Argon: Systematic Review on Neuro- and Organoprotective Properties of an “Inert” Gas
Abstract
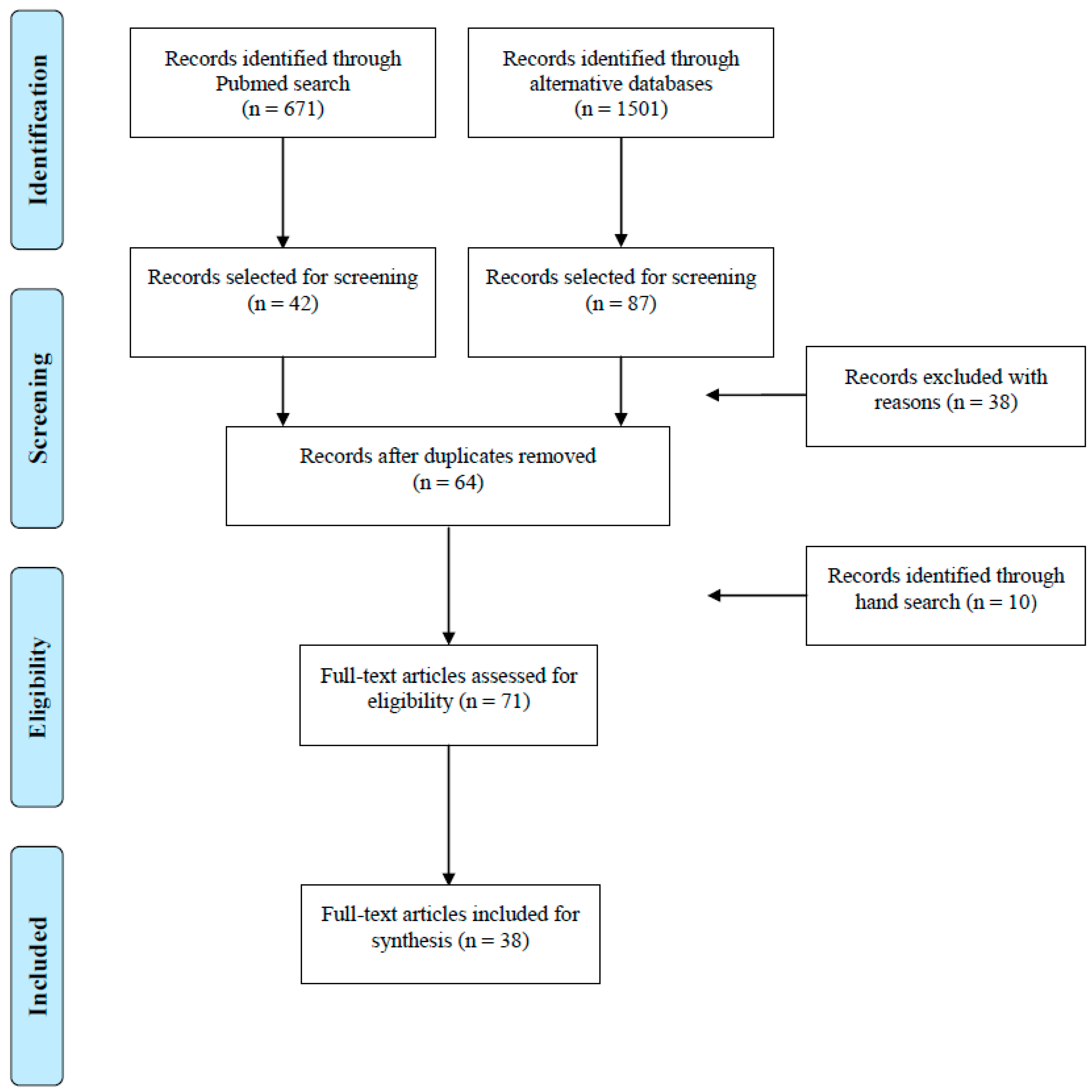
:1. Introduction
2. Results
3. Discussion
3.1. Physiological Studies
| Experimental Model | Number of Cases | Dose and Concentration | Outcome Parameter | Results of Experiments | Conclusion | Reference |
|---|---|---|---|---|---|---|
| Mental performance in Ar-N2-O2-atmosphere | n = 4, male | six days (5 m depths): 14% O2, 33% N2, 54% Ar, 0.2% CO2 followed by three days: 10% O2, 35% N2, 55% Ar, 0.2% CO2 | Adaptive biocontrol of cortical (ABC) bioelectric activity synchronization, emotional and mental performance (Luscher test), “Minesweeper” and “Tetris” performance | Partial improvement of performance, overall no decrease of ABC skill | Despite fluctuations of anxiety levels no influence on work performance, tendency to loose preservation of adaptation process with argon-mix | Antonov & Ershova (2009) [16] |
| Assessment of mental impairment breathing argon at different pressures (corresponding to 90–130 m diving depth) | n = 4 | 69% Ar, 11% N2, 20% O2, duration not specified | Self-assessment of diving depth | No effect on mental status for normobaric argon, mental impairment at pressure levels corresponding to depths of 90–130 m (tendency to overestimate diving depth) | Narcotic effect of argon is greater than that of nitrogen | Behnke & Yarbrough (1939) [1] |
| Comparison of argon and nitrogen narcosis at 1 to 10 ATA (0.1 resp. 1.1 MPa) | n = 10 | 80% Ar, 20% O2 or air (different pressure levels) | Assessment of narcosis: mental arithmetic, subjective estimate of narcosis, adjective checklist. | Arithmetic: numbers of errors increase with high pressure (with argon mix more than with air), subjective rating of narcosis: increases with higher pressure (with argon mix more than with air), adjective checklist: number of responses increases with pressure (highly variable) | Inert gases exert qualitavely identical effects | Fowler & Ackles (1972) [13] |
| (a) Exposition to white noise (85 dB) for 1 h; (b) Exposition of rats to hypoxic gas mix; (c) Exposition of hair cells (ex vivo) to hypoxic Ar-/N2-saturated medium | n = 10 | (a) 24% Ar, 60% N2, 16% O2, normobaric, duration not specified; (b) ≥25% Ar, 4%–5% O2 normobaric; (c) 95% Ar, 5% CO2 or 95% N2, 5% CO2 | (a) Pure-tone audiometry, TEOAE, DPOAE, BERA, EcohG; (b) Survivability of rats; (c) Survival time of hair cells in medium | (a) Improved condition of acoustic system in the argon treated group; (b) Increased survival in Ar-gas mix; (c) Increased survival of hair cells in Ar-containing medium | Oto- and neuroprotective effect of argon, attenuates effects of hypoxia | Matsnev et al. (2007) [23] |
| Long term (7 day) effects of hypoxic argon-oxygen mixture on human performance | n = 4 male | 7 days (10 m depths): 0.2 kg/cm2 O2, 0.8 kg/cm2 N2, 1.0 kg/cm2 Ar | Assessment of respiratory, cardiovascular and neurological parameters, evaluation of physical and mental work performance | Shift in lipid metabolism, better work performance with hyperbaric 15% Ar-O2 mixture | Argon is physiologically active causing increased resistance to hypoxic hypoxia (redox-reaction) | Pavlov et al. (1999) [14] |
| Oxygen consumption breathing Ar-containing gas mixtures during physical (submaximal) exercise | n = 7, male | 15% O2, 30% Ar, 55% N2 or 15% O2, 85% N2 | Oxygen consumption, heart rate, ventilation frequency during physical exercise breathing hypoxic gas mixtures | Increase of oxygen consumption during exercise breathing Ar-mix compared to N2-mix | Catalytic activity of argon on kinetics of oxygen consumption which might increase tolerance towards hypoxia | Shulagin et al. (2001) [15] |
| Experimental Model | Species, Age | Number of Cases | Pressure, Dose and Concentration | Outcome Parameter | Results of Experiments | Conclusion | Reference |
|---|---|---|---|---|---|---|---|
| Assessment of argon’s narcotic potency after pretreatment with GABA-antagonists (GABAA-receptor-antagonist gabazine; GABAB-receptor antagonist, GABAA-receptor antagonist benzodiazepine site) | Sprague Dawley rats, adult | n = 6 per group | Argon was dosed at 0.1 Mpa/min until narcosis was reached | Loss of righting reflex | Increase of argon threshold pressure after pretreatment with GABAA-receptor antagonist and GABAA-receptor antagonist for benzodiazepine site | Argon may interact directly with the GABAA receptor and partly with its benzodiazepine site | Abraini et al. (2003) [26] |
