRef] [Green Version]
- Kumada, Y.; Zhang, S. Significant type I and type III collagen production from human periodontal ligament fibroblasts in 3D peptide scaffolds without extra growth factors. PLoS ONE 2010, 5, e10305. [Google Scholar] [CrossRef] [Green Version]
- Kisiday, J.D.; Jin, M.; DiMicco, M.A.; Kurz, B.; Grodzinsky, A.J. Effects of dynamic compressive loading on chondrocyte biosynthesis in self-assembling peptide scaffolds. J. Biomech. 2004, 37, 595–604. [Google Scholar] [CrossRef]
- Mujeeb, A.; Miller, A.F.; Saiani, A.; Gough, J.E. Self-assembled octapeptide scaffolds for in vitro chondrocyte culture. Acta Biomater. 2013, 9, 4609–4617. [Google Scholar] [CrossRef]
- Senapati, S.; Mahanta, A.K.; Kumar, S.; Maiti, P. Controlled drug delivery vehicles for cancer treatment and their performance. Signal Transduct. Target Ther. 2018, 3, 7. [Google Scholar] [CrossRef] [Green Version]
- Zhang, X.X.; Eden, H.S.; Chen, X. Peptides in cancer nanomedicine: Drug carriers, targeting ligands and protease substrates. J. Control. Release 2012, 159, 2–13. [Google Scholar] [CrossRef] [Green Version]
- Torchilin, V. Multifunctional and stimuli-sensitive pharmaceutical nanocarriers. Eur. J. Pharm. Biopharm. 2009, 71, 431–444. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, F.; Ma, M.L.; Xu, B. Molecular hydrogels of therapeutic agents. Chem. Soc. Rev. 2009, 38, 883–891. [Google Scholar] [CrossRef] [PubMed]
- Sadatmousavi, P.; Soltani, M.; Nazarian, R.; Jafari, M.; Chen, P. Self-assembling peptides: Potential role in tumor targeting. Curr. Pharm. Biotechnol. 2011, 12, 1089–1100. [Google Scholar] [CrossRef] [PubMed]
- Matson, J.B.; Newcomb, C.J.; Bitton, R.; Stupp, S.I. Nanostructure-templated control of drug release from peptide amphiphile nanofiber gels. Soft Matter 2012, 8, 3586–3595. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fan, T.; Yu, X.; Shen, B.; Sun, L. Peptide self-assembled nanostructures for drug delivery applications. J. Nanomater. 2017, 2017, 1–16. [Google Scholar] [CrossRef] [Green Version]
- Cheng, T.Y.; Wu, H.C.; Huang, M.Y.; Chang, W.H.; Lee, C.H.; Wang, T.W. Self-assembling functionalized nanopeptides for immediate hemostasis and accelerative liver tissue regeneration. Nanoscale 2013, 5, 2734–2744. [Google Scholar] [CrossRef] [PubMed]
- Ryou, C. Prions and prion diseases: Fundamentals and mechanistic details. J. Microbiol. Biotechnol. 2007, 17, 1059–1070. [Google Scholar]
- Silva, G.A.; Czeisler, C.; Niece, K.L.; Beniash, E.; Harrington, D.A.; Kessler, J.A.; Stupp, S.I. Selective differentiation of neural progenitor cells by high-epitope density nanofibers. Science 2004, 303, 1352–1355. [Google Scholar] [CrossRef] [Green Version]
- Guo, J.; Leung, K.K.; Su, H.; Yuan, Q.; Wang, L.; Chu, T.H.; Zhang, W.; Pu, J.K.; Ng, G.K.; Wong, W.M.; et al. Self-assembling peptide nanofiber scaffold promotes the reconstruction of acutely injured brain. Nanomedicine 2009, 5, 345–351. [Google Scholar] [CrossRef]
- Chung, E.; Ricles, L.M.; Stowers, R.S.; Nam, S.Y.; Emelianov, S.Y.; Suggs, L.J. Multifunctional nanoscale strategies for enhancing and monitoring blood vessel regeneration. Nano. Today 2012, 7, 514–531. [Google Scholar] [CrossRef] [Green Version]
- Makovitzki, A.; Baram, J.; Shai, Y. Antimicrobial lipopolypeptides composed of palmitoyl Di- and tricationic peptides: In vitro and in vivo activities, self-assembly to nanostructures, and a plausible mode of action. Biochemistry 2008, 47, 10630–10636. [Google Scholar] [CrossRef] [PubMed]
- Yang, G.; Huang, T.; Wang, Y.; Wang, H.; Li, Y.; Yu, K.; Dong, L. Sustained release of antimicrobial peptide from self-assembling hydrogel enhanced osteogenesis. J. Biomater. Sci. Polym. Ed. 2018, 29, 1812–1824. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Xu, K.; Wang, H.; Tan, P.K.; Fan, W.; Venkatraman, S.S.; Li, L.; Yang, Y.Y. Self-assembled cationic peptide nanoparticles as an efficient antimicrobial agent. Nat. Nanotechnol. 2009, 4, 457–463. [Google Scholar] [CrossRef] [PubMed]
- Jeena, M.T.; Palanikumar, L.; Go, E.M.; Kim, I.; Kang, M.G.; Lee, S.; Park, S.; Choi, H.; Kim, C.; Jin, S.-M.; et al. Mitochondria localization induced self-assembly of peptide amphiphiles for cellular dysfunction. Nat. Commun. 2017, 8, 26. [Google Scholar] [CrossRef]
- Yang, Z.M.; Xu, K.M.; Guo, Z.F.; Guo, Z.H.; Xu, B. Intracellular enzymatic formation of nanofibers results in hydrogelation and regulated cell death. Adv. Mat. 2007, 19, 3152–3156. [Google Scholar] [CrossRef]
