Emerging Role of Circulating Tumor Cells in Gastric Cancer
Abstract
1. Introduction
2. Landscape of CTCs
2.1. CTC Migration
2.2. CTCs in Circulation
2.3. Morphologic Heterogeneity of CTCs
3. Clinical Implication of CTCs in GC
3.1. Diagnostic Potential of CTC in GC
3.2. CTC Assay for Prognosis and Treatment Management of GC
3.3. The Role of CTCs in GC Immunotherapy
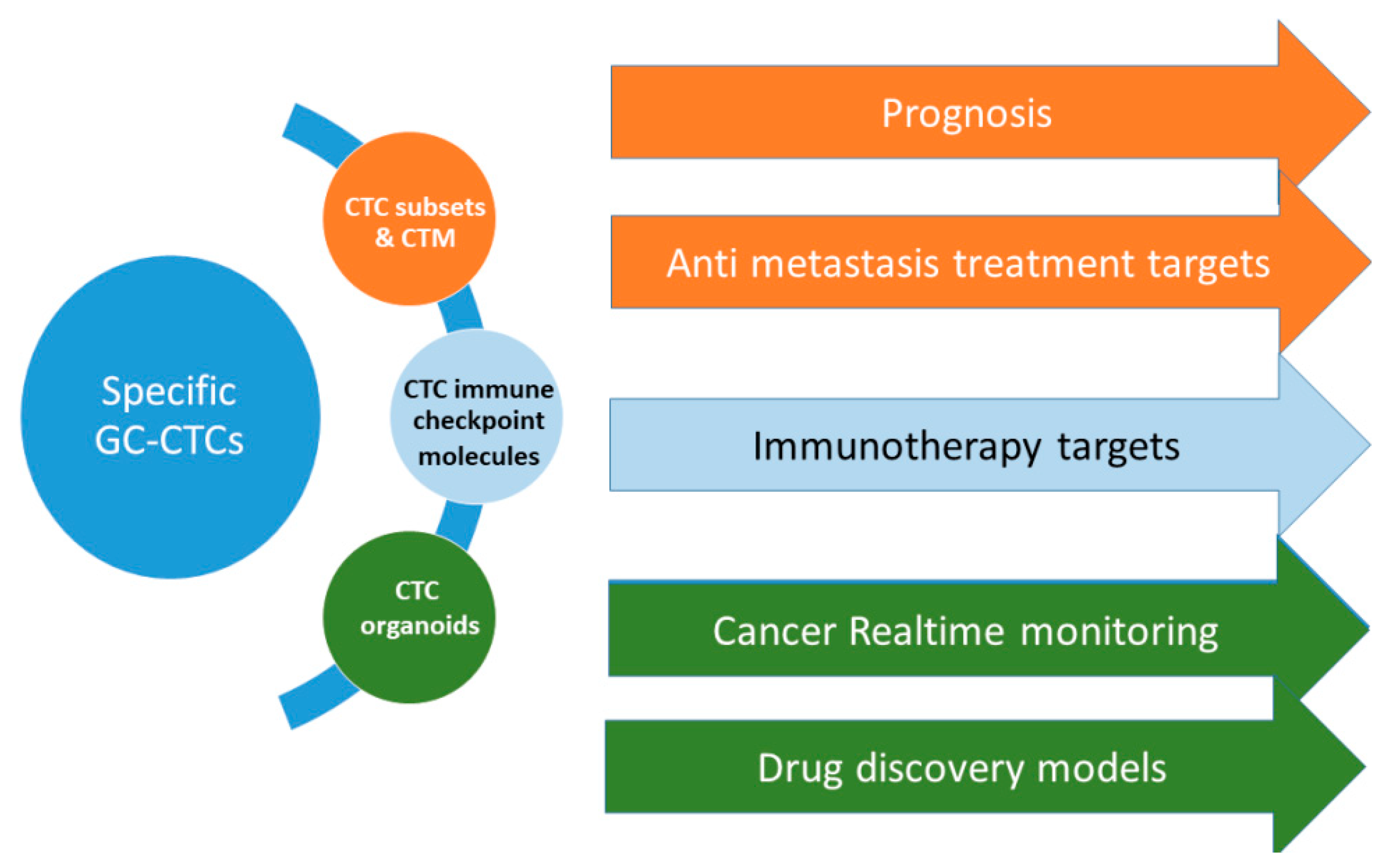
4. Challenge and Future Perspective
4.1. Challenge
4.2. Future Perspective
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| AUC | area under the curve |
| BCBM | breast cancer brain metastasis |
| CA19-9 | cancer antigen 19-9 |
| CEA | carcinoembryonic antigen |
| CI | confidence interval |
| CTC | circulating tumor cell |
| CTM | circulating tumor microembolies |
| DC | disease control |
| CA19-9 | cancer antigen 19-9 |
| CEA | carcinoembryonic antigen |
| CI | confidence interval |
| CTC | circulating tumor cell |
| CTM | circulating tumor microembolies |
| DC | disease control |
| DFS | disease-free survival |
| ECM | extracellular matrix |
| EMT | epithelial to mesenchymal transition |
| EpCAM | epithelial cell adhesion molecule |
| GC | gastric cancer |
| IHC | immunohistochemistry |
| HR | hazard ratio |
| MET | mesenchymal to epithelial cell transition |
| MHC | major histocompatibility complex |
| MP1-MMP | membrane type 1 matrix metalloproteinase |
| OS | overall survival |
| ROC | receiver operating characteristic |
| WBC | white blood cell |
References
- Lyons, K.; Le, L.C.; Pham, Y.T.H.; Borr/on, C.; Park, J.Y.; Tran, C.T.D.; Tran, T.V.; Tran, H.T.T.; Vu, K.T.; Do, C.D.; et al. Gastric cancer: Epidemiology, biology, and prevention: A mini review. Eur. J. Cancer Prev. 2019, 28. [Google Scholar]
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. Ca A Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef]
- Redaniel, M.T.; Laudico, A.; Mirasol-Lumague, M.R.; Gondos, A.; Pulte, D.; Mapua, C.; Brenner, H. Cancer survival discrepancies in developed and developing countries: Comparisons between the Philippines and the United States. Br. J. Cancer 2009, 100, 858. [Google Scholar] [CrossRef]
- Correa, P. Gastric cancer: Overview. Gastroenterol Clin. North. Am. 2013, 42, 211–217. [Google Scholar] [CrossRef] [PubMed]
- Ting-Ting, L.; Hao, L.; Jiang, Y.; Guang-Yao, S.; Li-Ying, Z.; Guo-Xin, L.; Ting-Ting, L.; Hao, L.; Jiang, Y.; Li-Ying, Z.; et al. Prognostic and predictive blood biomarkers in gastric cancer and the potential application of circulating tumor cells. World J. Gastroenterol. 2018, 24, 2236–2246. [Google Scholar]
- Li, Y.; Yang, Y.; Lu, M.; Shen, L. Predictive value of serum CEA, CA19-9 and CA72.4 in early diagnosis of recurrence after radical resection of gastric cancer. Hepatogastroenterology 2011, 58, 2166–2170. [Google Scholar] [PubMed]
- Jin, Z.; Jiang, W.; Wang, L. Biomarkers for gastric cancer: Progression in early diagnosis and prognosis (Review). Oncol. Lett. 2015, 9, 1502–1508. [Google Scholar] [CrossRef]
- Nakamura, K.; Iwatsuki, M.; Kurashige, J.; Ishimoto, T.; Baba, Y.; Miyamoto, Y.; Yoshida, N.; Watanabe, M.; Baba, H. Circulating tumor cells in gastric cancer. J. Cancer Metastasis Treat. 2018, 4, 32. [Google Scholar] [CrossRef]
- Zhou, J.; Ma, X.; Bi, F.; Liu, M. Clinical significance of circulating tumor cells in gastric cancer patients. Oncotarget 2017, 8, 25713–25720. [Google Scholar] [CrossRef]
- Palmirotta, R.; Lovero, D.; Cafforio, P.; Felici, C.; Mannavola, F.; Pellè, E.; Quaresmini, D.; Tucci, M.; Silvestris, F. Liquid biopsy of cancer: A multimodal diagnostic tool in clinical oncology. Ther. Adv. Med. Oncol. 2018, 10, 1758835918794630. [Google Scholar] [CrossRef]
- Yap, T.A.; Lorente, D.; Omlin, A.; Olmos, D.; de Bono, J.S. Circulating tumor cells: A multifunctional biomarker. Clin. Cancer Res. 2014, 20, 2553–2568. [Google Scholar] [CrossRef] [PubMed]
- Krebs, M.G.; Metcalf, R.L.; Carter, L.; Brady, G.; Blackhall, F.H.; Dive, C. Molecular analysis of circulating tumour cells-biology and biomarkers. Nat. Rev. Clin. Oncol. 2014, 11, 129–144. [Google Scholar] [CrossRef] [PubMed]
- Thiele, J.A.; Bethel, K.; Kralickova, M.; Kuhn, P. Circulating Tumor Cells: Fluid Surrogates of Solid Tumors. Annu. Rev. Pathol 2017, 12, 419–447. [Google Scholar] [CrossRef] [PubMed]
- Paterlini-Brechot, P. Organ-specific markers in circulating tumor cell screening: An early indicator of metastasis-capable malignancy. Future Oncol. 2011, 7, 849–871. [Google Scholar] [CrossRef]
- Pantel, K.; Alix-Panabières, C. Circulating tumour cells in cancer patients: Challenges and perspectives. Trends Mol. Med. 2010, 16, 398–406. [Google Scholar] [CrossRef]
- Giuliano, M.; Shaikh, A.; Lo, H.C.; Arpino, G.; de Placido, S.; Zhang, X.H.; Cristofanilli, M.; Schiff, R.; Trivedi, M.V. Perspective on Circulating Tumor Cell Clusters: Why It Takes a Village to Metastasize. Cancer Res. 2018, 78, 845–852. [Google Scholar] [CrossRef]
