Remote Sensing-Driven Climatic/Environmental Variables for Modelling Malaria Transmission in Sub-Saharan Africa
Abstract
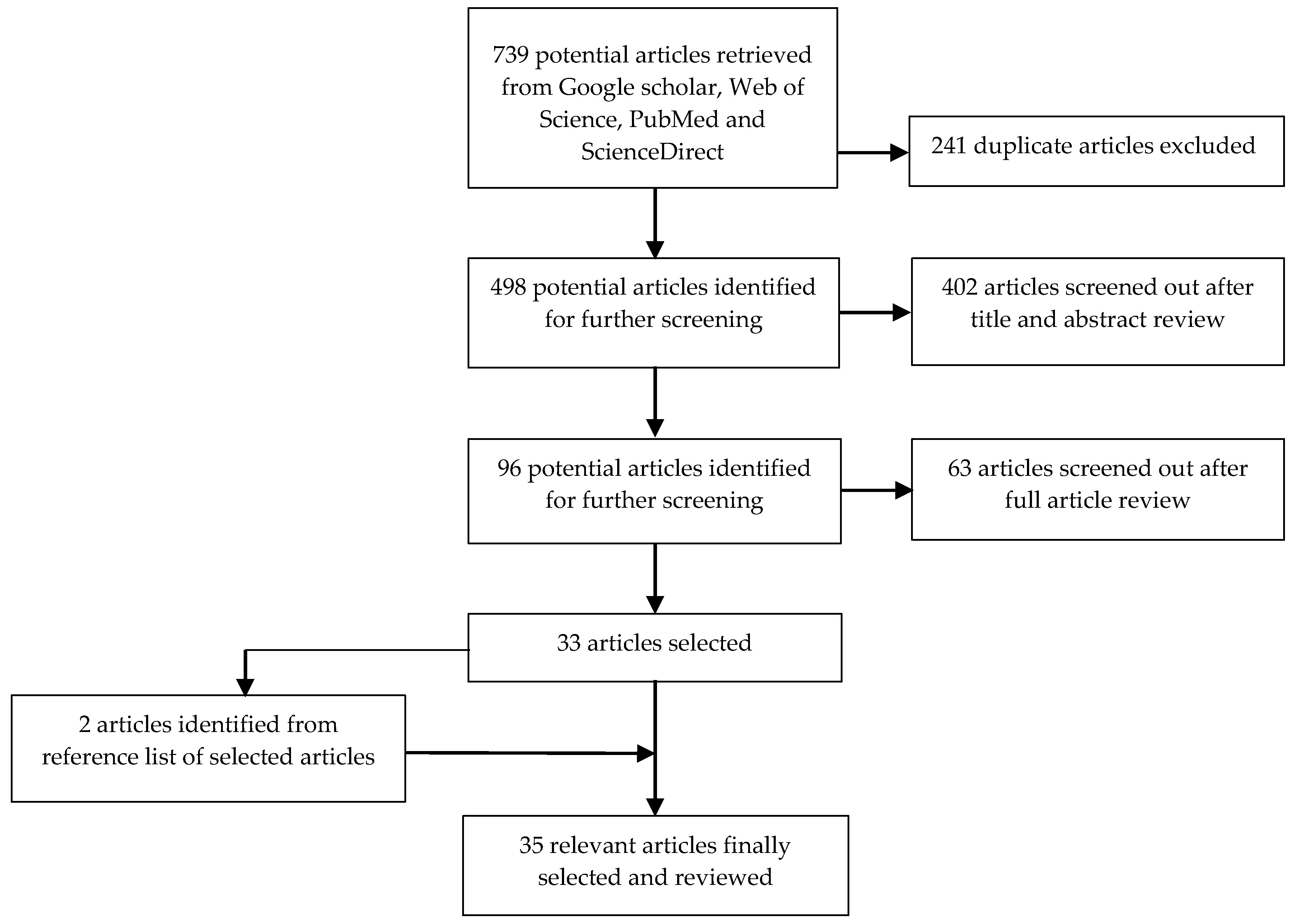
:1. Introduction
2. Materials and Methods
2.1. Search Strategy
2.2. Selection Criteria
- Original peer-reviewed articles published in English between 1 January 2000 and 31 December 2014. The search period was selected because since 2000, robust appreciation and application of RS in malaria studies occurred, which can be attributed to the easy access of RS data and the emergence of improved remote sensing sensors. Furthermore, this period coincides with the availability of MODIS data [33].
- Articles that applied RS-derived climatic/environmental variables and/or climatic proxy indicators in evaluating malaria risk, distribution, transmission and mapping.
- Studies that assessed the impact of inter-annual climate variability on malaria transmission. Studies in which climate change projections were used to estimate future malaria distribution were excluded.
- Publications that used malaria incidence and/or prevalence data in their epidemiological study design (descriptive/explorative, spatial and/or temporal analysis and time series analysis). Studies that used only entomological data were excluded.
- Studies conducted in Sub-Saharan Africa. Continental-wide studies were excluded because many African countries have made significant progress in fighting malaria, and malaria is clustered in small areas.