| Evaluation of relationship of locomotor and motor activity and striatal dopamine release under argon narcosis | Sprague Dawley rats, adult | Total n = 108 | 2 MPa (with 0.1 MPa/min) | Behavioral analysis, quantification of striatal dopamine release | Biphasic pattern with initial hyperactivity after compression; decrease of activity and dopamine release after 1 MPa | Dopamine release could be related to decrease of hyperactivity under argon narcosis | Balon et al. (2003) [27] |
| Assessment of reaction in response to minimal electroshock and antagonisation with antipsychotic drug (Frenquel) | Wistar rats | n = 46; 102 experiments | 12.6 atm abs (=1.3 MPa) | Reaction to minimal electroshock | Greater narcotic potency of argon compared with nitrogen, partly abolished by Frenquel | Argon narcosis may arise from histotoxic hypoxia; Frenquel somehow decreases the narcotic effect | Bennett (1963) [17] |
| Cardiac arrest for 7 min followed by 3 min resuscitation (CPR), postconditioning with argon | Sprague Dawley rats, adult | n = 7 per group | 1 h after CPR: 70% Ar, 30% O2 for 1 h | Neurological performance 7d after CPR, hippocampal cell loss | Better neurological performance (NDS score) and less neuronal damage of neocortex and hippocampus (C3/4), no difference in caspase 3/9 expression | Long lasting functional effect paralleled by less neuronal damage C3/4 | Brücken et al. (2013) [28] |
| Cardiac arrest for 7 min followed by 3 min resuscitation (CPR), postconditioning with argon, pretreatment with 5HD (KATP-Channel-Blocker) | Sprague Dawley rats, adult | n = 9 per group | 1 h after CPR: either 70% Ar and 30% O2 or 40% Ar, 30% O2 and 30% N2 | Neurological performance 8d after CPR, neuronal loss (neocortex, hippocampal C3/4) | Better neurological performance in argon –treated group (70% Ar > 40% Ar), less neuronal loss (regardless of Ar-concentration), no influence of 5HD on beneficial argon effect | Argon exerts dose dependent neuroprotective effect, KATP-Channels seem not to be involved in the mechanism of action | Brücken et al. (2014) [29] |
| Cardiac arrest for 7 min followed by 3 min resuscitation (CPR), postconditioning with argon | Sprague Dawley rats, adult | n = 8 per group | 1h of 70% Ar and 30% O2 either 1 or 3 h after CPR or no argon treatment | Neurological performance 8d after CPR, neuronal loss (neocortex, hippocampal C3/4, basal ganglia) | Better neurological performance and less neuronal loss in neocortex and hippocamplas C3/4 in both argon—treated groups, less neuronal damage in basal ganglia (3 h delay) | Argon exerts a neuroprotective effect even after treatment delayed for 3 h | Brücken et al. (2014) [30] |
| Assessment of oxygen consumption and development time of different species | Yeast, Drosophila, Mouse, Zootermopsis, Tenebrio, Cnemidophorus, Coloenyx | 80% Ar, 20% O2 | Oxygen consumption of different species, development time of larvae | Argon alters rate of metabolism and development (acceleration of metamorphosis) in some animals | Argon–either at atmospheric or high pressure is not inert | Cook (1950) [19] | |
| (a) OGD (brain slices); (b) NMDA-induced brain damage (in vivo); (c) MCAO (in vivo) | Sprague Dawley rats, adult | n = 8 to n= 14 per group | (a) 15%–75% Ar for 3 h after OGD; (b) 15%–75% Ar for 3 h (1 h after NMDA); (c) 50% Ar, 25% N2, 25% O2 for 3 h (2 h after MCAO) | (a) LDH release after OGD; (b) Extent of brain damage; (c) Neurologic outcome and extent of brain damage | (a) Most pronounced reduction of LDH release compared to N2 in 50% argon treated (less with 37.5% and 75% Ar); (b) Significantly attenuation of NMDA induced brain damage with 37.5 and 50% Ar; (c) Reduction of cortical ischemic volume by Ar, increase of subcortical brain damage, decrease of neurological score compared to sham | Argon shows antiexcitotoxic effects (oxygen like properties), but due the demonstrated adverse effects (increase of subcortical damage. and decrease of neurological function in the argon treated group after MCAO) results do not support therapeutic postischemic application of argon, protective effect after NMDA-induced brain injury and OGD. | David et al. (2012) [11] |