- Au, S.H.; Storey, B.D.; Moore, J.C.; Tang, Q.; Chen, Y.L.; Javaid, S.; Sarioglu, A.F.; Sullivan, R.; Madden, M.W.; O’Keefe, R.; et al. Clusters of circulating tumor cells traverse capillary-sized vessels. Proc. Natl. Acad. Sci. USA 2016, 113, 4947–4952. [Google Scholar] [CrossRef]
- Barriere, G.; Fici, P.; Gallerani, G.; Fabbri, F.; Zoli, W.; Rigaud, M. Circulating tumor cells and epithelial, mesenchymal and stemness markers: Characterization of cell subpopulations. Ann. Transl. Med. 2014, 2, 109. [Google Scholar]
- Maitre, J.L.; Heisenberg, C.P. Three functions of cadherins in cell adhesion. Curr. Biol. 2013, 23, R626–R633. [Google Scholar] [CrossRef]
- Nakamura, T.; Kato, Y.; Fuji, H.; Horiuchi, T.; Chiba, Y.; Tanaka, K. E-cadherin-dependent intercellular adhesion enhances chemoresistance. Int. J. Mol. Med. 2003, 12, 693–700. [Google Scholar] [CrossRef]
- Alonso-Alconada, L.; Muinelo-Romay, L.; Madissoo, K.; Diaz-Lopez, A.; Krakstad, C.; Trovik, J.; Wik, E.; Hapangama, D.; Coenegrachts, L.; Cano, A.; et al. Molecular profiling of circulating tumor cells links plasticity to the metastatic process in endometrial cancer. Mol. Cancer 2014, 13, 223. [Google Scholar] [CrossRef] [PubMed]
- Nagrath, S.; Sequist, L.V.; Maheswaran, S.; Bell, D.W.; Irimia, D.; Ulkus, L.; Smith, M.R.; Kwak, E.L.; Digumarthy, S.; Muzikansky, A.; et al. Isolation of rare circulating tumour cells in cancer patients by microchip technology. Nature 2007, 450, 1235–1239. [Google Scholar] [CrossRef] [PubMed]
- Galletti, G.; Sung, M.S.; Vahdat, L.T.; Shah, M.A.; Santana, S.M.; Altavilla, G.; Kirby, B.J.; Giannakakou, P. Isolation of breast cancer and gastric cancer circulating tumor cells by use of an anti HER2-based microfluidic device. Lab. Chip 2014, 14, 147–156. [Google Scholar] [CrossRef] [PubMed]
- Micalizzi, D.S.; Farabaugh, S.M.; Ford, H.L. Epithelial-mesenchymal transition in cancer: Parallels between normal development and tumor progression. J. Mammary Gland. Biol. Neoplasia 2010, 15, 117–134. [Google Scholar] [CrossRef] [PubMed]
- Petrova, Y.I.; Schecterson, L.; Gumbiner, B.M. Roles for E-cadherin cell surface regulation in cancer. Mol. Biol. Cell 2016, 27, 3233–3244. [Google Scholar] [CrossRef]
- Kalluri, R.; Weinberg, R.A. The basics of epithelial-mesenchymal transition. J. Clin. Investig. 2009, 119, 1420–1428. [Google Scholar] [CrossRef]
- Armstrong, A.J.; Marengo, M.S.; Oltean, S.; Kemeny, G.; Bitting, R.L.; Turnbull, J.D.; Herold, C.I.; Marcom, P.K.; George, D.J.; Garcia-Blanco, M.A. Circulating tumor cells from patients with advanced prostate and breast cancer display both epithelial and mesenchymal markers. Mol. Cancer Res. 2011, 9, 997–1007. [Google Scholar] [CrossRef]
- Williams, A.; Rawal, S.; Ao, Z.; Lu, B.; Torres-Munoz, J.; Rini, B.; Pelley, R.; Budd, G.T.; Borden, E.; Zheng, S.; et al. Abstract 2372: Capture and molecular characterization of CTC in metastatic breast, prostate, colorectal, and renal cancer. Cancer Res. 2012, 72, 2372. [Google Scholar]
- Francart, M.E.; Lambert, J.; Vanwynsberghe, A.M.; Thompson, E.W.; Bourcy, M.; Polette, M.; Gilles, C. Epithelial-mesenchymal plasticity and circulating tumor cells: Travel companions to metastases. Dev. Dyn. 2018, 247, 432–450. [Google Scholar] [CrossRef]
- Yang, Y.; Zheng, H.; Zhan, Y.; Fan, S. An emerging tumor invasion mechanism about the collective cell migration. Am. J. Transl. Res. 2019, 11, 5301–5312. [Google Scholar]
- Rejniak, K.A. Circulating Tumor Cells: When a Solid Tumor Meets a Fluid Microenvironment. Adv. Exp. Med. Biol. 2016, 936, 93–106. [Google Scholar] [PubMed]
- Kim, M.Y.; Oskarsson, T.; Acharyya, S.; Nguyen, D.X.; Zhang, X.H.; Norton, L.; Massague, J. Tumor self-seeding by circulating cancer cells. Cell 2009, 139, 1315–1326. [Google Scholar] [CrossRef]
- Jie, X.X.; Zhang, X.Y.; Xu, C.J. Epithelial-to-mesenchymal transition, circulating tumor cells and cancer metastasis: Mechanisms and clinical applications. Oncotarget 2017, 8, 81558–81571. [Google Scholar] [CrossRef]
- Zhao, X.H.; Wang, Z.R.; Chen, C.L.; Di, L.; Bi, Z.F.; Li, Z.H.; Liu, Y.M. Molecular detection of epithelial-mesenchymal transition markers in circulating tumor cells from pancreatic cancer patients: Potential role in clinical practice. World J. Gastroenterol. 2019, 25, 138–150. [Google Scholar] [CrossRef] [PubMed]
- Hong, B.; Zu, Y. Detecting circulating tumor cells: Current challenges and new trends. Theranostics 2013, 3, 377–394. [Google Scholar] [CrossRef] [PubMed]
- Hou, J.M.; Krebs, M.; Ward, T.; Sloane, R.; Priest, L.; Hughes, A.; Clack, G.; Ranson, M.; Blackhall, F.; Dive, C. Circulating tumor cells as a window on metastasis biology in lung cancer. Am. J. Pathol. 2011, 178, 989–996. [Google Scholar] [CrossRef]
- Jia, D.; Li, X.; Bocci, F.; Tripathi, S.; Deng, Y.; Jolly, M.K.; Onuchic, J.N.; Levine, H. Quantifying Cancer Epithelial-Mesenchymal Plasticity and its Association with Stemness and Immune Response. J. Clin. Med. 2019, 8, 725. [Google Scholar] [CrossRef]