2.3. Description of the Study Region
3. Results
3.1. East Africa
3.2. Southern Africa
3.3. West Africa
3.4. Central Africa
3.5. Commonly-Used RS Variables and Features of Satellites/Sensors Used by the Authors in the Articles Reviewed
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| SSA | Sub-Saharan Africa |
| RS | remote sensing |
| ISI | Institute for Scientific Information |
| NDVI | normalized difference vegetation index |
| NOAA | National Oceanic and Atmospheric Administration |
| AVHRR | Advanced Very High Resolution Radiometer |
| MODIS | Moderate-resolution Imaging Spectrometer |
| WHO | World Health Organisation |
| ARIMA | autoregressive integrated moving average |
| RFE | rainfall estimates |
| CMAP | Climate Prediction Centre Merged Analysis of Precipitation |
| LST | land surface temperature |
| USGS | United States Geological Service |
| DEM | digital elevation model |
| ADDS | Africa Data Dissemination Service |
| MIR | mid-infrared |
| CCD | cold cloud duration |
| ETa | actual evapotranspiration |
| EVI | enhanced vegetation index |
| MARA/ARMA | Mapping Malaria Risk in Africa |
| NDWI | normalized difference water index |
| TWI | topographic wetness index |
| ETM+ | Enhanced Thematic Mapper plus |
| SRTM | Shuttle radar Topography Mission |
| DWP | nocturnal dew point |
| TAMSAT | Tropical Application of Meteorology using satellite data and ground-based observations |
| GPM | Global Precipitation Measurement mission |
| SMAP | Soil Moisture Active/Passive mission |
References
- World Health Organization. WHO Factsheet: Vector Borne Diseases; World Health Organization: Geneva, Switzerland, 2014. [Google Scholar]
- World Health Organization. Malaria; World Health Organization: Geneva, Switzerland, 2015. [Google Scholar]
- Snow, R.; Hay, S.; Marsh, K. Malaria in Africa: Sources, Risks, Drivers and Disease Burden 2005–2030; Foresight Project, Office of Science and Technology: London, UK, 2006. [Google Scholar]
- Palaniyandi, M. The role of Remote Sensing and GIS for spatial prediction of vector-borne diseases transmission: A systematic review. J. Vector Borne Dis. 2012, 49, 197. [Google Scholar] [PubMed]
- Bai, L.; Morton, L.C.; Liu, Q. Climate change and mosquito-borne diseases in China: A review. Glob. Health 2013, 9, 1. [Google Scholar] [CrossRef] [PubMed]
- Gosoniu, L.; Vounatsou, P.; Sogoba, N.; Maire, N.; Smith, T. Mapping malaria risk in West Africa using a Bayesian nonparametric non-stationary model. Comput. Stat. Data Anal. 2009, 53, 3358–3371. [Google Scholar] [CrossRef]
- Yoo, E.-H. Exploring space-time models for West Nile virus mosquito abundance data. Appl. Geogr. 2013, 45, 203–210. [Google Scholar] [CrossRef]
- Grover-Kopec, E.; Kawano, M.; Klaver, R.W.; Blumenthal, B.; Ceccato, P.; Connor, S.J. An online operational rainfall-monitoring resource for epidemic malaria early warning systems in Africa. Malar. J. 2005, 4. [Google Scholar] [CrossRef] [PubMed]
- Teklehaimanot, H.D.; Lipsitch, M.; Teklehaimanot, A.; Schwartz, J. Weather-based prediction of Plasmodium falciparum malaria in epidemic-prone regions of Ethiopia I. Patterns of lagged weather effects reflect biological mechanisms. Malar. J. 2004, 3, 41. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Teklehaimanot, H.D.; Schwartz, J.; Teklehaimanot, A.; Lipsitch, M. Weather-based prediction of Plasmodium falciparum malaria in epidemic-prone regions of Ethiopia II. Weather-based prediction systems perform comparably to early detection systems in identifying times for interventions. Malar. J. 2004, 3. [Google Scholar] [CrossRef]
- Arab, A.; Jackson, M.C.; Kongoli, C. Modelling the effects of weather and climate on malaria distributions in West Africa. Malar. J. 2014, 13, 126. [Google Scholar] [CrossRef] [PubMed]
- Palaniyandi, M. GIS mapping of vector breeding habitats. Geospat. World 2013, 9, 1–4. [Google Scholar]
- Githeko, A.K. Malaria and Climate Change. Commonwealth Health Minister’s Update 2009; 2009; pp. 40–43. Available online: http://www.r4d.dfid.gov.uk/PDF/Outputs/ClimateChange/CommonwealthHealth MinistersUpdate2009Githeko.pdf (accessed on 17 April 2015).
- Centres for Disease Control and Prevention. Ecology of Malaria; Centres for Disease Control and Prevention: Atlanta, GA, USA, 2015.
- Beck-Johnson, L.M.; Nelson, W.A.; Paaijmans, K.P.; Read, A.F.; Thomas, M.B.; Bjørnstad, O.N. The effect of temperature on Anopheles mosquito population dynamics and the potential for malaria transmission. PLoS ONE 2013, 8, e79276. [Google Scholar]
- Lindsay, S.W.; Martens, W.J.M. Malaria in the African highlands: Past, present and future. Bull. World Health Organ. 1998, 76, 33–45. [Google Scholar] [PubMed]
- Yamana, T.K.; Eltahir, E.A.B. Incorporating the effects of humidity in a mechanistic model of Anopheles gambiae mosquito population dynamics in the Sahel region of Africa. Parasites Vectors 2013, 6, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Zacarias, O.P.; Andersson, M. Spatial and temporal patterns of malaria incidence in Mozambique. Malar. J. 2011, 10, 189. [Google Scholar] [CrossRef] [PubMed]
- Mills, A.; Lubell, Y.; Hanson, K. Malaria eradication: The economic, financial and institutional challenge. Malar. J. 2008, 7. [Google Scholar] [CrossRef] [PubMed]
- Midekisa, A.; Senay, G.; Henebry, G.M.; Semuniguse, P.; Wimberly, M.C. Remote sensing-based time series models for malaria early warning in the highlands of Ethiopia. Malar. J. 2012, 11. [Google Scholar] [CrossRef] [PubMed]
- Kuhn, K.; Campbell-Lendrum, D.; Haines, A.; Cox, J.; Corvalán, C.; Anker, M. Using Climate to Predict Infectious Disease Epidemics; WHO: Geneva, Switzerland, 2005. [Google Scholar]
- Dinku, T.; Ceccato, P.; Grover-Kopec, E.; Lemma, M.; Connor, S.J.; Ropelewski, C.F. Validation of satellite rainfall products over East Africa’s complex topography. Int. J. Remote Sens. 2007, 28, 1503–1526. [Google Scholar] [CrossRef]
- Gaudart, J.; Toure, O.; Dessay, N.; Dicko, A.L.; Ranque, S.; Forest, L.; Demongeot, J.; Doumbo, O.K. Modelling malaria incidence with environmental dependency in a locality of Sudanese savannah area, Mali. Malar. J. 2009, 8. [Google Scholar] [CrossRef] [PubMed]
- Hay, S.; Renshaw, M.; Ochola, S.A.; Noor, A.M.; Snow, R.W. Performance of forecasting, warning and detection of malaria epidemics in the highlands of western Kenya. Trends Parasitol. 2003, 19, 394–399. [Google Scholar] [CrossRef]
- Graves, P.M.; Osgood, D.E.; Thomson, M.C.; Sereke, K.; Araia, A.; Zerom, M.; Ceccato, P.; Bell, M.; del Corral, J.; Ghebreselassie, S.; et al. Effectiveness of malaria control during changing climate conditions in Eritrea, 1998–2003. Trop. Med. Int. Health 2008, 13, 218–228. [Google Scholar] [CrossRef] [PubMed]
- Nygren, D.; Stoyanov, C.; Lewold, C.; Mansson, F.; Miller, J.; Kamanga, A.; Shiff, C.J. Remotely-sensed, nocturnal, dew point correlates with malaria transmission in Southern Province, Zambia: A time-series study. Malar. J. 2014, 13, 231. [Google Scholar] [CrossRef] [PubMed]
- Gosoniu, L.; Vounatsou, P.; Sogoba, N.; Smith, T. Bayesian modelling of geostatistical malaria risk data. Geospat. Health 2006, 1, 127–139. [Google Scholar] [CrossRef] [PubMed]
- Gosoniu, L.; Veta, A.M.; Vounatsou, P. Bayesian geostatistical modeling of Malaria Indicator Survey data in Angola. PLoS ONE 2010, 5, e9322. [Google Scholar] [CrossRef] [PubMed]
- Palaniyandi, M.; Anand, P.; Maniyosai, R. Spatial cognition: A geospatial analysis of vector borne disease transmission and the environment, using remote sensing and GIS. Int. J. Mosq. Res. 2014, 1, 39–54. [Google Scholar]
- Onyiri, N. Estimating malaria burden in Nigeria: A geostatistical modelling approach. Geospat. Health 2015, 10. [Google Scholar] [CrossRef] [PubMed]
- Arksey, H.; O’Malley, L. Scoping studies: Towards a methodological framework. Int. J. Soc. Res. Methodol. 2005, 8, 19–32. [Google Scholar] [CrossRef]
- Levac, D.; Colquhoun, H.; O’Brien, K.K. Scoping studies: Advancing the methodology. Implement Sci. 2010, 5, 1–9. [Google Scholar] [CrossRef] [PubMed]
- European Space Agency. eoPortal Directory. European Space Agency, 2000–2005. Available online: https://directory.eoportal.org/web/eoportal/satellite-missions (accessed on 9 July 2015).
