Transcriptome Analysis Identifies ALCAM Overexpression as a Prognosis Biomarker in Laryngeal Squamous Cell Carcinoma
Abstract
1. Introduction
2. Results
2.1. Identifying Molecular Prognostic Biomarker for LSCC Patients
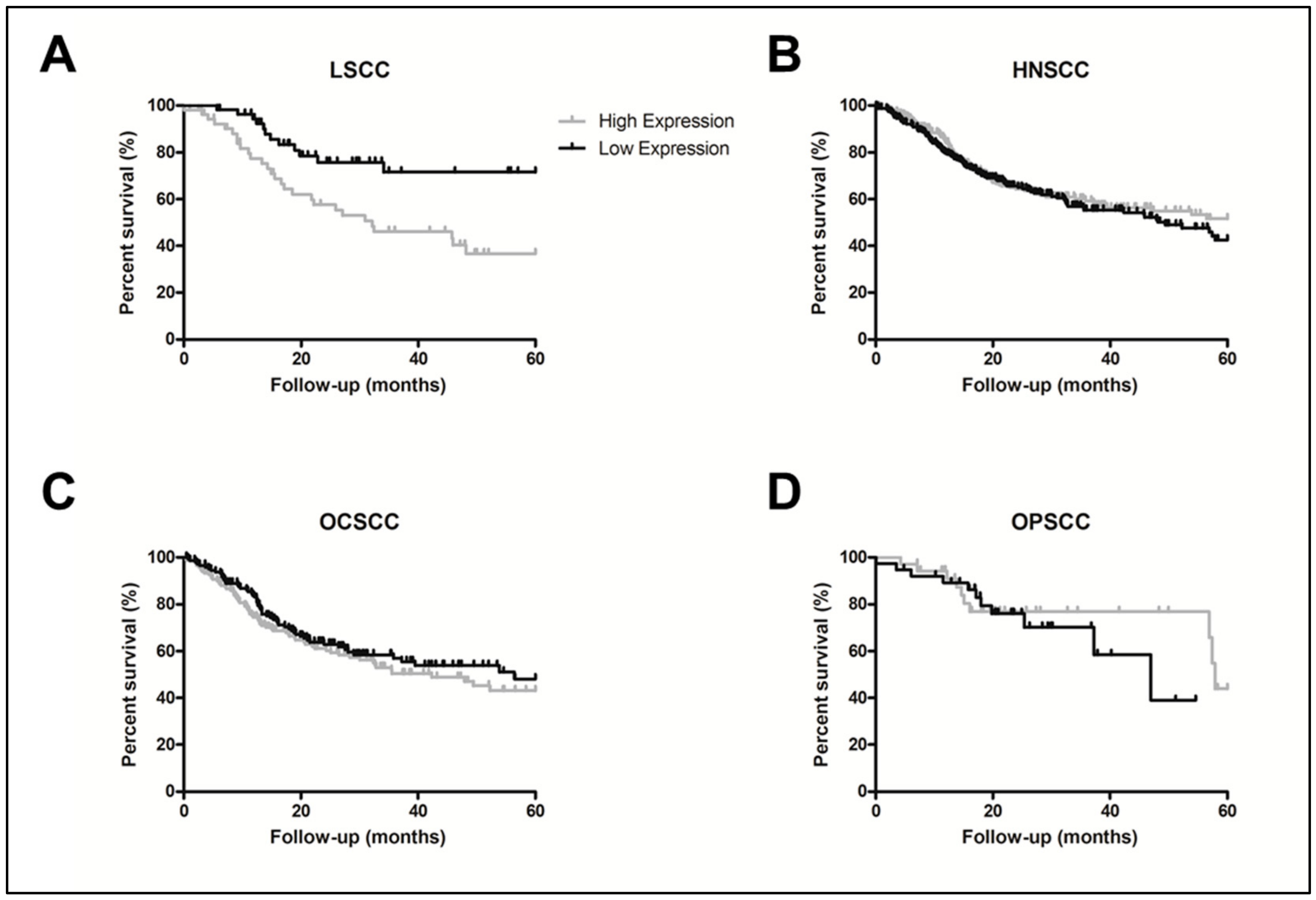
2.2. ALCAM Protein High Levels Was also Associated to LSCC Worse Prognosis
3. Discussion
4. Materials and Methods
4.1. LSCC Samples
4.2. LSCC Gene-Expression Profiling
4.3. Prognostic Gene-Pattern Signature
4.4. Gene-Expression Validation by Quantitative PCR
4.5. Immunohistochemistry Analysis
4.6. ALCAM Somatic Alterations in LSCC
4.7. Statistical Analyses
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2019. CA Cancer J. Clin. 2019, 69, 7–34. [Google Scholar] [CrossRef]
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef]
- Ferlay, J.; Ervik, M.; Lam, F.; Colombet, M.; Mery, L.; Piñeros, M.; Znaor, A.; Soerjomataram, I.; Bray, F. Global Cancer Observatory: Cancer Today; International Agency for Research on Cancer: Lyon, France, 2019; Available online: https://gco.iarc.fr/today (accessed on 6 January 2020).