- Yu, M.; Bardia, A.; Wittner, B.S.; Stott, S.L.; Smas, M.E.; Ting, D.T.; Isakoff, S.J.; Ciciliano, J.C.; Wells, M.N.; Shah, A.M.; et al. Circulating breast tumor cells exhibit dynamic changes in epithelial and mesenchymal composition. Science 2013, 339, 580–584. [Google Scholar] [CrossRef]
- Shliakhtunou, Y. Survivin gene expression in the primary tumor and circulating tumor cells—A new biomarker of tumor progression of breast cancer. Ann. Oncol. 2016, 27. [Google Scholar] [CrossRef]
- Végran, F.; Boidot, R. Survivin-3B promotes chemoresistance and immune escape by inhibiting caspase-8 and -6 in cancer cells. OncoImmunology 2013, 2, e26328. [Google Scholar] [CrossRef][Green Version]
- Huh, S.J.; Liang, S.; Sharma, A.; Dong, C.; Robertson, G.P. Transiently entrapped circulating tumor cells interact with neutrophils to facilitate lung metastasis development. Cancer Res. 2010, 70, 6071–6082. [Google Scholar] [CrossRef] [PubMed]
- Divella, R.; Daniele, A.; Abbate, I.; Bellizzi, A.; Savino, E.; Simone, G.; Giannone, G.; Giuliani, F.; Fazio, V.; Gadaleta-Caldarola, G.; et al. The presence of clustered circulating tumor cells (CTCs) and circulating cytokines define an aggressive phenotype in metastatic colorectal cancer. Cancer Causes Control. 2014, 25, 1531–1541. [Google Scholar] [CrossRef] [PubMed]
- Killock, D. CTCs ‘piggyback’ off neutrophils. Nat. Rev. Clin. Oncol 2019, 16, 208. [Google Scholar] [CrossRef] [PubMed]
- Xie, Z.; Gao, X.; Cheng, K.; Yu, L. Correlation between the presence of circulating tumor cells and the pathologic type and staging of non-small cell lung cancer during the early postoperative period. Oncol. Lett. 2017, 14, 5825–5830. [Google Scholar] [CrossRef]
- Vilsmaier, T.; Rack, B.; Konig, A.; Friese, K.; Janni, W.; Jeschke, U.; Weissenbacher, T.; Group, S.S. Influence of Circulating Tumour Cells on Production of IL-1alpha, IL-1beta and IL-12 in Sera of Patients with Primary Diagnosis of Breast Cancer Before Treatment. Anticancer Res. 2016, 36, 5227–5236. [Google Scholar] [CrossRef][Green Version]
- Wang, W.-C.; Zhang, X.-F.; Peng, J.; Li, X.-F.; Wang, A.-L.; Bie, Y.-Q.; Shi, L.-H.; Lin, M.-B.; -F, X. Zhang, Survival Mechanisms and Influence Factors of Circulating Tumor Cells. Biomed Res. Int. 2018, 2018, 9. [Google Scholar] [CrossRef]
- Mathewson, N.D.; Jenq, R.; Mathew, A.V.; Koenigsknecht, M.; Hanash, A.; Toubai, T.; Oravecz-Wilson, K.; Wu, S.R.; Sun, Y.; Rossi, C.; et al. Gut microbiome-derived metabolites modulate intestinal epithelial cell damage and mitigate graft-versus-host disease. Nat. Immunol. 2016, 17, 505–513. [Google Scholar] [CrossRef]
- Nieswandt, B.; Hafner, M.; Echtenacher, B.; Mannel, D.N. Lysis of tumor cells by natural killer cells in mice is impeded by platelets. Cancer Res. 1999, 59, 1295–1300. [Google Scholar]
- Placke, T.; Orgel, M.; Schaller, M.; Jung, G.; Rammensee, H.G.; Kopp, H.G.; Salih, H.R. Platelet-derived MHC class I confers a pseudonormal phenotype to cancer cells that subverts the antitumor reactivity of natural killer immune cells. Cancer Res. 2012, 72, 440–448. [Google Scholar] [CrossRef]
- Huong, P.T.; Nguyen, L.T.; Nguyen, X.-B.; Lee, S.K.; Bach, D.-H. The Role of Platelets in the Tumor-Microenvironment and the Drug Resistance of Cancer Cells. Cancers 2019, 11, 240. [Google Scholar] [CrossRef]
- Cleris, L.; Daidone, M.G.; Fina, E.; Cappelletti, V. The Detection and Morphological Analysis of Circulating Tumor and Host Cells in Breast Cancer Xenograft Models. Cells 2019, 8, 683. [Google Scholar] [CrossRef] [PubMed]
- Park, S.; Ang, R.R.; Duffy, S.P.; Bazov, J.; Chi, K.N.; Black, P.C.; Ma, H. Morphological differences between circulating tumor cells from prostate cancer patients and cultured prostate cancer cells. PLoS ONE 2014, 9, e85264. [Google Scholar] [CrossRef] [PubMed]
- Bulfoni, M.; Turetta, M.; del Ben, F.; di Loreto, C.; Beltrami, A.P.; Cesselli, D. Dissecting the Heterogeneity of Circulating Tumor Cells in Metastatic Breast Cancer: Going Far Beyond the Needle in the Haystack. Int. J. Mol. Sci. 2016, 17, 1775. [Google Scholar] [CrossRef] [PubMed]
- Kallergi, G.; Konstantinidis, G.; Markomanolaki, H.; Papadaki, M.A.; Mavroudis, D.; Stournaras, C.; Georgoulias, V.; Agelaki, S. Apoptotic circulating tumor cells in early and metastatic breast cancer patients. Mol. Cancer 2013, 12, 1886–1895. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.J.; Lee, J.; Kim, S.T.; Park, S.H.; Park, J.O.; Park, Y.S.; Lim, H.Y.; Kang, W.K. Circulating tumor cells are predictive of poor response to chemotherapy in metastatic gastric cancer. Int. J. Biol. Markers 2015, 30, e382–e386. [Google Scholar] [CrossRef]
- Okabe, H.; Tsunoda, S.; Hosogi, H.; Hisamori, S.; Tanaka, E.; Tanaka, S.; Sakai, Y. Circulating Tumor Cells as an Independent Predictor of Survival in Advanced Gastric Cancer. Ann. Surg. Oncol. 2015, 22, 3954–3961. [Google Scholar] [CrossRef][Green Version]