- MARA/ARMA. 2009. Available online: http://www.mara-database.org (accessed on 8 November 2014).
- Hellmuth, M.E.; Moorhead, A.; Thomson, M.C.; Williams, J. Cliamte Risk Managment in Africa: Learning from Practice; International Research Institute for Climate and Society (IRI), Columbia University: Palisades, NY, USA, 2007. [Google Scholar]
- United Nations Environmental Programme. Africa Environment Outlook 2: Our Evironment, Our Wealth. 2006. Available online: http://www.unep.org/dewa/Africa/publications/AEO-2/content/index.htm (accessed on 10 December 2015).
- Chenje, M.; Johnson, P. State of the Environment in Southern Africa; Southern African Research and Documentation Centre, IUCN (The World Conservation Union), and Southern African Development Community: Johannesburg, South Africa, 1996. [Google Scholar]
- Zhou, G.; Minakawa, N.; Githeko, A.K.; Yan, G. Association between climate variability and malaria epidemics in the East African highlands. Proc. Natl. Acad. Sci. USA 2004, 101, 2375–2380. [Google Scholar] [CrossRef] [PubMed]
- Omumbo, J.A.; Hay, S.I.; Goetz, S.J.; Snow, R.W.; Rogers, D.J. Updating historical maps of malaria transmission intensity in East Africa using remote sensing. Photogramm. Eng. Remote Sens. 2002, 68, 161–166. [Google Scholar]
- Omumbo, J.A.; Hay, S.I.; Snow, R.W.; Tatem, A.J.; Rogers, D.J. Modelling malaria risk in East Africa at high-spatial resolution. Trop. Med. Int. Health 2005, 10, 557–566. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. World Malaria Report 2014; World Health Organisation: Geneva, Switzerland, 2014. [Google Scholar]
- Gomez-Elipe, A.; Otero, A.; van Herp, M.; Aguirre-Jaime, A. Forecasting malaria incidence based on monthly case reports and environmental factors in Karuzi, Burundi, 1997–2003. Malar. J. 2007, 6. [Google Scholar] [CrossRef] [PubMed]
- Ceccato, P.; Ghebremeskel, T.; Jaiteh, M.; Graves, P.M.; Levy, M.; Ghebreselassie, S.; et al. Malaria stratification, climate, and epidemic early warning in Eritrea. Am. J. Trop.Med. Hyg. 2007, 77 (Suppl. 6), 61–68. [Google Scholar] [PubMed]
- Hay, S.I.; Myers, M.F.; Burke, D.S.; Vaughn, D.W.; Endy, T.; Ananda, N.; Shanks, G.D.; Snow, R.W.; Rogers, D.J. Etiology of interepidemic periods of mosquito-borne disease. Proc. Natl. Acad. Sci. USA 2000, 97, 9335–9339. [Google Scholar] [CrossRef] [PubMed]
- Hay, S.I.; Were, E.C.; Renshaw, M.; Noor, A.M.; Ochola, S.A.; Olusanmi, L.; Alipui, N.; Snow, R.W. Forecasting, warning, and detection of malaria epidemics: A case study. Lancet 2003, 361, 1705–1706. [Google Scholar] [CrossRef]
- Noor, A.M.; Clements, A.C.; Gething, P.W.; Moloney, G.; Borle, M.; Shewchuk, T.; Hay, S.I.; Snow, R.W. Spatial prediction of Plasmodium falciparum prevalence in Somalia. Malar. J. 2008, 7, 159. [Google Scholar] [CrossRef] [PubMed]
- Hashizume, M.; Terao, T.; Minakawa, N. The Indian Ocean Dipole and malaria risk in the highlands of western Kenya. Proc. Natl. Acad. Sci. USA 2009, 106, 1857–1862. [Google Scholar] [CrossRef] [PubMed]
- Gosoniu, L.; Msengwa, A.; Lengeler, C.; Vounatsou, P. Spatially explicit burden estimates of malaria in Tanzania: Bayesian geostatistical modeling of the malaria indicator survey data. PLoS ONE 2012, 7, e23966. [Google Scholar] [CrossRef] [PubMed]
- Noor, A.M.; Alegana, V.A.; Patil, A.P.; Moloney, G.; Borle, M.; Yusuf, F.; Amran, J.; Snow, R.W. Mapping the receptivity of malaria risk to plan the future of control in Somalia. BMJ Open 2012, 2. [Google Scholar] [CrossRef] [PubMed]
- Mabaso, M.L.; Craig, M.; Vounatsou, P.; Smith, T. Towards empirical description of malaria seasonality in Southern Africa: The example of Zimbabwe. Trop. Med. Int. Health 2005, 10, 909–918. [Google Scholar] [CrossRef] [PubMed]
- Thomson, M.C.; Mason, S.J.; Phindela, T.; Connor, S.J. Use of rainfall and sea surface temperature monitoring for malaria early warning in Botswana. Am. J. Trop. Med. Hyg. 2005, 73, 214–221. [Google Scholar] [PubMed]
- Mabaso, M.L.; Vounatsou, P.; Midzi, S.; Da Silva, J.; Smith, T. Spatio-temporal analysis of the role of climate in inter-annual variation of malaria incidence in Zimbabwe. Int. J. Health Geogr. 2006, 5, 20. [Google Scholar] [CrossRef] [PubMed]
- Thomson, M.C.; Doblas-Reyes, F.J.; Mason, S.J.; Hagedorn, R.; Connor, S.J.; Phindela, T.; Morse, A.P.; Palmer, T.N. Malaria early warnings based on seasonal climate forecasts from multi-model ensembles. Nature 2006, 439, 576–579. [Google Scholar] [CrossRef] [PubMed]
- Craig, M.H.; Sharp, B.L.; Mabaso, M.L.; Kleinschmidt, I. Developing a spatial-statistical model and map of historical malaria prevalence in Botswana using a staged variable selection procedure. Int. J. Health Geogr. 2007, 6, 44. [Google Scholar] [CrossRef] [PubMed]
- Riedel, N.; Vounatsou, P.; Miller, J.M.; Gosoniu, L.; Chizema-Kawesha, E.; Mukonka, V.; Steketee, R.W. Geographical patterns and predictors of malaria risk in Zambia: Bayesian geostatistical modelling of the 2006 Zambia national malaria indicator survey (ZMIS). Malar. J. 2010, 9, 37. [Google Scholar] [CrossRef] [PubMed]
- Alegana, V.A.; Atkinson, P.M.; Wright, J.A.; Kamwi, R.; Uusiku, P.; Katokele, S.; Snow, R.W.; Noor, A.M. Estimation of malaria incidence in northern Namibia in 2009 using Bayesian conditional-autoregressive spatial-temporal models. Spat. Spatiotemporal. Epidemiol. 2013, 7, 25–36. [Google Scholar] [CrossRef] [PubMed]
- Cohen, J.M.; Dlamini, S.; Novotny, J.M.; Kandula, D.; Kunene, S.; Tatem, A.J. Rapid case-based mapping of seasonal malaria transmission risk for strategic elimination planning in Swaziland. Malar. J. 2013, 12, 61. [Google Scholar] [CrossRef] [PubMed]
- Lowe, R.; Chirombo, J.; Tompkins, A.M. Relative importance of climatic, geographic and socio-economic determinants of malaria in Malawi. Malar. J. 2013, 12. [Google Scholar] [CrossRef] [PubMed]