- Strojan, P.; Haigentz, M.; Bradford, C.R.; Wolf, G.T.; Hartl, D.M.; Langendijk, J.A.; Rinaldo, A.; Eisbruch, A.; Mendenhall, W.M.; Forastiere, A.A.; et al. Chemoradiotherapy vs. total laryngectomy for primary treatment of advanced laryngeal squamous cell carcinoma. Oral Oncol. 2013, 49, 283–286. [Google Scholar] [CrossRef]
- Steuer, C.E.; El-Deiry, M.; Parks, J.R.; Higgins, K.A.; Saba, N.F. An update on larynx cancer. CA Cancer J. Clin. 2017, 67, 31–50. [Google Scholar] [CrossRef]
- Logothetis, C.J.; Gallick, G.E.; Maity, S.N.; Kim, J.; Aparicio, A.; Efstathiou, E.; Lin, S.H. Molecular classification of prostate cancer progression: Foundation for marker-driven treatment of prostate cancer. Cancer Discov. 2013, 3, 849–861. [Google Scholar] [CrossRef]
- Sørlie, T.; Perou, C.M.; Tibshirani, R.; Aas, T.; Geisler, S.; Johnsen, H.; Hastie, T.; Eisen, M.B.; van de Rijn, M.; Jeffrey, S.S.; et al. Gene expression patterns of breast carcinomas distinguish tumor subclasses with clinical implications. Proc. Natl. Acad. Sci. USA 2001, 98, 10869–10874. [Google Scholar] [CrossRef]
- Linnekamp, J.F.; Hooff, S.R.V.; Prasetyanti, P.R.; Kandimalla, R.; Buikhuisen, J.Y.; Fessler, E.; Ramesh, P.; Lee, K.A.S.T.; Bochove, G.G.W.; de Jong, J.H.; et al. Consensus molecular subtypes of colorectal cancer are recapitulated in in vitro and in vivo models. Cell Death Differ. 2018, 25, 616–633. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Yan, M.; Li, R.R.; Chen, W.T. Sonic Hedgehog Signalling Activation Contributes to ALCAM Over-Expression and Poor Clinical Outcome in Patients with Oral Squamous Cell Carcinoma. Chin. J. Dent. Res. 2018, 21, 31–40. [Google Scholar] [CrossRef] [PubMed]
- Network, C.G.A. Comprehensive genomic characterization of head and neck squamous cell carcinomas. Nature 2015, 517, 576–582. [Google Scholar] [CrossRef] [PubMed]
- Leemans, C.R.; Snijders, P.J.F.; Brakenhoff, R.H. The molecular landscape of head and neck cancer. Nat. Rev. Cancer 2018, 18, 269–282. [Google Scholar] [CrossRef] [PubMed]
- Larque, A.B.; Conde, L.; Hakim, S.; Alos, L.; Jares, P.; Vilaseca, I.; Cardesa, A.; Nadal, A. P16(INK⁴a) overexpression is associated with CDKN2A mutation and worse prognosis in HPV-negative laryngeal squamous cell carcinomas. Virchows Arch. 2015, 466, 375–382. [Google Scholar] [CrossRef]
- Manterola, L.; Aguirre, P.; Larrea, E.; Arestín, M.; Gaafar, A.; Elorriaga, K.; Goicoechea, I.; Armesto, M.; Fernández-Mercado, M.; Zabalza, I.; et al. Mutational profiling can identify laryngeal dysplasia at risk of progression to invasive carcinoma. Sci. Rep. 2018, 8, 6613. [Google Scholar] [CrossRef] [PubMed]
- Scheel, A.; Bellile, E.; McHugh, J.B.; Walline, H.M.; Prince, M.E.; Urba, S.; Wolf, G.T.; Eisbruch, A.; Worden, F.; Carey, T.E.; et al. Classification of TP53 mutations and HPV predict survival in advanced larynx cancer. Laryngoscope 2016, 126, E292–E299. [Google Scholar] [CrossRef] [PubMed]
- Ben-Dayan, M.M.; Ow, T.J.; Belbin, T.J.; Wetzler, J.; Smith, R.V.; Childs, G.; Diergaarde, B.; Hayes, D.N.; Grandis, J.R.; Prystowsky, M.B.; et al. Nonpromoter methylation of the CDKN2A gene with active transcription is associated with improved locoregional control in laryngeal squamous cell carcinoma. Cancer Med. 2017, 6, 397–407. [Google Scholar] [CrossRef]