- Zou, K.; Yang, S.; Zheng, L.; Wang, S.; Xiong, B. Prognostic Role of the Circulating Tumor Cells Detected by Cytological Methods in Gastric Cancer: A Meta-Analysis. Biomed Res. Int. 2016, 2016, 2765464. [Google Scholar] [CrossRef]
- Mishima, Y.; Matsusaka, S.; Chin, K.; Mikuniya, M.; Minowa, S.; Takayama, T.; Shibata, H.; Kuniyoshi, R.; Ogura, M.; Terui, Y. Detection of HER2 amplification in circulating tumor cells of HER2-negative gastric cancer patients. Target. Oncol. 2017, 12, 341–351. [Google Scholar] [CrossRef]
- Liu, Y.; Ling, Y.; Qi, Q.; Lan, F.; Zhu, M.; Zhang, Y.; Bao, Y.; Zhang, C. Prognostic value of circulating tumor cells in advanced gastric cancer patients receiving chemotherapy. Mol. Clin. Oncol. 2017, 6, 235–242. [Google Scholar] [CrossRef]
- Zheng, X.; Fan, L.; Zhou, P.; Ma, H.; Huang, S.; Yu, D.; Zhao, L.; Yang, S.; Liu, J.; Huang, A.; et al. Detection of Circulating Tumor Cells and Circulating Tumor Microemboli in Gastric Cancer. Transl. Oncol. 2017, 10, 431–441. [Google Scholar] [CrossRef]
- Kang, H.M.; Kim, G.H. Circulating tumor cells detected by lab-on-a-disc: Role in early diagnosis of gastric cancer. PLoS ONE 2017, 12, e0180251. [Google Scholar] [CrossRef] [PubMed]
- Yue, C.; Jiang, Y.; Li, P.; Wang, Y.; Xue, J.; Li, N.; Li, D.; Wang, R.; Dang, Y.; Hu, Z.; et al. Dynamic change of PD-L1 expression on circulating tumor cells in advanced solid tumor patients undergoing PD-1 blockade therapy. OncoImmunology 2018, 7, e1438111. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Zhang, N.; Wang, S.; Shi, D.; Zhang, C.; Liu, K.; Xiong, B. Wedge-shaped microfluidic chip for circulating tumor cells isolation and its clinical significance in gastric cancer. J. Transl. Med. 2018, 16, 139. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zhang, X.; Liu, D.; Gong, J.; Wang, D.D.; Li, S.; Peng, Z.; Li, Y.; Wang, X.; Lin, P.P.; et al. Evolutionary Expression of HER2 Conferred by Chromosome Aneuploidy on Circulating Gastric Cancer Cells Contributes to Developing Targeted and Chemotherapeutic Resistance. Clin. Cancer Res. 2018, 24, 5261–5271. [Google Scholar] [CrossRef]
- Cheng, B.; Tong, G.; Wu, X.; Cai, W.; Li, Z.; Tong, Z.; He, L.; Yu, S.; Wang, S. Enumeration and Characterization Of Circulating Tumor Cells And Its Application In Advanced Gastric Cancer. Oncotargets Ther. 2019, 12, 7887–7896. [Google Scholar] [CrossRef]
- Lu, R.; Chen, Q.; Liu, X.; Shen, S.; Pan, Z.; Shi, C. Detection of circulating stage III–IV gastric cancer tumor cells based on isolation by size of epithelial tumor: Using the circulating tumor cell biopsy technology. Transl. Cancer Res. 2019, 8, 1342–1350. [Google Scholar] [CrossRef]
- Abdallah, E.A.; Braun, A.C.; Flores, B.; Senda, L.; Urvanegia, A.C.; Calsavara, V.; de Jesus, V.H.F.; Almeida, M.F.A.; Begnami, M.D.; Coimbra, F.J.F.; et al. The Potential Clinical Implications of Circulating Tumor Cells and Circulating Tumor Microemboli in Gastric Cancer. Oncologist 2019, 24, e854–e863. [Google Scholar] [CrossRef]
- Gao, Y.; Xi, H.; Wei, B.; Cui, J.; Zhang, K.; Li, H.; Cai, A.; Shen, W.; Li, J.; Rosell, R.; et al. Association Between Liquid Biopsy and Prognosis of Gastric Cancer Patients: A Systematic Review and Meta-Analysis. Front. Oncol. 2019, 9, 1222. [Google Scholar] [CrossRef]
- Venerito, M.; Vasapolli, R.; Rokkas, T.; Malfertheiner, P. Gastric cancer: Epidemiology, prevention, and therapy. Helicobacter 2018, 23, e12518. [Google Scholar] [CrossRef]
- Tang, L.; Zhao, S.; Liu, W.; Parchim, N.F.; Huang, J.; Tang, Y.; Gan, P.; Zhong, M. Diagnostic accuracy of circulating tumor cells detection in gastric cancer: Systematic review and meta-analysis. Bmc Cancer 2013, 13, 314. [Google Scholar] [CrossRef]
- Vaiopoulos, A.G.; Kostakis, I.D.; Gkioka, E.; Athanasoula, K.; Pikoulis, E.; Papalambros, A.; Christopoulos, P.; Gogas, H.; Kouraklis, G.; Koutsilieris, M. Detection of circulating tumor cells in colorectal and gastric cancer using a multiplex PCR assay. Anticancer Res. 2014, 34, 3083–3092. [Google Scholar] [PubMed]
- Hao, N.-B.; He, Y.-F.; Li, X.-Q.; Wang, K.; Wang, R.-L. The role of miRNA and lncRNA in gastric cancer. Oncotarget 2017, 8, 81572–81582. [Google Scholar] [CrossRef] [PubMed]
- Sierzega, M.; Kaczor, M.; Kolodziejczyk, P.; Kulig, J.; Sanak, M.; Richter, P. Evaluation of serum microRNA biomarkers for gastric cancer based on blood and tissue pools profiling: The importance of miR-21 and miR-331. Br. J. Cancer 2017, 117, 266. [Google Scholar] [CrossRef] [PubMed]
- Ge, X.; Liu, X.; Lin, F.; Li, P.; Liu, K.; Geng, R.; Dai, C.; Lin, Y.; Tang, W.; Wu, Z.; et al. MicroRNA-421 regulated by HIF-1alpha promotes metastasis, inhibits apoptosis, and induces cisplatin resistance by targeting E-cadherin and caspase-3 in gastric cancer. Oncotarget 2016, 7, 24466–24482. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; Xiao, B.; Zhou, F.; Deng, H.; Zhang, X.; Lou, Y.; Gong, Z.; Du, C.; Guo, J. MiR-421 is a functional marker of circulating tumor cells in gastric cancer patients. Biomarkers 2012, 17, 104–110. [Google Scholar] [CrossRef]