| 2 h of MCAO, 1 h after MCAO either 50% Ar/50% O2 or 50% N2/50% O2 | Sprague-Dawley rats, adult | n = 53 | 50% Ar/50% O2 or 50% N2/50% O2 for 1 h, normobaric | 24 h after MCAO, expression analysis of inflammatory and growth factors, cell count of neurons, astrocytes and microglia | In argon-treated MCAO significantly higher expression levels of IL-1beta, IL-6, iNOS, TGF-beta, and NGF were found compared to MCAO. VEGF was significantly elevated compared to sham. Significant reduction of neurons only occurred in the penumbra after MCAO | An elevated expression of several inflammatory and growth factors following MCAO + argon compared to MCAO + placebo and sham | Fahlenkamp et al. (2014) [31] |
| Effect of hypoxic argon containing gas mix (for 4 days) on early embryogenic development | Japanese quail eggs | n = 30 | 15% O2, 30% N2, 55% Ar or 15% O2, 85% N2 for 4 days | Assessment of survival and development | With argon containing gas mix up to 60% development, normal morphology, without argon only 17% reached adequate developmental state | Positive effect of argon on embryonic development in hypoxic atmosphere | Gur’eva et al. (2008) [24] |
| Transplantation of harvested kidneys after storage in Ar-, Xe- or N2-saturated solution | Wistar rats, adult | n = 60 | Storage in Ar-, Xe- or N2-saturated solution for 6 h | Assessment of renal function (Creatinine clearance, urinary albumin) 7 and 14 days after transplantation, histological examination of transplanted kidneys 14 days after transplantation | Creatinine clearance higher and urinary albumin lower as well as better renal architecture in Ar-treated group compared to N2 treated with a more pronounced effect by argon than by xenon treatment | Decrease of ischemia-reperfusion injury, improved graft function and maintained anatomical structure after Ar- treatment (compared to Xe and N2) | Irani et al. (2011) [32] |
| LAD occlusion for 30 min, preconditioning with 70% Ar/He/Ne/30% O2 or hypoxic preconditioning | New Zealand white, rabbit | n = 98 | Preconditioning with 3 cycles each 5 min (70% Ar/He/Ne, 30% O2), normobaric | Assessment of infarct size compared to hypoxic preconditioning compared to control (no preconditioning) | Significant reduction of infarct size after preconditioning with Ar, He and Ne | More pronounced cardioprotection with Ar-preconditioning compared to hypoxic preconditioning | Pagel et al. (2007) [33] |
| LAD occlusion, cardiac arrest for 8 min, CPR for 5 min followed by defibrillation, postconditioning for 4 h with either Ar/O2 or N2/O2. | Domestic pig, male | n = 12 | 70% Ar, 30% O2 or 70% N2, 30% O2 for 4 h, normobaric | Assessment of survival and neurological function 72 h after CPR, serum neuron-specific enolase (NSE) and troponin, Immunohistochemistry of brain slices | Better neurological performance in argon-treated group, significantly lower increase in serum NSE and minimal histological brain injury | Faster, complete neurologic recovery with argon treatment, no detrimental side effects, mainly functional improvement assessed | Ristagno et al. (2014) [12] |
| Narcotic effect of compression in argon atmosphere | Rats, 15 weeks | n = 15 | Ar 100–800 kPa | Assessment of behavior during compression and decompression | First signs of narcosis from 500 kPa on, subsequently falling asleep at 800 kPa (8 of 10 animals) | Demonstration of narcotic properties of argon | Ružička et al. (2007) [18] |
| 2 h of MCAO, 1 h after MCAO either 50% Ar/50% O2 or 50% N2/50% O2 | Sprague Dawley rats, adult | n = 22 | 50% Ar/50% O2 or 50% N2/50% O2 for 1 h, normobaric | 24 h after MCAO: neurological assessment, evaluation of infarct size | Improved composite adverse outcome, reduction of infarct volume (overall, cortical and subcortical) in argon-treated group | Argon demonstrates in vivo neuroprotective properties (reduced infarct size), but no improvement concerning neurological outcome and mortality | Ryang et al. (2011) [34] |
| Survivability of rats in hypoxic argon containing atmosphere | Wistar rats | Hypoxic atmosphere: O2 (4%–8%), different concentrations of Ar (0%–80%), N2 (15%–87%) and CO2 (0%–8%) | Survival rate of rats in hypoxic atmospheres with different gas mix | Adding argon increases survival rate, adding CO2 and increasing temperature reduces survival rate | Adding argon improves hypoxic tolerance | Soldatov et al. (1998) [20] | |
| Effect of hypoxic environment on development | Japanese quail eggs | 10% O2, 55% Ar, 35% N2 or 10% O2, 90% N2 | Assessment of survival rate and occurrence of teratogenic pathologies | Argon containing gas mixture reduces occurrence of teratogenic events, 100% mortality after 7 days with both mixtures | Argon reduces incidence of teratogenic events probably by stimulation of metabolism | Soldatov et al. (2002) [25] | |