- Kleinschmidt, I.; Bagayoko, M.; Clarke, G.P.; Craig, M.; Le Sueur, D. A spatial statistical approach to malaria mapping. Int. J. Epidemiol. 2000, 29, 355–361. [Google Scholar] [CrossRef] [PubMed]
- Gemperli, A.; Vounatsou, P.; Sogoba, N.; Smith, T. Malaria mapping using transmission models: Application to survey data from Mali. Am. J. Epidemiol. 2006, 163, 289–297. [Google Scholar] [CrossRef] [PubMed]
- Silue, K.D.; Raso, G.; Yapi, A.; Vounatsou, P.; Tanner, M.; N’Goran, E.K.; Utzinger, J. Spatially-explicit risk profiling of Plasmodium falciparum infections at a small scale: A geostatistical modelling approach. Malar. J. 2008, 7, 111. [Google Scholar] [CrossRef] [PubMed]
- Raso, G.; Silue, K.D.; Vounatsou, P.; Singer, B.H.; Yapi, A.; Tanner, M.; Utzinger, J.; N’Goran, E.K. Spatial risk profiling of Plasmodium falciparum parasitaemia in a high endemicity area in Cote d’Ivoire. Malar. J. 2009, 8, 252. [Google Scholar] [CrossRef] [PubMed]
- Giardina, F.; Gosoniu, L.; Konate, L.; Diouf, M.B.; Perry, R.; Gaye, O.; Faye, O.; Vounatsou, P. Estimating the burden of malaria in Senegal: Bayesian zero-inflated binomial geostatistical modeling of the MIS 2008 data. PLoS ONE 2012, 7, e32625. [Google Scholar]
- Raso, G.; Schur, N.; Utzinger, J.; Koudou, B.G.; Tchicaya, E.S.; Rohner, F.; N’Goran, E.K.; Silue, K.D.; Mattys, B.; Assi, S.; et al. Mapping malaria risk among children in Cote d’Ivoire using Bayesian geo-statistical models. Malar. J. 2012, 11, 160. [Google Scholar] [CrossRef] [PubMed]
- Gemperli, A.; Sogoba, N.; Fondjo, E.; Mabaso, M.; Bagayoko, M.; Briet, O.J.; Anderegg, D.; Liebe, J.; Smith, T.; Vounatsou, P. Mapping malaria transmission in West and Central Africa. Trop. Med. Int. Health 2006, 11, 1032–1046. [Google Scholar] [CrossRef] [PubMed]
- Wardlow, B.D.; Egbert, S.L.; Kastens, J.H. Analysis of time-series MODIS 250 m vegetation index data for crop classification in the U.S. Central Great Plains. Remote Sens. Environ. 2007, 108, 290–310. [Google Scholar] [CrossRef]
- Jiang, Z.; Huete, A.R.; Didan, K.; Miura, T. Development of a two-band enhanced vegetation index without a blue band. Remote Sens. Environ. 2008, 112, 3833–3845. [Google Scholar] [CrossRef]
- Walz, Y.; Wegmann, M.; Dech, S.; Raso, G.; Utzinger, J. Risk profiling of schistosomiasis using remote sensing: Approaches, challenges and outlook. Parasites Vectors 2015, 8. [Google Scholar] [CrossRef] [PubMed]
- Machault, V.; Vignolles, C.; Borchi, F.; Vounatsou, P.; Pages, F.; Briolant, S.; Lacaux, J.P.; Rogier, C. The use of remotely sensed environmental data in the study of malaria. Geospat. Health 2011, 5, 151–168. [Google Scholar] [CrossRef] [PubMed]
- Roll Back Malaria. Roll Back Malaria Progress and Impact Series: Focus on Swaziland; Roll Back Malaria: Geneva, Swaziland, 2014. [Google Scholar]
- Maidment, R.I.; Grimes, D.; Allan, R.P.; Tarnavsky, E.; Stringer, M.; Hewison, T.; Roebeling, R.; Black, E. The 30 year TAMSAT African rainfall climatology and time series (TARCAT) data set. J. Geophys. Res. Atmos. 2014, 119. [Google Scholar] [CrossRef]
- Tarnavsky, E.; Grimes, D.; Maidment, R.; Black, E.; Allan, R.P.; Stringer, M.; Chadwick, R.; Kayitakire, F. Extension of the TAMSAT satellite-based rainfall monitoring over Africa and from 1983 to present. J. Appl. Meteorol. Climatol. 2014, 53, 2805–2822. [Google Scholar] [CrossRef]
- Hutchinson, M.; Nix, H.; McMahon, J.; Ord, K. Africa—A Topographic and Climate Database (CD-ROM); The Australian National University: Canberra, Australia, 1996. [Google Scholar]
- World Resources Institute. African Data Sampler (CD-ROM), 1st ed.; World Resources Institute: Washington, DC, USA, 1995. [Google Scholar]
- Hay, S.I.; Snow, R.W.; Rogers, D.J. Predicting malaria seasons in Kenya using multitemporal meteorological satellite sensor data. Trans. R. Soc. Trop. Med. Hyg. 1998, 92, 12–20. [Google Scholar] [CrossRef]
- Stefani, A.; Dusfour, I.; Cruz, M.C.B.; Dessay, N.; Galardo, A.K.R.; Galardo, C.D.; Girod, R.; Gomes, M.S.M.; Gurgel, H.; Lime, A.C.F.; et al. Land cover, land use and malaria in the Amazon: A systematic literature review of studies using remotely sensed data. Malar. J. 2013, 12. [Google Scholar] [CrossRef] [PubMed]
- Britch, S.C.; Linthicum, K.J.; Anyamba, A.; Tucker, C.J.; Pak, E.W.; Maloney, F.A., Jr.; Cobb, K.; Stanwix, E.; Humphries, J.; Spring, A.; et al. Satellite vegetation index data as a tool to forecast population dynamics of medically important mosquitoes at military installations in the continental United States. Mil. Med. 2008, 173, 677–683. [Google Scholar] [CrossRef] [PubMed]
- Matsushita, B.; Yang, W.; Chen, J.; Onda, Y.; Qiu, G. Sensitivity of the Enhanced Vegetation Index (EVI) and Normalized Difference Vegetation Index (NDVI) to topographic effects: A case study in high-density cypress forest. Sensors 2007, 7, 2636–2651. [Google Scholar] [CrossRef]
- Huete, A.; Didan, K.; Miura, T.; Rodriguez, E.P.; Gao, X.; Ferreira, L.G. Overview of the radiometric and biophysical performance of the MODIS vegetation indices. Remote Sens. Environ. 2002, 83, 195–213. [Google Scholar] [CrossRef]
- Gebreslasie, M.; Naidoo, I. Earth Observation in Malaria Vector Control and Management: South African Medical Research Council (SAMRC) 2011; Report No. 262887; Medical Research Council: Durban South, Africa, 2011. [Google Scholar]
- Franke, J.; Gebreslasie, M.; Bauwens, I.; Deleu, J.; Siegert, F. Earth observation in support of malaria control and epidemiology: MALAREO monitoring approaches. Geospat. Health 2015, 10. [Google Scholar] [CrossRef] [PubMed]
- National Aeronautics and Space Administration. Reverb Echo. Available online: http://reverb.echo.nasa.gov/reverb/#utf8=%E2%9C%93&spatial_map=satellite&spatial_type=rectangle (accessed on 10 December 2014).
- Zhang, Z.; Ward, M.; Gao, J.; Wang, Z.; Yao, B.; Zhang, T.; Jiang, Q. Remote sensing and disease control in China: Past, present and future. Parasites Vectors 2013, 6. [Google Scholar] [CrossRef] [PubMed]