- Cao, Y.; Fan, Y.; Zhuang, W.; Sun, H.; Xuan, B.; Chen, Y.; Xu, W.; Sheng, H. miR-543 functions as a new marker of circulating tumorcells in gastric cancer patients. Int. J. Clin. Exp. Pathol. 2017, 10, 473–478. [Google Scholar]
- Zhu, M.; Zhang, N.; He, S.; Lui, Y.; Lu, G.; Zhao, L. MicroRNA-106a targets TIMP2 to regulate invasion and metastasis of gastric cance. FEBS Lett. 2014, 588, 600–607. [Google Scholar] [CrossRef]
- Hu, G.; Lv, Q.; Yan, J.; Chen, L.; Du, J.; Zhao, K.; Xu, W. MicroRNA-17 as a promising diagnostic biomarker of gastric cancer: An investigation combining TCGA, GEO, meta-analysis, and bioinformatics. FEBS Open Bio. 2018, 8, 1508–1523. [Google Scholar] [CrossRef]
- Zhou, H.; Guo, J.M.; Lou, Y.R.; Zhang, X.J.; Zhong, F.D.; Jiang, Z.; Cheng, J.; Xiao, B.X. Detection of circulating tumor cells in peripheral blood from patients with gastric cancer using microRNA as a marker. J. Mol. Med. (Berl. Ger.) 2010, 88, 709–717. [Google Scholar] [CrossRef]
- Huang, X.; Gao, P.; Sun, J.; Chen, X.; Song, Y.; Zhao, J.; Xu, H.; Wang, Z. Clinicopathological and prognostic significance of circulating tumor cells in patients with gastric cancer: A meta-analysis. Int. J. Cancer 2015, 136, 21–33. [Google Scholar] [CrossRef]
- Li, Y.; Gong, J.; Zhang, Q.; Lu, Z.; Gao, J.; Li, Y.; Cao, Y.; Shen, L. Dynamic monitoring of circulating tumour cells to evaluate therapeutic efficacy in advanced gastric cancer. Br. J. Cancer 2016, 114, 138–145. [Google Scholar] [CrossRef] [PubMed]
- Ishiguro, Y. Prognostic significance of circulating tumor cells in patients with gastric cancer: Epithelial mesenchymal transition and perioperative kinetics. J. Clin. Oncol. 2019, 37, 59. [Google Scholar] [CrossRef]
- Szczepanik, A.; Sierzega, M.; Drabik, G.; Pituch-Noworolska, A.; Kołodziejczyk, P.; Zembala, M. CD44+ cytokeratin-positive tumor cells in blood and bone marrow are associated with poor prognosis of patients with gastric cancer. Gastric Cancer 2019, 22, 264–272. [Google Scholar] [CrossRef] [PubMed]
- Takaishi, S.; Okumura, T.; Tu, S.; Wang, S.S.; Shibata, W.; Vigneshwaran, R.; Gordon, S.A.; Shimada, Y.; Wang, T.C. Identification of gastric cancer stem cells using the cell surface marker CD44. Stem Cells 2009, 27, 1006–1020. [Google Scholar] [CrossRef]
- Yuan, D.; Chen, L.; Li, M.; Xia, H.; Zhang, Y.; Chen, T.; Xia, R.; Tang, Q.; Gao, F.; Mo, X.; et al. Isolation and characterization of circulating tumor cells from human gastric cancer patients. J. Cancer Res. Clin. Oncol. 2015, 141, 647–660. [Google Scholar] [CrossRef]
- Brown, H.K.; Tellez-Gabriel, M.; Cartron, P.F.; Vallette, F.M.; Heymann, M.F.; Heymann, D. Characterization of circulating tumor cells as a reflection of the tumor heterogeneity: Myth or reality? Drug Discov. Today 2019, 24, 763–772. [Google Scholar] [CrossRef]
- Grillet, F.; Bayet, E.; Villeronce, O.; Zappia, L.; Lagerqvist, E.L.; Lunke, S.; Charafe-Jauffret, E.; Pham, K.; Molck, C.; Rolland, N.; et al. Circulating tumour cells from patients with colorectal cancer have cancer stem cell hallmarks in ex vivo culture. Gut 2017, 66, 1802–1810. [Google Scholar] [CrossRef]
- Yu, M.; Bardia, A.; Aceto, N.; Bersani, F.; Madden, M.W.; Donaldson, M.C.; Desai, R.; Zhu, H.; Comaills, V.; Zheng, Z.; et al. Cancer therapy. Ex vivo culture of circulating breast tumor cells for individualized testing of drug susceptibility. Science (N. Y.) 2014, 345, 216–220. [Google Scholar] [CrossRef]
- Fu, X.; Zhang, Y.; Yang, J.; Qi, Y.; Ming, Y.; Sun, M.; Shang, Y.; Yang, Y.; Zhu, X.; Gao, Q. Efficacy and safety of trastuzumab as maintenance or palliative therapy in advanced HER2-positive gastric cancer. Oncotargets Ther. 2018, 11, 6091–6100. [Google Scholar] [CrossRef]
- Bang, Y.J.; van Cutsem, E.; Feyereislova, A.; Chung, H.C.; Shen, L.; Sawaki, A.; Lordick, F.; Ohtsu, A.; Omuro, Y.; Satoh, T.; et al. Trastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of HER2-positive advanced gastric or gastro-oesophageal junction cancer (ToGA): A phase 3, open-label, randomised controlled trial. Lancet (Lond. Engl.) 2010, 376, 687–697. [Google Scholar] [CrossRef]
- Bernards, R.; Weinberg, R.A. A progression puzzle. Nature 2002, 418, 823. [Google Scholar] [CrossRef] [PubMed]
- Mimori, K.; Fukagawa, T.; Kosaka, Y.; Ishikawa, K.; Iwatsuki, M.; Yokobori, T.; Hirasaki, S.; Takatsuno, Y.; Sakashita, H.; Ishii, H.; et al. A large-scale study of MT1-MMP as a marker for isolated tumor cells in peripheral blood and bone marrow in gastric cancer cases. Ann. Surg Oncol. 2008, 15, 2934–2942. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zhang, X.; Gong, J.; Zhang, Q.; Gao, J.; Cao, Y.; Wang, D.D.; Lin, P.P.; Shen, L. Aneuploidy of chromosome 8 in circulating tumor cells correlates with prognosis in patients with advanced gastric cancer. Chin. J. Cancer Res. 2016, 28, 579–588. [Google Scholar] [CrossRef] [PubMed]