- Fan, L.; Zhang, A.; Deng, P. LMX1B mRNA expression and its gene body CpG methylation are valuable prognostic biomarkers for laryngeal squamous cell carcinoma. Biomed. Pharmacother. 2019, 117, 109174. [Google Scholar] [CrossRef]
- Cossu, A.M.; Mosca, L.; Zappavigna, S.; Misso, G.; Bocchetti, M.; De Micco, F.; Quagliuolo, L.; Porcelli, M.; Caraglia, M.; Boccellino, M. Long Non-coding RNAs as Important Biomarkers in Laryngeal Cancer and Other Head and Neck Tumours. Int. J. Mol. Sci. 2019, 20, 3444. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Li, Y.; Gao, W.; Huangfu, H.; Wen, S.; Zhang, C.; Zhao, Q.; Dong, Z.; Qu, C.; Li, G.; et al. Assessment of tumor-associated immune cells in laryngeal squamous cell carcinoma. J. Cancer Res. Clin. Oncol. 2019, 145, 1761–1772. [Google Scholar] [CrossRef] [PubMed]
- Zhou, S.; Piao, X.; Wang, C.; Wang, R.; Song, Z. Identification of claudin-1, -3, -7 and -8 as prognostic markers in human laryngeal carcinoma. Mol. Med. Rep. 2019, 20, 393–400. [Google Scholar] [CrossRef] [PubMed]
- Swart, G.W.; Lunter, P.C.; Kilsdonk, J.W.; Kempen, L.C. Activated leukocyte cell adhesion molecule (ALCAM/CD166): Signaling at the divide of melanoma cell clustering and cell migration? Cancer Metastasis Rev. 2005, 24, 223–236. [Google Scholar] [CrossRef]
- Bowen, M.A.; Bajorath, J.; D’Egidio, M.; Whitney, G.S.; Palmer, D.; Kobarg, J.; Starling, G.C.; Siadak, A.W.; Aruffo, A. Characterization of mouse ALCAM (CD166): The CD6-binding domain is conserved in different homologs and mediates cross-species binding. Eur. J. Immunol. 1997, 27, 1469–1478. [Google Scholar] [CrossRef]
- Swart, G.W. Activated leukocyte cell adhesion molecule (CD166/ALCAM): Developmental and mechanistic aspects of cell clustering and cell migration. Eur. J. Cell Biol. 2002, 81, 313–321. [Google Scholar] [CrossRef] [PubMed]
- Masedunskas, A.; King, J.A.; Tan, F.; Cochran, R.; Stevens, T.; Sviridov, D.; Ofori-Acquah, S.F. Activated leukocyte cell adhesion molecule is a component of the endothelial junction involved in transendothelial monocyte migration. FEBS Lett. 2006, 580, 2637–2645. [Google Scholar] [CrossRef] [PubMed]
- Zimmerman, A.W.; Joosten, B.; Torensma, R.; Parnes, J.R.; van Leeuwen, F.N.; Figdor, C.G. Long-term engagement of CD6 and ALCAM is essential for T-cell proliferation induced by dendritic cells. Blood 2006, 107, 3212–3220. [Google Scholar] [CrossRef] [PubMed]
- Diekmann, H.; Stuermer, C.A. Zebrafish neurolin-a and -b, orthologs of ALCAM, are involved in retinal ganglion cell differentiation and retinal axon pathfinding. J. Comp. Neurol. 2009, 513, 38–50. [Google Scholar] [CrossRef] [PubMed]
- Aruffo, A.; Bowen, M.A.; Patel, D.D.; Haynes, B.F.; Starling, G.C.; Gebe, J.A.; Bajorath, J. CD6-ligand interactions: A paradigm for SRCR domain function? Immunol Today 1997, 18, 498–504. [Google Scholar] [CrossRef]
- Santos, R.F.; Oliveira, L.; Carmo, A.M. Tuning T Cell Activation: The Function of CD6 At the Immunological Synapse and in T Cell Responses. Curr. Drug Targets. 2016, 17, 630–639. [Google Scholar] [CrossRef] [PubMed]
- Pinto, M.; Carmo, A.M. CD6 as a therapeutic target in autoimmune diseases: Successes and challenges. BioDrugs 2013, 27, 191–202. [Google Scholar] [CrossRef]
- Ibáñez, A.; Sarrias, M.R.; Farnós, M.; Gimferrer, I.; Serra-Pagès, C.; Vives, J.; Lozano, F. Mitogen-activated protein kinase pathway activation by the CD6 lymphocyte surface receptor. J. Immunol. 2006, 177, 1152–1159. [Google Scholar] [CrossRef]