| Influence of hypoxic atmosphere (O2/Ar or O2/N2) on brain metabolism | White rats | Hypoxic atmosphere: O2 (7%) with Ar or N2 | Detection of NADH/NAD in brain slices | Argon attenuates hypoxia induced metabolic impairment | Positive effect on cerebral energy metabolism by argon | Vdovin et al. (1998) [21] | |
| Decompression in atmospheres containing Ar or He | Male albino mice | Total n= 231 | 79% Ar/ He, 21% O2, decompression to 179 mmHg | Survival rate during decompression at different temperatures, assessment of oxygen consumption | Survival rate in argon containing atmosphere similar to air during decompression, higher survival rate in helium containing atmosphere | Helium promotes hypoxic resistance of mice, but none observed for argon | Witherspoon et al. (1964) [9] |
| Hypoxic ischemic brain injury: ligation of right carotid artery, hypoxia (8% O2, 92% N2) 1h after ligation for 90 min (moderate) or 120 min (severe) followed by postconditioning with Ar/He/Xe or control | Sprague Dawley rats, age: 7 days | n = 5 per group | 120 min after hypoxia: 70% Ar, 30% O2 for 90 min, normobaric | Cell viability after moderate and severe hypoxia (7 and 14 days thereafter), infarct volume, neurologic/motor performance, protein analysis contralateral hemisphere | Improved cell viability with postconditiong (Ar > Xe, He) after moderate hypoxia, improvement after severe hypoxia by Ar and Xe, induction of Bcl-2 (contralateral hemisphere) after Ar-postconditioning, neurologic function in noble gas treated animals better than control | Pronounced neuroprotective effect by argon after mild and severe hypoxia, possibly acts via upregulation of Bcl-2 expression | Zhuang et al. (2012) [35] |
| Experimental Model | Studied Material | Pressure, Dose and Concentration | Outcome Parameter | Results of Experiments | Conclusion | Reference |
|---|---|---|---|---|---|---|
| Evaluation of interaction of argon and tPA (tissue plasminogen activator) on enzymatic and thrombolytic efficiency: catalytic efficiency of tPA, blood clot formation and thrombolysis | Whole blood (Sprague Dawley rats) | 25%–75% Ar, 25% O2 | Catalytic and thrombolytic efficiency of tPA | Concentration dependent dual effect of argon on tPA effect: at concentrations higher than 50% argon increases catalytic and thrombolytic efficiency, but decreases them at concentrations lower 50% | Effect may be due to elastase binding of argon or to its interaction with oxygen competing for tPA binding and overcoming the hypoxic effect with higher concentrations (oxygen synergism) | David et al. (2013) [36] |
| Nitrogen or argon hypoxia (OGD) for 90 min followed by postconditioning with argon or nitrogen (each 75%) | Foetal (18 days) BALB/c mice, brain slices | OGD: 75% Ar, 20% N2 or 95% N2, 5% CO2; followed by: 75% Ar or 75% N2, 20% O2, 5% CO2, normobaric | Cell viability quantified by MTT assay | Neuroprotective effect of argon after OGD (less than Xe, also tested), in the absence of OGD: improved cell viability with argon compared to control (naïve) | Argon shows a significant neuroprotective effect but less pronounced than with xenon | Jawad et al. (2009) [37] |
| Exposure of primary neuronal and astroglial cell cultures and the microglial cell line BV-2 to 50% argon, additionally stimulation of microglia with LPS | BALB/c mice (primary cultures), BV-2 cell line | Exposure of primary cultures to 50% Ar for 15–120 min (vs. control N2 instead of Ar) | Protein analysis after treatment and stimulation with LPS, analysis of RNA-expression | Increase of ERK 1/2 phosphorylation in microglia by argon (mediated by upstream kinase MEK1/2), no phosphotyrosine phosphatase inhibition, no augmentation of LPS-mediated ERK 1/2 activation, no relevant modification of LPS-induced cytokine expression by argon | Short enhanced activation of ERK1/2 via MEK by argon (in primary cultures and microglia), activation does not take part via interference with phosphotyrosine phosphatases. No substantial modification of cytokine expression after LPS-exposure in microglia | Fahlenkamp et al. (2012) [38] |