- Hong, S.H.; Cha, J.M.; Lee, J.I.; Joo, K.R.; Shin, H.P.; Park, J.J.; Jeun, J.W.; Lim, J.U. Association of hyper-LDL cholesterolemia with increased risk of colorectal adenoma. Hepatogastroenterology 2014, 61, 1588–1594. [Google Scholar]
- Dolcetti, R.; de Re, V. Immunotherapy for Gastric Cancer: Time for a Personalized Approach? IJMS 2018, 19, 1602. [Google Scholar] [CrossRef]
- Fuchs, C.S.; Doi, T.; Jang, R.W.; Muro, K.; Satoh, T.; Machado, M.; Sun, W.; Jalal, S.I.; Shah, M.A.; Metges, J.P.; et al. Safety and Efficacy of Pembrolizumab Monotherapy in Patients With Previously Treated Advanced Gastric and Gastroesophageal Junction Cancer: Phase 2 Clinical KEYNOTE-059 Trial. JAMA Oncol. 2018, 4, e180013. [Google Scholar] [CrossRef]
- Gandini, S.; Massi, D.; Mandala, M. PD-L1 expression in cancer patients receiving anti PD-1/PD-L1 antibodies: A systematic review and meta-analysis. Crit. Rev. Oncol. Hematol. 2016, 100, 88–98. [Google Scholar] [CrossRef]
- Kloten, V.; Lampignano, R.; Krahn, T.; Schlange, T. Circulating Tumor Cell PD-L1 Expression as Biomarker for Therapeutic Efficacy of Immune Checkpoint Inhibition in NSCLC. Cells 2019, 8, 9. [Google Scholar] [CrossRef]
- Schehr, J.L.; Schultz, Z.D.; Warrick, J.W.; Guckenberger, D.J.; Pezzi, H.M.; Sperger, E.; Heninger, A.; Saeed, T.; Leal, K.; Matto, A.M.; et al. Lang, High Specificity in Circulating Tumor Cell Identification Is Required for Accurate Evaluation of Programmed Death-Ligand 1. PLoS ONE 2016, 11, e0159397. [Google Scholar] [CrossRef]
- Wang, Y.; Kim, T.H.; Fouladdel, S.; Zhang, Z.; Soni, P.; Qin, A.; Zhao, L.; Azizi, E.; Lawrence, T.S.; Ramnath, N.; et al. PD-L1 Expression in Circulating Tumor Cells Increases during Radio(chemo)therapy and Indicates Poor Prognosis in Non-small Cell Lung Cancer. Sci. Rep. 2019, 9, 566. [Google Scholar] [CrossRef]
- Kallergi, G.; Vetsika, E.K.; Aggouraki, D.; Lagoudaki, E.; Koutsopoulos, A.; Koinis, F.; Katsarlinos, P.; Trypaki, M.; Messaritakis, I.; Stournaras, C.; et al. Evaluation of PD-L1/PD-1 on circulating tumor cells in patients with advanced non-small cell lung cancer. Adv. Med. Oncol 2018, 10, 1758834017750121. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Z.; Bu, Z.; Liu, X.; Zhang, L.; Li, Z.; Wu, A.; Wu, X.; Cheng, X.; Xing, X.; Du, H.; et al. Level of circulating PD-L1 expression in patients with advanced gastric cancer and its clinical implications. Chin. J. Cancer Res. 2014, 26, 104–111. [Google Scholar] [PubMed]
- Wu, S.; Liu, S.; Liu, Z.; Huang, J.; Pu, X.; Li, J.; Yang, D.; Deng, H.; Yang, N.; Xu, J. Classification of circulating tumor cells by epithelial-mesenchymal transition markers. PLoS ONE 2015, 10, e0123976. [Google Scholar] [CrossRef] [PubMed]
- Calabuig-Fariñas, S.; Jantus-Lewintre, E.; Herreros-Pomares, A.; Camps, C. Circulating tumor cells versus circulating tumor DNA in lung cancer-which one will win? Transl. Lung Cancer Res. 2016, 5, 466–482. [Google Scholar] [CrossRef]
- Keup, C.; Storbeck, M.; Hauch, S.; Hahn, P.; Sprenger-Haussels, M.; Tewes, M.; Mach, P.; Hoffmann, O.; Kimmig, R.; Kasimir-Bauer, S. Cell-Free DNA Variant Sequencing Using CTC-Depleted Blood for Comprehensive Liquid Biopsy Testing in Metastatic Breast Cancer. Cancers 2019, 11, 238. [Google Scholar] [CrossRef]
- Kulasinghe, A.; Zhou, J.; Kenny, L.; Papautsky, I.; Punyadeera, C. Capture of Circulating Tumour Cell Clusters Using Straight Microfluidic Chips. Cancers 2019, 11, 89. [Google Scholar] [CrossRef]
- Leone, K.; Poggiana, C.; Zamarchi, R. The Interplay between Circulating Tumor Cells and the Immune System: From Immune Escape to Cancer Immunotherapy. Diagnostics 2018, 8, 59. [Google Scholar] [CrossRef]
- Lian, S.; Xie, R.; Ye, Y.; Lu, Y.; Cheng, Y.; Xie, X.; Li, S.; Jia, L. Dual blockage of both PD-L1 and CD47 enhances immunotherapy against circulating tumor cells. Sci. Rep. 2019, 9, 4532. [Google Scholar] [CrossRef]
- Wong, C.H.; Siah, K.W.; Lo, A.W. Estimation of clinical trial success rates and related parameters. Biostatistics 2018, 20, 273–286. [Google Scholar] [CrossRef]
- Praharaj, P.P.; Bhutia, S.K.; Nagrath, S.; Bitting, R.L.; Deep, G. Circulating tumor cell-derived organoids: Current challenges and promises in medical research and precision medicine. Biochim. Biophys. Acta Rev. Cancer 2018, 1869, 117–127. [Google Scholar] [CrossRef]
- Seidlitz, T.; Merker, S.R.; Rothe, A.; Zakrzewski, F.; von Neubeck, C.; Grützmann, K.; Sommer, U.; Schweitzer, C.; Schölch, S.; Uhlemann, H.; et al. Human gastric cancer modelling using organoids. Gut 2019, 68, 207–217. [Google Scholar] [CrossRef] [PubMed]
- Bartucci, M.; Ferrari, A.C.; Kim, I.Y.; Ploss, A.; Yarmush, M.; Sabaawy, H.E. Personalized Medicine Approaches in Prostate Cancer Employing Patient Derived 3D Organoids and Humanized Mice. Front. Cell Dev. Biol. 2016, 4, 64. [Google Scholar] [PubMed]