- McCulloch, C.E.; Searle, S.R.; Neuhaus, J.M. Generalized Linear Mixed Models, 2nd ed.; Wiley: New York, NY, USA, 2008. [Google Scholar]
- Chatfield, C. The Analysis of Time Series: An Introduction, 6th ed.; CRC Press: Boca Raton, FL, USA, 2003. [Google Scholar]
- De Jong, R.M.; Davidson, J. Consistency of kernel estimators of heteroscedastic and autocorrelated covariance matrices. Econometrica 2000, 68, 407–423. [Google Scholar] [CrossRef]
- Zinszer, K.; Verma, A.D.; Charland, K.; Brewer, T.F.; Brownstein, J.S.; Sun, Z.; Buckeridge, D.L. A scoping review of malaria forecasting: Past work and future directions. BMJ Open 2012, 2. [Google Scholar] [CrossRef] [PubMed]
- Sternberg, R.J.; Grigorenko, E.L. The General Factor of Intelligence: How General Is It? Erlbaum Associates Pulishers: Mahwah, NJ, USA, 2002. [Google Scholar]


| Reference(s) | Study Area(s) | Malaria Epidemiological Data | Climatic/Environmental Data Gotten via RS Technology | Environmental/Climatic Data from Other Sources | Statistical Method(s) | Main Findings | |
|---|---|---|---|---|---|---|---|
| Climatic/Environmental Data | Source(s) of RS Data | ||||||
| [44] | Kenya: Western Kenya | Monthly inpatient confirmed cases | Multivariate El Nino Southern Oscillation Index (MENSOI) | NOAA | Monthly rainfall, mean monthly temperature | Time-series technique of spectral density analysis (SDA) | MENSOI did not influence teleconnection with monthly malaria incidence. |
| [39] | Kenya | Historic malaria distribution maps | NDVI, MIR, LST | NOAA-AVH R, Meteosat, USGS-DEM | - | Temporal Fourier analysis (TFA), discriminant analysis | LST was noted to be the best predictor of malaria transmission intensity. NDVI and CCD were identified as secondary predictors of transmission intensity. Altitude significantly improved the predictions. |
| Uganda | CCD, | ||||||
| Tanzania | altitude | ||||||
| [45] | Kenya: Kisii Central, Gucha, Nandi, and Kericho | Malaria cases (outpatients) | RFE | USGS | Seasonal climate forecast | WHO quartile, Cullen and cumulative sum (C-SUM) epidemic detection methods | Rainfall was able to forecast an epidemic one month in advance, but the outcome of seasonal climate forecast was erroneous and unreliable. |
| [24] | Kenya: Kisii Central, Gucha, Nandi, and Kericho | Malaria cases (outpatients) | RFE | USGS | Seasonal climate forecast | WHO quartile, Cullen and C-SUM epidemic detection methods | Seasonal climate forecasts did not predict the heavy rainfall. Rainfall estimates gave timely and reliable early warning, but monthly surveillance of malaria cases gave no effective warning. |
| [38] | Kenya | Malaria cases (outpatients) | Maximum temperature, minimum temperature and monthly rainfall | National Climate data Centre, NOAA | - | t-test, WHO Cullen epidemic detection methods, forward stepwise regression | Malaria incidence was significantly associated with monthly rainfall and maximum and minimum temperature at a time lag of 1–2 and 2–5 months, respectively. |
| Ethiopia | |||||||
| Uganda | |||||||
| [40] | Kenya Uganda Tanzania | Malariometric data from Mapping Malaria Risk in Africa (MARA/ARMA) (children between 0 and 15 years) | NDVI, MIR, LST CCD, altitude, land cover | NOAA-AVHRR, Meteosat, USGS-DEM, Landsat TM | TFA, discriminant analysis | NDVI, CCD and water body area were associated with malaria in the dry Ecozone 1. In Ecozone 2 where it was assumed that water was not generally limiting, LST and MIR were most abundant among the predictor variables selected. | |
| [43] | Eritrea | Monthly clinical malaria cases | RFE, NDVI | CMAP, NOAA-AVHRR | Interpolated rainfall gauge data | Spearman and Pearson rank correlations, principal component analysis (PCA), non-hierarchical clustering analysis. | NDVI anomalies were highly correlated with malaria incidence anomalies, particularly in the semi-arid north of the country and along the northern Red Sea coast, which is a highly epidemic-prone area. CMAP rainfall correlated with malaria incidence anomalies, with a lead time of 2–3 months; while weather station rainfall correlated with malaria anomalies with a lag of 2 months. |
| [42] | Burundi: Karuzi | Monthly inpatient confirmed and unconfirmed cases | NDVI | AVHRR-NOAA | Rainfall, minimum and maximum temperature | ARIMA | NDVI, rainfall, mean maximum temperature and number of cases constituted the formation of the best predicting model (R2adj = 82%, p < 0.0001 and 93% forecasting accuracy in the range ±4 cases per 100 inhabitants). NDVI, rainfall and maximum temperature were noted to correlate with malaria cases. |
| [25] | Eritrea | Monthly clinical malaria cases | RFE, NDVI | CMAP NOAA-AVHRR | Regression analysis | The Poisson regression analysis showed that CMAP rainfall estimates were significantly associated with malaria with a lead time of 2–3 months in Gash Barka. NDVI showed a similar relationship in Anseba. | |
| [46] | Somalia | Survey of P. falciparum parasite rate (PfPR) | EVI | MODIS | Precipitation, temperature, distance to permanent water bodies | Logistic regression models, kriging, Bayesian binomial generalized linear geostatistical models | The non-spatial bivariate logistic regression analysis showed that EVI, precipitation, maximum and minimum temperature and distance to water were highly significantly associated with PfPR. After employing the above covariates in the multivariate Bayesian geostatistical model, only temperature and precipitation remained significant (odds 95% confidence interval (CI)) at the southern part of Somalia. |
| [47] | Kenya: Nandi and Kisii | Confirmed and unconfirmed, monthly inpatient and outpatient cases | Dipole mode index (DMI), El Nino-Southern Oscillation (ENSO) index Nino 3 region (NINO3) | NOAA | Rainfall | Time series regression, Poisson generalized linear model (GLM), Pearson’s correlation | No strong association was found between NINO3 and the number of malaria cases after adjusting for the effect of DMI. Malaria cases increased by 3.4%–17.9% for each 0.1 increase above a DMI threshold value lagged at 3–4 months. Malaria cases increased by 1.4%–10.7% for each 10-mm increase in monthly rainfall lagged at 1–3 months. |