| Membrane stability of peritoneal macrophages (mice) under argon or nitrogen saturated medium after UV-induced damage | Peritoneal macrophages (mice) | Normobaric, hypoxic Ar or N2 saturated medium | Measurement of intracellular pH, ability to build up fluorescein | Normobaric environment with Ar or N2 protects plasmatic membranes from UV-induced damage | Resistance against UV-induced damage is elevated by hypoxic Ar or N2 containing environment | Galchuk et al. (2001) [39] |
| (a) In vitro traumatic brain injury (hippocampal brain slices), effect of glycine administration; postconditioning with noble gas; (b) Patch clamp study to evaluate receptor effect. | (a) C57BL/6 mice (brain slices); (b) HEK293 cells | Different concentrations | (a) Extent of cell injury after trauma; (b) Effect on NMDA-mediated or TREK-1 currents | (a) Argon at 50% atm shows neuroprotective effects attenuate secondary injury after trauma (but less than xenon), glycine does not reverse argon’s positive effect; (b) NMDA-mediated or Trek-1 currents are not influenced by argon | Argon’s neuroprotective effect seems not to be mediated by NMDA-receptor glycine site, potassium channels neither seem to be involved | Harris et al. (2013) [40] |
| (a) In vitro trauma (hippocampal brain slice); (b) OGD for 30 min followed by postconditioning with argon. | Brain slices C57BL/6 | Postconditioning with 25%-74% argon (directly after lesion) or with 50% argon up to 3 h delayed | Extent of cell injury 72 h after lesion | (a) Neuroprotective effect of argon after TBI even if applied with delay up to 3 h (most effective at 50% argon concentration); (b) Dose dependent neuroprotective effect of argon after OGD even if applied with delay up to 3 h | Neuroprotective effect of argon in two types of brain lesions, effect even noticeable with 50% argon after delayed application | Loetscher et al. (2009) [41] |
| Oxygen consumption of yeast and liver slices (rat) in inert gas mixture | Yeast, liver slices (Sprague Dawley rats) | 20%–80% Ar | Oxygen consumption | Reduced oxygen consumption of yeast and live slices in buffer bubbled with argon, no effect on homogenized liver slices | Depression of oxygen consumption under argon may be due to cell membrane mediated effect as not noticeable with homogenized samples | Maio et al. (1967) [42] |
| Preconditioning with noble gas (75% for 3 h), 24 h thereafter OGD for 3 h | Cultured human renal tubular cells (HEK2) | 75% argon, helium, neon, krypton or xenon for 3 h (24 h after injury) | Cell viability 24 h after OGD, without OGD: protein analysis for p-Akt, HIF-1α and Bcl-2 | No protection from injury by argon, decrease of HIF-1α with argon | No protective effect with argon (but with xenon), for argon: no influence on Bcl-2 expression and decrease of HIF-1α expression | Rizvi et al. (2010) [10] |
| Effect of gas mixtures on induction of apoptotic cell death (by tyrosine kinase inhibitors, DNA-damaging agents and mitochondrial toxins) | Human osteosarcoma cells (U2OS) | 75% Ar or Xe or He or Ne or Kr or N2, 20% O2, 5% CO2 | Automated fluorescence microscopy to reveal cell death | Argon (and xenon) prevent cell loss after damaging agents, activation of signal transduction pathway sensitive to Z-VAD-fmk, suppresses pathways of intrinsic apoptosis (cytochrome C, caspase 3) | Argon suppresses multiple manifestations of the intrinsic apoptotic pathway | Spaggiari et al. (2013) [43] |
| Trauma of organotypic cultures (organ of Corti, rat) with (a) hypoxia; (b) cisplatin or gentamycin | Organotypic cultures (organ of Corti), Wistar rat | (a) 95% Ar or N2, 5% CO2 vs. normoxia; (b) 74% Ar or N2, 21% O2, 5% CO2 | Assessment of cell viability after 48 h | Lower damage in argon treated group after hypoxia as well as cisplatin or gentamycin damage | Protective effect of argon probably affecting Ca+ metabolism | Yarin et al. (2005) [44] |
3.2. Neuroprotective and Organoprotective Properties
3.3. Mechanism of Action
3.4. Lack of Clarity
In one in vivo study under hypoxic argon atmosphere, mice did not survive longer than the control group [9]. Another in vitro study using OGD as experimental model did not disclose a beneficial effect of argon preconditioning [10]. Finally, argon treatment in rats applying MCAO resulted in one study in reduced infarct volume (including subcortical area) but in the other in increased infarct volume of subcortical area and worse neurological outcome [11,34]. During one trial the application of argon occurred within the intraischemic phase, and on another occasion after reperfusion, as David and colleagues clearly pointed out [11].