- Wiiger, M.T.; Gehrken, H.B.; Fodstad, Ø.; Maelandsmo, G.M.; Andersson, Y. A novel human recombinant single-chain antibody targeting CD166/ALCAM inhibits cancer cell invasion in vitro and in vivo tumour growth. Cancer Immunol. Immunother. 2010, 59, 1665–1674. [Google Scholar] [CrossRef]
- Kozbor, D. Cancer vaccine with mimotopes of tumor-associated carbohydrate antigens. Immunol. Res. 2010, 46, 23–31. [Google Scholar] [CrossRef]
- Levin, T.G.; Powell, A.E.; Davies, P.S.; Silk, A.D.; Dismuke, A.D.; Anderson, E.C.; Swain, J.R.; Wong, M.H. Characterization of the intestinal cancer stem cell marker CD166 in the human and mouse gastrointestinal tract. Gastroenterology 2010, 139, 2072–2082. [Google Scholar] [CrossRef] [PubMed]
- Satar, N.A.; Fakiruddin, K.S.; Lim, M.N.; Mok, P.L.; Zakaria, N.; Fakharuzi, N.A.; Abd Rahman, A.Z.; Zakaria, Z.; Yahaya, B.H.; Baharuddin, P. Novel triple-positive markers identified in human non-small cell lung cancer cell line with chemotherapy-resistant and putative cancer stem cell characteristics. Oncol. Rep. 2018, 40, 669–681. [Google Scholar] [CrossRef] [PubMed]
- Xiao, M.; Yan, M.; Zhang, J.; Xu, Q.; Qi, S.; Wang, X.; Chen, W. Cancer stem-like cell related protein CD166 degrades through E3 ubiquitin ligase CHIP in head and neck cancer. Exp. Cell Res. 2017, 353, 46–53. [Google Scholar] [CrossRef] [PubMed]
- Yan, M.; Yang, X.; Wang, L.; Clark, D.; Zuo, H.; Ye, D.; Chen, W.; Zhang, P. Plasma membrane proteomics of tumor spheres identify CD166 as a novel marker for cancer stem-like cells in head and neck squamous cell carcinoma. Mol. Cell Proteomics 2013, 12, 3271–3284. [Google Scholar] [CrossRef] [PubMed]
- Jeong, Y.J.; Oh, H.K.; Park, S.H.; Bong, J.G. Prognostic Significance of Activated Leukocyte Cell Adhesion Molecule (ALCAM) in Association with Promoter Methylation of the ALCAM Gene in Breast Cancer. Molecules 2018, 23, 131. [Google Scholar] [CrossRef] [PubMed]
- Chen, F.; Zhang, Y.; Parra, E.; Rodriguez, J.; Behrens, C.; Akbani, R.; Lu, Y.; Kurie, J.M.; Gibbons, D.L.; Mills, G.B.; et al. Multiplatform-based molecular subtypes of non-small-cell lung cancer. Oncogene 2017, 36, 1384–1393. [Google Scholar] [CrossRef]
- Jia, G.; Wang, X.; Yan, M.; Chen, W.; Zhang, P. CD166-mediated epidermal growth factor receptor phosphorylation promotes the growth of oral squamous cell carcinoma. Oral Oncol. 2016, 59, 1–11. [Google Scholar] [CrossRef]
- Andisheh-Tadbir, A.; Ashraf, M.J.; Khademi, B.; Ahmadi, S. Clinical implication of CD166 expression in salivary gland tumor. Tumour Biol. 2015, 36, 2793–2799. [Google Scholar] [CrossRef]
- Clauditz, T.S.; von Rheinbaben, K.; Lebok, P.; Minner, S.; Tachezy, M.; Borgmann, K.; Knecht, R.; Sauter, G.; Wilczak, W.; Blessmann, M.; et al. Activated leukocyte cell adhesion molecule (ALCAM/CD166) expression in head and neck squamous cell carcinoma (HNSSC). Pathol. Res. Pract. 2014, 210, 649–655. [Google Scholar] [CrossRef]
- Eustace, A.; Mani, N.; Span, P.N.; Irlam, J.J.; Taylor, J.; Betts, G.N.; Denley, H.; Miller, C.J.; Homer, J.J.; Rojas, A.M.; et al. A 26-gene hypoxia signature predicts benefit from hypoxia-modifying therapy in laryngeal cancer but not bladder cancer. Clin. Cancer Res. 2013, 19, 4879–4888. [Google Scholar] [CrossRef]