- Eso, Y.; Shimizu, T.; Takeda, H.; Takai, A.; Marusawa, H. Microsatellite instability and immune checkpoint inhibitors: Toward precision medicine against gastrointestinal and hepatobiliary cancers. J. Gatroenterol. 2020, 55, 15–26. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.T.; Cristescu, R.; Bass, A.J.; Kim, K.M.; Odegaard, J.I.; Kim, K.; Liu, X.Q.; Sher, X.; Jung, H.; Lee, M.; et al. Comprehensive molecular characterization of clinical responses to PD-1 inhibition in metastatic gastric cancer. Nat. Med. 2018, 24, 1449–1458. [Google Scholar] [CrossRef] [PubMed]
- Steinert, G.; Scholch, S.; Niemietz, T.; Iwata, N.; Garcia, S.A.; Behrens, B.; Voigt, A.; Kloor, M.; Benner, A.; Bork, U.; et al. Immune escape and survival mechanisms in circulating tumor cells of colorectal cancer. Cancer Res. 2014, 74, 1694–1704. [Google Scholar] [CrossRef]
- Boral, D.; Vishnoi, M.; Liu, H.N.; Yin, W.; Sprouse, M.L.; Scamardo, A.; Hong, D.S.; Tan, T.Z.; Thiery, J.P.; Chang, J.C.; et al. Molecular characterization of breast cancer CTCs associated with brain metastasis. Nat. Commun. 2017, 8, 196. [Google Scholar] [CrossRef]
- Guimarães, C.T.U.; Martins, N.N.F.; Oliveira, K.C.d.; Almeida, C.M.; Pinheiro, T.M.; Gigek, C.O.; Cavallero, S.R.d.; Assumpção, P.P.; Smith, M.A.C.; Burbano, R.R.; et al. Liquid biopsy provides new insights into gastric cancer. Oncotarget 2018, 9, 15144–15156. [Google Scholar] [CrossRef]
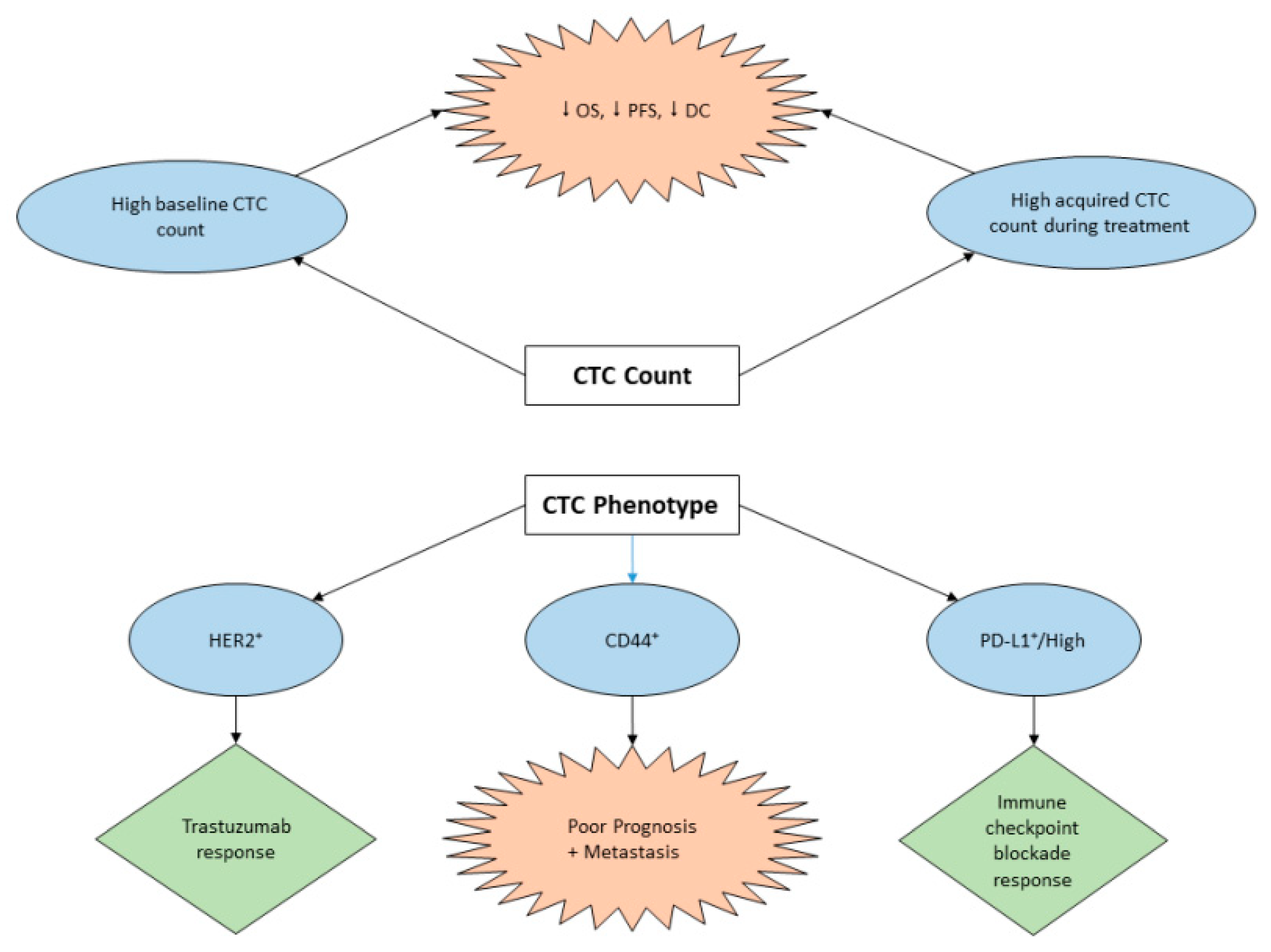
| Author, Country, Year | Cases | Isolation and Identification Tool | Markers | OS and PFS of CTC(+) vs. CTC(-) | Positive Cutoff | Clinical Implications | Reference |
|---|---|---|---|---|---|---|---|
| Lee (South Korea), 2015 | 100 metastatic GC patients | anti-EpCAM antibody coated magnetic particles CTC-Profiler (Veridex) | EpCAM, CK8/18/19, CD45 | OS: 120 days vs. 220 days; p = 0.03) PFS: 59 days vs. 141 days; p = 0.004 | ≥ 5CTCs/7.5 mL | CTCs are associated with poor response to chemotherapy in metastatic gastric cancer patients. CTC positivity was an independent adverse factor for PFS and OS. | [55] |
| Okabe (Japan), 2016 | 136 advanced GC patients | semi-automated immunomagnetic separation system CellSearch | EpCAM, CK8/18/19CD45, DAPI | OS: HR 2.20 [95%CI: 1.120–4.03]; p = 0.009 PFS: HR 2.03 [95%CI: 1.13–3.66]; p = 0.016 | ≥ 1 CTCs/7.5 mL | Detection of CTCs was an independent predictor of a shorter PFS in advanced gastric cancer. Patients who require intensive treatment: CTCs could be a valuable biomarker. The combined status of CTC and CY would be useful in selecting patients for radical surgery. | [56] |
| Zhou 2016 | 1110 GC patients in meta-analysis | - | - | OS: HR = 2.23, 95% CI: 1.86–2.66 PFS: HR = 2.02, 95% CI: 1.36–2.99 | - | High CTCs count was associated with depth of infiltration regional lymph nodes metastasis and distant metastasis. For un-resectable GC patients, high CTCs count before and during chemotherapy was significantly correlated with poor OS, PFS, and DC rate. | [57] |
| Mishima(Japan), 2017 | 101 GC patients 15 advanced GC patients whose primary tumors were HER2-, but CTCs were HER2+ | both 3 D-IF-FISH method & CellSearch System3D-IF-FISH only | EpCAM, CK8/18/19, CD45 HER2 | OS and PFS of 15 advanced GC patients with CTC- HER2+: 6.1 months (95% CI: 2.1–10.0) and 14.4 months (11.0–17.8), respectively | ≥ 1 CTCs/7.5 mL | New, non-invasive strategy to select patients who are likely to benefit from trastuzumab-based therapies, despite their primary biopsy being HER2-negative. | [58] |
| Liu (China), 2017 | 59 GC patients of stage II-IV | CELLection™ Epithelial Enrich kit | EpCAM, CK8/18/19, DAPI | OS: HR = 3.59, 95% CI:1.655-7.817, p = 0.001 PFS: = 2.81, 95% CI:1.313-5.999, p = 0.008 | ≥ 2 CTCs/5 mL | The baseline CTC count of >2 cells/5 mL and an increase of the CTC count after the first cycle of chemotherapy was an independent prognostic marker of poor PFS and OS→ patients with a low baseline CTC count or decrease of the CTC count after the first cycle of chemotherapy may benefit significantly from palliative chemotherapy | [59] |
| Zheng (China), 2017 | 81 GC patients | ISET-immunofluorescence | CK8/18/19, vimentin | CTM positivity was an independent factor for determining the PFS (p = 0.016) and OS (p = 0.003) of stage IV patients CTM correlated with shorter PFS and OS than single CTCs (p < 0.05) | ≥ 1 CTCs/5 mL For CTM: ≥ 3 CTCs | In stage IV patients, CTM positivity was correlated with serum CA125 level. CTM were an independent predictor of shorter PFS and OS in stage IV patients. → CTM detection may be a useful tool to predict prognosis in stage IV patients. | [60] |
| Kang (South Korea), 2017 | 116 patients with gastric cancer patients & 31 healthy volunteers | “FAST disc” centrifugal microfluidic system | EpCAM, CK8/18/19, CD45 DAPI | - | ≥ 2 CTCs/7.5 mL Sensitivity: 85.3 Specificity: 90.3 | Although the clinical feasibility of CTCs for gastric cancer staging was not proved, these results suggest a potential role of CTCs as an early diagnostic biomarker of gastric cancer. | [61] |