4. Methods
5. Conclusions
Acknowledgments
Author Contributions
Appendix
Conflicts of Interest
References
- Behnke, A.R.; Yarbrough, O.D. Respiratory resistance, oil water solubility and mental effects of argon compared with helium and nitrogen. Am. J. Physiol. 1939, 126, 409–415. [Google Scholar]
- Nowrangi, D.S.; Tang, J.; Zhang, J.H. Argon gas: A potential neuroprotectant and promising medical therapy. Med. Gas Res. 2014, 4, 3. [Google Scholar] [CrossRef]
- Deng, J.; Lei, C.; Chen, Y.; Fang, Z.; Yang, Q.; Zhang, H.; Cai, M.; Shi, L.; Dong, H.; Xiong, L. Neuroprotective gases—Fantasy or reality for clinical use? Prog. Neurobiol. 2014, 115, 210–245. [Google Scholar]
- Coburn, M.; Sanders, R.D.; Ma, D.; Fries, M.; Rex, S.; Magalon, G.; Rossaint, R. Argon: The “lazy” noble gas with organoprotective properties. Eur. J. Anaesthesiol. 2012, 29, 549–551. [Google Scholar] [CrossRef] [PubMed]
- Coburn, M.; Rossaint, R. Argon in the fast lane: Noble gases and their neuroprotective effects. Crit. Care Med. 2012, 40, 1965–1966. [Google Scholar] [CrossRef] [PubMed]
- Pavlov, N.B. Argon—A biologically active component of atmosphere. Aerosp. Environ. Med. 2006, 40, 3–6. [Google Scholar]
- Fisher, M. New approaches to neuroprotective drug development. Stroke; J. Cereb. Circ. 2011, 42, S24–S27. [Google Scholar] [CrossRef]
- O’Collins, V.E.; Macleod, M.R.; Donnan, G.A.; Horky, L.L.; van der Worp, B.H.; Howells, D.W. 1026 experimental treatments in acute stroke. Ann. Neurol. 2006, 59, 467–477. [Google Scholar] [CrossRef] [PubMed]
- Witherspoon, J.D.; Wiebers, J.E.; Hiestand, W.A.; Heimlich, A.H. Decompression of mice in atmospheres containing helium or argon in place of nitrogen. Aerosp. Med. 1964, 35, 529–532. [Google Scholar] [PubMed]
- Rizvi, M.; Jawad, N.; Li, Y.; Vizcaychipi, M.P.; Maze, M.; Ma, D. Effect of noble gases on oxygen and glucose deprived injury in human tubular kidney cells. Exp. Biol. Med. 2010, 235, 886–891. [Google Scholar] [CrossRef]
- David, H.N.; Haelewyn, B.; Degoulet, M.; Colomb, D.G., Jr.; Risso, J.J.; Abraini, J.H. Ex vivo and in vivo neuroprotection induced by argon when given after an excitotoxic or ischemic insult. PLoS One 2012, 7, e30934. [Google Scholar]
- Ristagno, G.; Fumagalli, F.; Russo, I.; Tantillo, S.; Zani, D.D.; Locatelli, V.; de Maglie, M.; Novelli, D.; Staszewsky, L.; Vago, T.; et al. Postresuscitation treatment with argon improves early neurological recovery in a porcine model of cardiac arrest. Shock 2014, 41, 72–78. [Google Scholar] [CrossRef] [PubMed]
- Fowler, B.; Ackles, K.N. Narcotic effects in man of breathing 80–20 argon-oxygen and air under hyperbaric conditions. Aerosp. Med. 1972, 43, 1219–1224. [Google Scholar] [PubMed]
- Pavlov, B.N.; Buravkov, S.V.; Soldatov, P.E.; Vdovin, A.V.; Deviatova, N.V. The effects of oxygen-argon gaseous mixtures on humans under long-term hyperbaric condition. In Advances in High Pressure Bioscience and Biotechnology; Springer: Berlin Heidelberg, Germany, 1999; pp. 561–564. [Google Scholar]
- Shulagin, Iu.A.; D’Iachenko, A.I.; Pavlov, B.N. Effect of argon on oxygen consumption during physical load under hypoxic conditions in humans. Fiziol. Cheloveka 2001, 27, 95–101. [Google Scholar] [PubMed]
- Antonov, A.A.; Ershova, T.A. Retention of the skill to perform adaptive bio-control of bioelectrical activity synchronization in the human brain cortex in an argon-nitrogen-oxygen atmosphere with various oxygen content. Aerosp. Environ. Med. 2009, 43, 27–31. [Google Scholar]
- Bennett, P.B. Prevention in rats of narcosis produced by inert gases at high pressures. Am. J. Physiol. 1963, 205, 1013–1018. [Google Scholar] [PubMed]
- Ruzicka, J.; Benes, J.; Bolek, L.; Markvartova, V. Biological effects of noble gases. Physiol. Res./Acad. Sci. Bohemoslov. 2007, 56, S39–S44. [Google Scholar]
- Cook, S.F. The effect of helium and argon on metabolism and metamorphosis. J. Cell. Physiol. 1950, 36, 115–127. [Google Scholar] [CrossRef] [PubMed]
- Soldatov, P.E.; D’Iachenko, A.I.; Pavlov, B.N.; Fedotov, A.P.; Chuguev, A.P. Survival of laboratory animals in argon-containing hypoxic gaseous environments. Aerosp. Environ. Med. 1998, 32, 33–37. [Google Scholar]
- Vdovin, A.V.; Nozdracheva, L.V.; Pavlov, B.N. Parameters of energy metabolism of the rat brain during inhalation of hypoxic mixtures containing nitrogen and argon. Bull. Exp. Biol. Med. 1998, 125, 618–619. [Google Scholar] [CrossRef]