| [48] | Tanzania | Survey of confirmed malaria cases among children less than 5 years old | LST, NDVI, altitude | MODIS DEM-USGS | Rainfall, permanent water bodies | Multivariate logistic regression, Bayesian kriging | The bivariate analyses showed that altitude was negatively associated with malaria risk at the 5% significance level, indicating that children at above 1500 m had a lower risk of malaria. Rainfall, NDVI, day and night LST were positively associated with parasitemia risk. |
| [20] | Ethiopia: Amhara region | Monthly confirmed outpatients cases | LST, NDVI, enhanced vegetation index (EVI), actual evapotranspiration (ETa), RFE | MODIS TRMM, NASA, and the Japan Aerospace Exploration Agency (JAXA) | Seasonal autoregressive integrated moving average (SARIMA) | RFE, EVI, LST and ETa served as suitable malaria predictor as they improved the model fit, and they revealed a lagged positive association with malaria cases. ETa, which was utilized in malaria epidemiological study for the first time, showed a significant positive correlation with malaria at lags from 1–3 months in 3 of the 12 sites studied. EVI had a 3-month lag at 3 sites, while rainfall lagged by 1–3 months at 5 sites. LST exhibited a positive association lagged by 1–6 month at 6 sites. | |
| [49] | Somalia | Survey of PfPR data among children of 2 to less than 10 years | EVI | MODIS | Annual mean precipitation, temperature suitability index (TSI), distance to larva breeding sites. | Linear regression, Space-time model-based geostatistical (MBG) method | The inclusion of 1 km2 MODIS EVI (odds ratio (OR) = 0.81, 95% CI = 0.19–1.44, p-value = 0.011) and other covariates (precipitation, floodplains, distance to main water bodies) in the analysis served as the best predictor for PfPR. |
| Reference(s) | Study Area(s) | Malaria Epidemiological Data | Climatic/Environmental Data Obtained via RS Technology | Environmental/Climatic Data from Other Sources | Statistical Method(s) | Main Findings | |
|---|---|---|---|---|---|---|---|
| Climatic/Environmental Data | Source(s) of RS Data | ||||||
| [50] | Zimbabwe | Monthly confirmed and unconfirmed cases (children less than 5 years old) | NDVI | NOAA-AVHRR | Rainfall, maximum temperature, minimum temperature, vapor pressure | Bayesian Poisson model | Vapor pressure, rainfall, mean monthly (28–32 °C) and maximum temperature (24–28 °C), showed a significant positive correlation with malaria incidence, while NDVI, high monthly maximum and minimum temperatures showed a negative association. |
| [51] | Botswana | Confirmed malaria incidence data | RFE, sea surface temperature (SST) | CMAP | Stepwise regression, Spearman’s rank order, Pearson’s product moment correlation, quadratic test, logistic regression, Mann–Whitney U-tests | Negative anomalies of December–January SSTs were significantly associated with December–January rainfall estimates (Pearson’s R = −0.55 (−0.76 to −0.22) and Spearman’s R = −0.59 (−0.81 to −0.18)), as well as with the standardized malaria incidence anomalies and accounted for nearly 25% of the inter-annual variance in malaria incidence. | |
| [52] | Zimbabwe | Annual confirmed and unconfirmed malaria case (children less than 5 years old) | NDVI | NOAA-AVHRR (NASA) | Rainfall, vapor pressure, mean temperature, maximum temperature, minimum temperature | Markham’s seasonality index, Negative binomial regression analysis, Bayesian negative binomial models | In the bivariate analysis NDVI, vapor pressure, rainfall, average monthly (28 °C–32 °C) and maximum (24 °C–29 °C) temperature range revealed a significant positive correlation (p < 0.001) with malaria incidence. After employing the spatiotemporal model, NDVI became insignificant. |
| [53] | Botswana | Confirmed malaria incidence data | RFE | CMAP | SST | Probabilistic prediction, Kolmogorov–Smirnov test, quadratic test | Higher than expected malaria years were associated with above-average rainfall, while the lowest malaria years were associated with below average rainfall. |
| [54] | Botswana | Malaria prevalence data (children between 1 and 14 years age) | NDVI, RFE | NOAA-AVHRR, CMAP | Elevation, surface water land cover, temperature vapor pressure | Univariate logistic regression analysis, stepwise bootstrap method | RFE (OR = 2.01, 95% CI = 1.47–2.70), annual mean temperature (OR = 5.75, 95% CI = 4.14–8.08) and elevation (OR = 1.82, 95% CI = 1.49–2.22) were significantly associated with malaria prevalence after allowing for spatial correlation. |
| [28] | Angola | Survey of confirmed malaria cases (children less than 5 years old) | Day LST, night LST, NDVI, altitude | MODIS, USGS-DEM | Rainfall | Bayesian logistic regression, Bayesian kriging | NDVI (95% CI = 6.28, 17.94; OR = 10.62) and rainfall (95% CI = 6.00, 19.43; OR = 10.80) showed a significantly positive relationship with malaria incidence after carrying out a bivariate analysis. |
| [55] | Zambia | Survey of confirmed malaria cases among children less than 5 years old | Day LST, night LST, NDVI, land cover, altitude | MODIS, USGS-DEM | RFE, water bodies (lakes, rivers and wetlands) | Lag time analysis, bivariate and multiple geostatistical logistic regression analysis, Bayesian kriging | NDVI, night LST at 1-km2 spatial resolution and rainfall within the last 2.7 months showed positive significant association, while day LST reflected a significant negative relationship. |