- Zhou, J.; Du, X.; Berciu, C.; He, H.; Shi, J.; Nicastro, D.; Xu, B. Enzyme-instructed self-assembly for spatiotemporal profiling of the activities of alkaline phosphatases on live cells. Chem 2016, 1, 246–263. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gao, Y.; Kuang, Y.; Guo, Z.-F.; Guo, Z.; Krauss, I.J.; Xu, B. Enzyme-instructed molecular self-assembly confers nanofibers and a supramolecular hydrogel of taxol derivative. J. Am. Chem. Soc. 2009, 131, 13576–13577. [Google Scholar] [CrossRef]




| Peptide Building Blocks | Characteristics | References |
|---|---|---|
| Dipeptides | Simple phenylalanine dipeptides with or without N-terminal modifications, such as N-fluorenylmethoxycarbonyl (Fmoc) and naphthyl | [16,17,18,19] |
| Surfactant-like peptides | Amphiphilic structure with both hydrophilic and hydrophobic amino acids included in the peptide head and tail | [20,21,22] |
| Repeated sequence of hydrophobic amino acids | ||
| Peptide amphiphiles with an alkyl group | An alkyl tail linked to the N- or C-terminus | [23,24] |
| A hydrophilic functional region | ||
| Form a stable β-sheet, providing hydrogen bonds for self-assembly | ||
| Glycine linker residues support flexibility | ||
| Bolaamphiphilic peptides | Two hydrophilic heads connected by a hydrophobic region that is generally composed of alkyls | [25,26,27,28,29,30] |
| Ionic-complementary self-assembling peptides | A hydrophobic tail promotes self-assembly in water | [31,32,33,34] |
| A hydrophilic tail with charged amino acids residues forms an ionic bond | ||
| Classified by the number of repeated ion charges: Type I has a charge pattern of “+-+-+-”, Type II has “++--++--“, Type III has “+++---+++”, and Type IV has “++++----“. | ||
| Cyclic peptides | Even number of alternating D and L amino acids stacked by hydrogen bonding | [35,36,37,38,39] |
| Other types of cyclic peptides are characterized by amphiphilic characteristics, i.e., one side of the cycle is hydrophilic, whereas the other side contains hydrophobic and/or aggregation-prone amino acids |
| Structure | Sequence | Applications | Reference |
|---|---|---|---|
| Nanofibers | VEVK9 (VEVKVEVKV) and VEVK12 (VEVKVEVKVEVK)/combined with RGD | Increase fibroblast migration | [88] |
| V3A3E3 (VVVAAAEEE) | Stem cell culture and differentiation | [23,46] | |
| Nanotubes | Heparin-binding peptide amphiphile (HBPA) | Hierarchical structure | [89,90] |
| Q11 (QQKFQFQFEQQ) | Endothelial cell proliferation | [91] | |
| Nano particle, vesicle, micelle, suspension | Lyp-1 (CGNKRTRGC) | Increase drug cellular uptake | [92] |
| MAX8 (VKVKVKVKVDPPTKVEVKVKV) | Drug delivery | [93] | |
| RADA16 with LRKKLGKA | Vascular endothelial growth factor (VEGF) delivery to the myocardium | [94] | |
| Tat/Tat combined with PEG/Cholesterol | Cross blood brain barrier (BBB)drug delivery | [95,96] | |
| cRGDfK | Drug targeting | [97] | |
| C16V2A2E2K(Hyd) | Drug stabilization | [98] | |
| V6K2(VVVVVVKK) combined with PLA | Drug delivery | [99] | |
| EAK16II (AEAEAKAKAEAEAKAK) | Drug stabilization | [100] | |
| Hydrogel | RADA16I (RADARADARADARADA) | Controlled drug release | [101,102] |
| RADA16I (RADARADARADARADA) | Hepatocyte regeneration | [103,104] | |
| RADA16 II (RARADADARARADADA) | Neuron regeneration | [105] | |
| RADA16-I combined with RGD motif | Neuron regeneration | [106,107] | |
| RADA16-I combined with RGD motif | Ligament regeneration | [108] | |
| KLD12 (KFDLKKDLKLDL) | Hepatocyte regeneration | [103] | |
| KLD12 (KFDLKKDLKLDL) | Chondrocyte regeneration | [69,109] | |
| KFE8 (FKFEFKFF) | Hepatocyte regeneration | [103] | |
| FEFEFKFK octarepeat | Extracellular matrix (ECM) accumulation | [110] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, S.; Trinh, T.H.T.; Yoo, M.; Shin, J.; Lee, H.; Kim, J.; Hwang, E.; Lim, Y.-b.; Ryou, C. Self-Assembling Peptides and Their Application in the Treatment of Diseases. Int. J. Mol. Sci. 2019, 20, 5850. https://doi.org/10.3390/ijms20235850
Lee S, Trinh THT, Yoo M, Shin J, Lee H, Kim J, Hwang E, Lim Y-b, Ryou C. Self-Assembling Peptides and Their Application in the Treatment of Diseases. International Journal of Molecular Sciences. 2019; 20(23):5850. https://doi.org/10.3390/ijms20235850
Chicago/Turabian StyleLee, Sungeun, Trang H.T. Trinh, Miryeong Yoo, Junwu Shin, Hakmin Lee, Jaehyeon Kim, Euimin Hwang, Yong-beom Lim, and Chongsuk Ryou. 2019. "Self-Assembling Peptides and Their Application in the Treatment of Diseases" International Journal of Molecular Sciences 20, no. 23: 5850. https://doi.org/10.3390/ijms20235850