- Pavlov, B.N.; Grigoriev, A.I.; Smolin, V.V.; Komordin, I.P.; Sokolov, G.M.; Ramazanov, R.R.; Spirkov, P.S.; Soldatov, P.E.; Vdovin, A.V.; Buravkova, L.B. Investigations of different hyperoxic, hypoxic and normoxic oxygen-argon gaseous mixtures under different barometric pressure and respiration period. In Proceedings of the 5th International Meeting on High Pressure Biology, St. Petersburg, Russia, 1997; University of Rochester Press: Rochester, NY; USA, 1998; pp. 133–142. [Google Scholar]
- Matsnev, E.I.; Sigaleva, E.E.; Tikhonova, G.A.; Buravkova, L.B. Otoprotective effect of argon in exposure to noise. Vestn. Otorinolaringol. 2007, 3, 22–26. [Google Scholar] [PubMed]
- Gur’eva, T.S.; Dadasheva, O.A.; Soldatov, P.E.; Sychev, V.N.; Mednikova, E.I.; Smirnov, I.A.; Smolenskaia, T.S.; Dadasheva, M.T. Effect of hypoxic argon-containing gas mixtures on developing organism. Aerosp. Environ. Med. 2008, 42, 40–43. [Google Scholar]
- Soldatov, P.E.; Dadasheva, O.A.; Gur’eva, T.S.; Lysenko, L.A.; Remizova, S.E. The effect of argon-containing hypoxic gas environment on development of Japanese quail embryos. Aerosp. Environ. Med. 2002, 36, 25–28. [Google Scholar]
- Abraini, J.H.; Kriem, B.; Balon, N.; Rostain, J.C.; Risso, J.J. Gamma-aminobutyric acid neuropharmacological investigations on narcosis produced by nitrogen, argon, or nitrous oxide. Anesth. Analg. 2003, 96, 746–749. [Google Scholar] [CrossRef] [PubMed]
- Balon, N.; Risso, J.J.; Blanc, F.; Rostain, J.C.; Weiss, M. Striatal dopamine release and biphasic pattern of locomotor and motor activity under gas narcosis. Life Sci. 2003, 72, 2731–2740. [Google Scholar] [CrossRef] [PubMed]
- Brucken, A.; Cizen, A.; Fera, C.; Meinhardt, A.; Weis, J.; Nolte, K.; Rossaint, R.; Pufe, T.; Marx, G.; Fries, M. Argon reduces neurohistopathological damage and preserves functional recovery after cardiac arrest in rats. Br. J. Anaesth. 2013, 110, i106–i112. [Google Scholar] [CrossRef]
- Brucken, A.; Kurnaz, P.; Bleilevens, C.; Derwall, M.; Weis, J.; Nolte, K.; Rossaint, R.; Fries, M. Dose dependent neuroprotection of the noble gas argon after cardiac arrest in rats is not mediated by K(ATP)-channel opening. Resuscitation 2014, 85, 826–832. [Google Scholar] [CrossRef] [PubMed]
- Brucken, A.; Kurnaz, P.; Bleilevens, C.; Derwall, M.; Weis, J.; Nolte, K.; Rossaint, R.; Fries, M. Delayed argon administration provides robust protection against cardiac arrest-induced neurological damage. Neurocrit. Care 2014. [Google Scholar] [CrossRef]
- Fahlenkamp, A.V.; Coburn, M.; de Prada, A.; Gereitzig, N.; Beyer, C.; Haase, H.; Rossaint, R.; Gempt, J.; Ryang, Y.M. Expression analysis following argon treatment in an in vivo model of transient middle cerebral artery occlusion in rats. Med. Gas Res. 2014, 4, 11. [Google Scholar] [CrossRef] [PubMed]
- Irani, Y.; Pype, J.L.; Martin, A.R.; Chong, C.F.; Daniel, L.; Gaudart, J.; Ibrahim, Z.; Magalon, G.; Lemaire, M.; Hardwigsen, J. Noble gas (argon and xenon)-saturated cold storage solutions reduce ischemia-reperfusion injury in a rat model of renal transplantation. Nephron Extra 2011, 1, 272–282. [Google Scholar] [CrossRef] [PubMed]
- Pagel, P.S.; Krolikowski, J.G.; Shim, Y.H.; Venkatapuram, S.; Kersten, J.R.; Weihrauch, D.; Warltier, D.C.; Pratt, P.F., Jr. Noble gases without anesthetic properties protect myocardium against infarction by activating prosurvival signaling kinases and inhibiting mitochondrial permeability transition in vivo. Anesth. Analg. 2007, 105, 562–569. [Google Scholar] [CrossRef] [PubMed]
- Ryang, Y.M.; Fahlenkamp, A.V.; Rossaint, R.; Wesp, D.; Loetscher, P.D.; Beyer, C.; Coburn, M. Neuroprotective effects of argon in an in vivo model of transient middle cerebral artery occlusion in rats. Crit. Care Med. 2011, 39, 1448–1453. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, L.; Yang, T.; Zhao, H.; Fidalgo, A.R.; Vizcaychipi, M.P.; Sanders, R.D.; Yu, B.; Takata, M.; Johnson, M.R.; Ma, D. The protective profile of argon, helium, and xenon in a model of neonatal asphyxia in rats. Crit. Care Med. 2012, 40, 1724–1730. [Google Scholar] [CrossRef] [PubMed]
- David, H.N.; Haelewyn, B.; Risso, J.J.; Abraini, J.H. Modulation by the noble gas argon of the catalytic and thrombolytic efficiency of tissue plasminogen activator. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2013, 386, 91–95. [Google Scholar] [CrossRef]
- Jawad, N.; Rizvi, M.; Gu, J.; Adeyi, O.; Tao, G.; Maze, M.; Ma, D. Neuroprotection (and lack of neuroprotection) afforded by a series of noble gases in an in vitro model of neuronal injury. Neurosci. Lett. 2009, 460, 232–236. [Google Scholar] [CrossRef] [PubMed]