| [56] | Namibia: Northern Namibia | Monthly confirmed malaria cases | EVI, precipitation | MODIS, TRMM-NASA and JAXA | Temperature suitability index (TSI) | Non-spatial Poisson regression, Bayesian spatio-temporal zero-inflated conditional autoregressive (CAR) model, zero-Inflated Poisson (ZIP) model | Initially, the univariate non-spatial regression analysis indicated that the EVI (coefficient of regression, 95% CI: 6.55, 4.25–8.87, p < 0.001), the temperature suitability index acquired from the Malaria Atlas project (7.57, 5.34–9.96, p < 0.001) and precipitation (0.02, 0.01–0.03, p = 0.002) were significant predictors. However, after employing the best performing predictive model (the multivariate model), only EVI (coefficient of regression, 95% CI: 14.29, 9.24–19.42, p < 0.001) was positively correlated. |
| [57] | Swaziland | Monthly confirmed malaria cases (imported and locally-acquired) | NDVI, NDWI, elevation, TWI | Landsat-7 ETM+, SRTM | Temperature, rainfall, distance to nearest water body | Satterthwaite t-tests, logistic regression mixed model, random forest | Case households during the high transmission season tended to be located in areas of lower elevation, closer to bodies of water, in more sparsely-populated areas, with lower rainfall and warmer temperatures and closer to imported cases than random background points (all p < 0.001). In relation to model accuracy, NDWI was the most important RS-derived variable followed by NDVI and, lastly, TWI. |
| [58] | Malawi | Monthly confirmed and unconfirmed cases | Precipitation, altitude | NOAA Climate Prediction Centre SRTM | temperature | Negative binomial generalized linear model (GLM), generalized linear mixed model (GLMM), Kernel density | The negative binomial with only fixed effects was used to determine the best time lags between climatic variables and malaria. It showed that at the 0.05 significance level, precipitation and temperature were statistically significant at Lag 1–3. The maximum relative malaria risk is observed to be the maximum temperature of 28 °C and precipitation of 6.24 mm·day−1. |
| [26] | Zambia: Southern Province | Weekly confirmed malaria cases | Rainfall, NDVI, DWP, LST, elevation | TAMSAT, MODIS, ASTER | - | Kruskal-Wallis tests, Ljung–Box Q statistics, Kriging, ARIMAX | NDVI, DWP and night LST were the highly significant predictors at the high and low malaria transmission malaria zones partitioned in the study area. |
| Reference(s) | Study Area(s) | Malaria Epidemiological Data | Climatic/Environmental Data Gotten via RS Technology | Environmental/Climatic Data from Other Sources | Statistical Method(s) | Main Findings | |
|---|---|---|---|---|---|---|---|
| Climatic/Environmental Data | Source(s) of RS Data | ||||||
| [59] | Mali | Malaria prevalence data extracted from the MARA/ARMA database | NDVI | NOAA-AVHRR | Rainfall, average maximum temperature, average minimum temperature, distance to the nearest water body | Logistic regression analysis, kriging | Mean NDVI from June–November (wet season), mean maximum temperature from March–May, months with more than 60 mm of rainfall and distance to water bodies were the significant independent variables for predicting malaria prevalence. |
| [60] | Mali | Malaria prevalence data extracted from the MARA/ARMA database | NDVI | NOAA/NASA-AVHRR | Temperature, duration of rainy season, distance to water | Garki mode, Bayesian models and kriging | During the raining season, NDVI and temperature had no statistical relationship with entomological inoculation rate (EIR). Distance to water was significantly related to transmission intensity, indicating high transmission in the areas within 4 km of the water source. |
| [27] | Mali | Malaria prevalence data from the MARA/ARMA database (children between 1 and 10 years old) | NDVI | NASA-AVHRR | Temperature, rainfall, water bodies, season length | Bayesian logistic regression, Bayesian non-stationary model, Bayesian kriging | The non-stationary model showed that NDVI and minimum temperature had a positive statistical relationship with malaria risk, awhile rainfall had a negative statistical relationship. |
| [61] | Côte d’Ivoire: Man | Confirmed P. falciparum survey in children between 6 and 16 years | NDVI, LST, RFE | MODIS-USGS Meteosat 7 | Distance to the nearest river | Bivariate logistic regression models | In bivariate non-spatial models, NDVI, RFE and distance to rivers, were significantly associated with a P. falciparum infection. However, after employing the spatial correlation, NDVI showed only a ‘borderline’ significance with P. falciparum prevalence. |
| [23] | Mali: Bancoumana | Confirmed P. falciparum survey in children between 0 and 12 years | NDVI | NOAA-AVHRR | ARIMA | The seasonal analytical approach revealed that the seasonality of P. falciparum incidence was significantly explained by NDVI with s 15-day lag (p = 0.001). The NDVI threshold was 0.361 (p = 0.007). | |
| [6] | West Africa | MARA/ARMA Malaria prevalence date among children between 1 and 10 years | NDVI, land use | NOAA-AVHRR USGS | Temperature, rainfall, soil water storage index (SWS), water bodies, agro-ecological zones | Logistic regression model, non-parametric regression models | NDVI was not associated with malaria in any of the four defined agro-ecological zones (Equatorial forest, Guinea savannah, Sahel region, Sudanese savannah). |
| [62] | Côte d’Ivoire: Man | Survey of confirmed malaria cases among school children of Grades 3–5 | NDVI, LST, RFE DEM | MODIS-USGS Meteosat 7 SRTM | Bayesian negative binomial regression models, Bayesian kriging | The bivariate non-spatial analysis identified NDVI, RFE, LST and close proximity to standing water (rivers, swamps and irrigated fields) as significant risk malaria factors. After employing the spatial analyses, only mean RFE remained significant over the malaria transmission season (June–August). | |
| [63] | Senegal | Survey of confirmed malaria cases among children less than 5 years old | Day LST, night LST, NDVI, altitude | MODIS USGS-DEM | Rainfall, permanent rivers and lakes | Bayesian geostatistical zero-inflated binomial (ZIB), Bayesian kriging | Night LST (OR 1.16; 95% CI (0.66, 1.86)) and NDVI (OR 1.48; 95% CI (0.88, 2.48)) were noted to have a positive association with malaria parasitemia. |