| Yue (China), 2018 | 35 patients with different advanced gastrointestinal tumors | Pep MNPs isolated system | CK19, CD45 DAPI, PD-L1 | PFS based on baseline PD-L1high CTC count: 4.27 vs. 2.07 months HR = 3.342; 95%CI 1.488–7.505; p= 0.002 PFS based on post-therapeutic PD-L1high CTC count: 3.4 vs. 2.1 months; HR= 0.412; 95%CI 0.177–0.962;, p= 0.031) | ≥ 2 PD-L1high CTCs/4 mL | The abundance of PD-L1high CTCs at baseline might serve as a predictor to screen patients for PD-1/PD-L1 blockade therapies. Measuring the dynamic changes of CTC could indicate the therapeutic response at early time. | [62] |
| Yang (China), 2018 | 40 GC patients | wedge-shaped microfluidic chip (CTC-ΔChip) & three-color immunocytochemistry method | (CK, CD45, Nucleus marker | - | - | CTC-ΔChip exhibited the feasibility of detecting CTCs from different types of solid tumor, and it identified 7.30 ± 7.29 CTCs from 2 mL peripheral blood with a positive rate of 75% (30/40) in GC patients. Novel CTC-ΔChip shows high performance for detecting CTCs from less volume of blood samples of cancer patients and important clinical significance in GC. | [63] |
| Li (China), 2018 | 115 advanced GC patients, including 56 tumor HER2+ subjects who received first-line HER2-targeted therapy plus chemotherapy and 59 tumor HER2− subjects who received chemotherapy alone | IF-FISH Cytelligen system | DAPI, HER2, CEP8, and CD45 | - | - | CTC HER2+ was found in 91.0% of tumor HER2+ and 76.2% tumor HER2− patients and was correlated with development of resistance to trastuzumab for the tumor HER2+ patients and chemotherapy alone for the tumor HER2− patients. Determining of CTC HER2 showed advantages in real-time monitoring of therapeutic resistance. | [64] |
| Cheng (China), 2019 | 32 advanced GC patients | CanPatrol CTC enrichment technique Multiplex RNA in situ hybridization assay | EpCAM, CK8/18/19, CD45 DAPI, PD-L1, Vimentin and Twist | - | ≥ 2 PD-L1+ CTCs/5 mL | CTCs count was well correlated with clinicopathology parameters. Enumeration of epithelial CTC subset and its relative abundance in the total CTC pool are highly correlated with clinical efficacy. Monitoring CTC subtypes exhibits higher sensitivity of evaluating the disease status, compared to the traditional methods. | [65] |
| Lu (China), 2019 | 42 GC patients of stage III-IV | ISET-ICC method followed by IHC | EpCAM, CK8/18/19, CD45, Vimentin and Twist, E-cadherin | - | - | The threshold number of CTCs is significantly associated with different clinical stages and was positively correlated with the value in U/mL of CA724. CTCs technology based on ISET method has a high detection rate. CTCs are promising predictor for the evaluation and prediction of treatment responses in stage III–IV gastric cancer. | [66] |
| Abdallah (Brazil), 2019 | At diagnosis (55 samples before neoadjuvant treatment) After surgery and before adjuvant therapy (33 samples) | ISET and immunocytochemistry & microscopy | HER2 and plakoglobin, CD45 | -PFS between CTM-positive patients vs. CTM-negative patients (18.7 months vs. 21.6 months; p = 0.258 -PFS between plakoglobin-positive CTM patients vs. plakoglobin-positive CTM patients: 15.9 months vs. 21.3 months; p= 0.114 | ≥ 1 CTM (2 CTCs)/4 mL | The analysis of CTM plakoglobin expression is a promising tool in the understanding the biology and prognosis of GC. | [67] |
| Gao, 2019 | 3814 GC patients in meta-analysis | - | - | HR = 1.84, 95%CI 1.50–2.26, p < 0.001 | - | CTC positivity was associated with poorer OS. | [68] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Thanh Huong, P.; Gurshaney, S.; Thanh Binh, N.; Gia Pham, A.; Hoang Nguyen, H.; Thanh Nguyen, X.; Pham-The, H.; Tran, P.-T.; Truong Vu, K.; Xuan Duong, N.; et al. Emerging Role of Circulating Tumor Cells in Gastric Cancer. Cancers 2020, 12, 695. https://doi.org/10.3390/cancers12030695
Thanh Huong P, Gurshaney S, Thanh Binh N, Gia Pham A, Hoang Nguyen H, Thanh Nguyen X, Pham-The H, Tran P-T, Truong Vu K, Xuan Duong N, et al. Emerging Role of Circulating Tumor Cells in Gastric Cancer. Cancers. 2020; 12(3):695. https://doi.org/10.3390/cancers12030695
Chicago/Turabian StyleThanh Huong, Phung, Sanjeev Gurshaney, Nguyen Thanh Binh, Anh Gia Pham, Huy Hoang Nguyen, Xuan Thanh Nguyen, Hai Pham-The, Phuong-Thao Tran, Khanh Truong Vu, Nhuong Xuan Duong, and et al. 2020. "Emerging Role of Circulating Tumor Cells in Gastric Cancer" Cancers 12, no. 3: 695. https://doi.org/10.3390/cancers12030695
APA StyleThanh Huong, P., Gurshaney, S., Thanh Binh, N., Gia Pham, A., Hoang Nguyen, H., Thanh Nguyen, X., Pham-The, H., Tran, P.-T., Truong Vu, K., Xuan Duong, N., Pelucchi, C., La Vecchia, C., Boffetta, P., Nguyen, H. D., & Luu, H. N. (2020). Emerging Role of Circulating Tumor Cells in Gastric Cancer. Cancers, 12(3), 695. https://doi.org/10.3390/cancers12030695