- Fahlenkamp, A.V.; Rossaint, R.; Haase, H.; Al Kassam, H.; Ryang, Y.M.; Beyer, C.; Coburn, M. The noble gas argon modifies extracellular signal-regulated kinase 1/2 signaling in neurons and glial cells. Eur. J. Pharmacol. 2012, 674, 104–111. [Google Scholar] [CrossRef] [PubMed]
- Galchuk, S.V.; Turovetskii, V.B.; Andreev, A.I.; Buravkova, L.B. Effect of argon and nitrogen on the peritoneal macrophages in mice and their resistance to the UV damaging effect in vitro. Aerosp. Environ. Med. 2001, 35, 39–43. [Google Scholar]
- Harris, K.; Armstrong, S.P.; Campos-Pires, R.; Kiru, L.; Franks, N.P.; Dickinson, R. Neuroprotection against traumatic brain injury by xenon, but not argon, is mediated by inhibition at the N-methyl-d-aspartate receptor glycine site. Anesthesiology 2013, 119, 1137–1148. [Google Scholar] [CrossRef] [PubMed]
- Loetscher, P.D.; Rossaint, J.; Rossaint, R.; Weis, J.; Fries, M.; Fahlenkamp, A.; Ryang, Y.M.; Grottke, O.; Coburn, M. Argon: Neuroprotection in in vitro models of cerebral ischemia and traumatic brain injury. Crit. Care 2009, 13, R206. [Google Scholar] [CrossRef] [Green Version]
- Maio, D.A.; Neville, J.R. Effect of chemically inert gases on oxygen consumption in living tissues. Aerosp. Med. 1967, 38, 1049–1056. [Google Scholar] [PubMed]
- Spaggiari, S.; Kepp, O.; Rello-Varona, S.; Chaba, K.; Adjemian, S.; Pype, J.; Galluzzi, L.; Lemaire, M.; Kroemer, G. Antiapoptotic activity of argon and xenon. Cell Cycle 2013, 12, 2636–2642. [Google Scholar] [CrossRef] [PubMed]
- Yarin, Y.M.; Amarjargal, N.; Fuchs, J.; Haupt, H.; Mazurek, B.; Morozova, S.V.; Gross, J. Argon protects hypoxia-, cisplatin- and gentamycin-exposed hair cells in the newborn rat’s organ of Corti. Hear. Res. 2005, 201, 1–9. [Google Scholar] [CrossRef] [PubMed]
- David, H.N.; Haelewyn, B.; Rouillon, C.; Lecoq, M.; Chazalviel, L.; Apiou, G.; Risso, J.J.; Lemaire, M.; Abraini, J.H. Neuroprotective effects of xenon: A therapeutic window of opportunity in rats subjected to transient cerebral ischemia. FASEB J. 2008, 22, 1275–1286. [Google Scholar] [CrossRef] [PubMed]
- Sanders, R.D.; Godlee, A.; Fujimori, T.; Goulding, J.; Xin, G.; Salek-Ardakani, S.; Snelgrove, R.J.; Ma, D.; Maze, M.; Hussell, T. Benzodiazepine augmented gamma-amino-butyric acid signaling increases mortality from pneumonia in mice. Crit. Care Med. 2013, 41, 1627–1636. [Google Scholar] [CrossRef] [PubMed]
- Dickinson, R.; Peterson, B.K.; Banks, P.; Simillis, C.; Martin, J.C.; Valenzuela, C.A.; Maze, M.; Franks, N.P. Competitive inhibition at the glycine site of the N-methyl-D-aspartate receptor by the anesthetics xenon and isoflurane: Evidence from molecular modeling and electrophysiology. Anesthesiology 2007, 107, 756–767. [Google Scholar] [CrossRef] [PubMed]
- Colloc’h, N.; Marassio, G.; Prange, T. Protein-noble gas interactions investigated by crystallography on three enzymes—implication on anesthesia and neuroprotection mechanisms. In Current Trends in X-ray Crystallography; Chandrasekaran, Annamalai, Ed.; InTech: Rijeka, Croatia, 2011. [Google Scholar]
- Quillin, M.L.; Breyer, W.A.; Griswold, I.J.; Matthews, B.W. Size versus polarizability in protein-ligand interactions: Binding of noble gases within engineered cavities in phage T4 lysozyme. J. Mol. Biol. 2000, 302, 955–977. [Google Scholar] [CrossRef] [PubMed]
- Soviet’s first Nobelist. In New Scientist; New Science Publications: London, UK, 19 September 1974; Volume 63, Number 915.
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Höllig, A.; Schug, A.; Fahlenkamp, A.V.; Rossaint, R.; Coburn, M.; Argon Organo-Protective Network. Argon: Systematic Review on Neuro- and Organoprotective Properties of an “Inert” Gas. Int. J. Mol. Sci. 2014, 15, 18175-18196. https://doi.org/10.3390/ijms151018175
Höllig A, Schug A, Fahlenkamp AV, Rossaint R, Coburn M, Argon Organo-Protective Network. Argon: Systematic Review on Neuro- and Organoprotective Properties of an “Inert” Gas. International Journal of Molecular Sciences. 2014; 15(10):18175-18196. https://doi.org/10.3390/ijms151018175
Chicago/Turabian StyleHöllig, Anke, Anita Schug, Astrid V. Fahlenkamp, Rolf Rossaint, Mark Coburn, and Argon Organo-Protective Network (AON). 2014. "Argon: Systematic Review on Neuro- and Organoprotective Properties of an “Inert” Gas" International Journal of Molecular Sciences 15, no. 10: 18175-18196. https://doi.org/10.3390/ijms151018175
APA StyleHöllig, A., Schug, A., Fahlenkamp, A. V., Rossaint, R., Coburn, M., & Argon Organo-Protective Network. (2014). Argon: Systematic Review on Neuro- and Organoprotective Properties of an “Inert” Gas. International Journal of Molecular Sciences, 15(10), 18175-18196. https://doi.org/10.3390/ijms151018175