| [64] | Côte d’Ivoire | Malaria prevalence data for children aged less than 16 years | LST, NDVI Elevation | MODIS, USGS-DEM | Rainfall, distance to the nearest water body | Binomial regression models, Bayesian non-spatial and geo-statistical logistic regression models, Bayesian kriging | In the non-stationary spatial model (the best model), the covariates rainfall (OR = 0.76; Bayesian credible interval (BCI) = 0.70, 0.83) and maximum LST (OR = 0.72; BCI = 0.64, 0.79) were significantly negatively associated with Plasmodium prevalence. |
| Reference(s) | Study Area(s) | Malaria Epidemiological Data | Climatic/Environmental Data Gotten via RS Technology | Environmental/Climatic Data from Other Sources | Statistical Method(s) | Main Findings | |
|---|---|---|---|---|---|---|---|
| Climatic/Environmental Data | Source(s) of RS Data | ||||||
| [65] | West Africa and Central Africa | Malaria prevalence data extracted from the MARA/ARMA database | NDVI, land use | NASA-AVHRR USGS-NASA | Temperature, rainfall, soil water storage index, water bodies, agro-ecological zones, transmission seasonality | Multivariate analysis, Garki model, Bayesian linear geostatistical model, Bayesian kriging | NDVI, distance from water, length of season, rainfall and maximum temperature correlated significantly with malaria transmission intensity and were included in the best fitting model. NDVI had a significant positive association with malaria transmission, except for areas distant from water bodies. This negative association between malaria transmission and distance to water was observed in regions with NDVI values greater than 0.6. |
| RS Variables | Description | Sources |
|---|---|---|
| NDVI | This is an indicator of the greenness of the biomass and varies between −1 and +1. It is calculated as [66,67]: | MODIS, NOAA-AVHRR |
| LST (day and night) | This can be estimated from thermal infrared sensors. It is sensitive to the thermal characteristics of the ground and atmospheric effects of spectral radiation [68]. | MODIS, NOAA-AVHRR |
| RFE/CCD | This provides indirect estimates of rainfall based on the detection of precipitation particles or the duration a cloud top is below a threshold temperature [69]. | TRMM, CMAP, Meteosat |
| EVI | EVI provides an alternative to NDVI because it improves sensitivity over areas of denser vegetation. It is calculated as [66]: , where G is a gain factor, C1 and C2 are aerosol resistance coefficients and L is the canopy background adjustment that addresses nonlinear, differential NIR and red radiant transfer through a canopy. | MODIS |
| Elevation/altitude | This correlates negatively with temperature and positively with precipitation and can be applied as a surrogate indicator [69]. | USGS-DEM, ASTER, SRTM |
| Land use and land cover | This is related to the natural and physical environment and the human activities on the landscape [66]. | MODIS, Landsat TM, USGS-NASA |
| Satellite/Sensors | Spectral Range | Spatial Resolution | Revisit Time | Swath Width | Radiometric Resolution |
|---|---|---|---|---|---|
| NOAA/NASA-AVHRR | 0.58–12.50 µm | 1.1 km | 12 h | 2900 km | 10 bit |
| MODIS | 0.40–14.50 µm | 250 m, 500 m, 1 km | 1–2 days | 2330 km | 12 bit |
| Landsat TM 1 | 0.45–12.5 µm | 30 m, 120 m | 16 days | 185 km | 8 bit |
| Landsat-7 ETM+ 2 | 0.45–12.5 µm | 15 m, 30 m, 60 m | 16 days | 185 km | 9 bit (8 bit transmitted) |
| Meteosat 1–7 | 0.50–12.5 µm | 2.5 km, 5 km | 30 min | - | 8 bit |
| Meteosat 8–10 | 0.40–14.40 µm | 1 km, 3 km | 15 min | 10 bit | |
| TRMM | VIRS 3: 0.63 µm, 1.60 µm, 3.75 µm, 10.7 µm, and 12 µm | VIRS: 2 km TMI 4: 5–45 km PR 5: 4.3 km | 3 hourly, daily, monthly | VIRS: 720 km TMI: 780 km PR: 215 km | - |
| SRTM | - | 30 m | 16 times per day | C-radar: 225 km X-radar: 50 km | C-radar: 8 bit X-radar: 6 bit |
| ASTER | VNIR 6: 0.52–0.86 µm SWIR 7: 1.60–2.43 µm TIR 8: 8.125–11.65 µm | VNIR: 15 m SWIR: 30 m TIR: 90 m | 5 days 16 days 16 days | 60 km 60 km 60 km | VNIR: 8 bit SWIR: 8 bit TIR: 12 bit |
| CMAP | - | 0.25° × 0.25° | 5 days, monthly | - | - |
| Satellite/Sensors | Spectral Range | Spatial Resolution | Revisit Time | Swath Width | Radiometric Resolution |
|---|---|---|---|---|---|
| Landsat-8 | 0.43–12.5 µm | 15 m, 30 m, 100 m | 16 days | 185 km | 12 bit |
| Copernicus: Sentinel-2 | 0.43–2.28 µm | 10 m, 20 m, 60 m | 5 days | 290 km | 12 bit |
| GPM | - | 250 m, 500 m | 3 h | 120 km, 245 km, 885 km | - |
| SMAP | - | 3 km, 10 km, 40 km | 2 days, 3 days | 1000 km | - |
| SPOT 6 and SPOT 7 | 0.45–0.89 µm | 1.5 m, 2 m, 6 m, 8 m | 1–5 days | 60 km | 12 bit |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ebhuoma, O.; Gebreslasie, M. Remote Sensing-Driven Climatic/Environmental Variables for Modelling Malaria Transmission in Sub-Saharan Africa. Int. J. Environ. Res. Public Health 2016, 13, 584. https://doi.org/10.3390/ijerph13060584
Ebhuoma O, Gebreslasie M. Remote Sensing-Driven Climatic/Environmental Variables for Modelling Malaria Transmission in Sub-Saharan Africa. International Journal of Environmental Research and Public Health. 2016; 13(6):584. https://doi.org/10.3390/ijerph13060584
Chicago/Turabian StyleEbhuoma, Osadolor, and Michael Gebreslasie. 2016. "Remote Sensing-Driven Climatic/Environmental Variables for Modelling Malaria Transmission in Sub-Saharan Africa" International Journal of Environmental Research and Public Health 13, no. 6: 584. https://doi.org/10.3390/ijerph13060584
APA StyleEbhuoma, O., & Gebreslasie, M. (2016). Remote Sensing-Driven Climatic/Environmental Variables for Modelling Malaria Transmission in Sub-Saharan Africa. International Journal of Environmental Research and Public Health, 13(6), 584. https://doi.org/10.3390/ijerph13060584