- He, Q.; Tian, L.; Jiang, H.; Zhang, J.; Li, Q.; Sun, Y.; Zhao, J.; Li, H.; Liu, M. Identification of laryngeal cancer prognostic biomarkers using an inflammatory gene-related, competitive endogenous RNA network. Oncotarget 2017, 8, 9525–9534. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Bai, Z.; Shi, E.; Wang, Q.; Dong, Z.; Xu, P. A potential panel of two-long non-coding RNA signature to predict recurrence of patients with laryngeal cancer. Oncotarget 2017, 8, 69641–69650. [Google Scholar] [CrossRef] [PubMed]
- Cerami, E.; Gao, J.; Dogrusoz, U.; Gross, B.E.; Sumer, S.O.; Aksoy, B.A.; Jacobsen, A.; Byrne, C.J.; Heuer, M.L.; Larsson, E.; et al. The cBio cancer genomics portal: An open platform for exploring multidimensional cancer genomics data. Cancer Discov. 2012, 2, 401–404. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.; Aksoy, B.A.; Dogrusoz, U.; Dresdner, G.; Gross, B.; Sumer, S.O.; Sun, Y.; Jacobsen, A.; Sinha, R.; Larsson, E.; et al. Integrative analysis of complex cancer genomics and clinical profiles using the cBioPortal. Sci. Signal 2013, 6, pl1. [Google Scholar] [CrossRef] [PubMed]
- Nicolau-Neto, P.; Da Costa, N.M.; de Souza Santos, P.T.; Gonzaga, I.M.; Ferreira, M.A.; Guaraldi, S.; Moreira, M.A.; Seuánez, H.N.; Brewer, L.; Bergmann, A.; et al. Esophageal squamous cell carcinoma transcriptome reveals the effect of. Oncotarget 2018, 9, 16634–16647. [Google Scholar] [CrossRef]
- Li, C.; Wong, W.H. Model-based analysis of oligonucleotide arrays: Expression index computation and outlier detection. Proc. Natl. Acad. Sci. USA 2001, 98, 31–36. [Google Scholar] [CrossRef]
- Davis, S.; Meltzer, P.S. GEOquery: A bridge between the Gene Expression Omnibus (GEO) and BioConductor. Bioinformatics 2007, 23, 1846–1847. [Google Scholar] [CrossRef]
- Wettenhall, J.M.; Smyth, G.K. limmaGUI: A graphical user interface for linear modeling of microarray data. Bioinformatics 2004, 20, 3705–3706. [Google Scholar] [CrossRef]
- Carstensen, B.; Plummer, M.; Laara, E.; Hills, M. Epi: A Package for Statistical Analysis in Epidemiology. R package Version 2.40. Available online: https://CRAN.R-project.org/package=Epi (accessed on 9 January 2020).
- Therneau, T. A Package for Survival Analysis in S. Version 2.38. Available online: https://CRAN.R-project.org/package=survival (accessed on 9 January 2020).
- Savage, R.; Cooke, E.; Darkins, R.; Xu, Y. BHC: Bayesian Hierarchical Clustering. R Package Version 1.38.0. Available online: https://www.bioconductor.org/packages/release/bioc/manuals/BHC/man/BHC.pdf (accessed on 9 January 2020).
- De A Simão, T.; Souza-Santos, P.T.; de Oliveira, D.S.; Bernardo, V.; Lima, S.C.; Rapozo, D.C.; Kruel, C.D.; Faria, P.A.; Ribeiro Pinto, L.F.; Albano, R.M. Quantitative evaluation of SPRR3 expression in esophageal squamous cell carcinoma by qPCR and its potential use as a biomarker. Exp. Mol. Pathol. 2011, 91, 584–589. [Google Scholar] [CrossRef]
- Mermel, C.H.; Schumacher, S.E.; Hill, B.; Meyerson, M.L.; Beroukhim, R.; Getz, G. GISTIC2.0 facilitates sensitive and confident localization of the targets of focal somatic copy-number alteration in human cancers. Genome Biol. 2011, 12, R41. [Google Scholar] [CrossRef]
- Bradburn, M.J.; Clark, T.G.; Love, S.B.; Altman, D.G. Survival analysis Part III: Multivariate data analysis—Choosing a model and assessing its adequacy and fit. Br. J. Cancer 2003, 89, 605–611. [Google Scholar] [CrossRef] [PubMed]
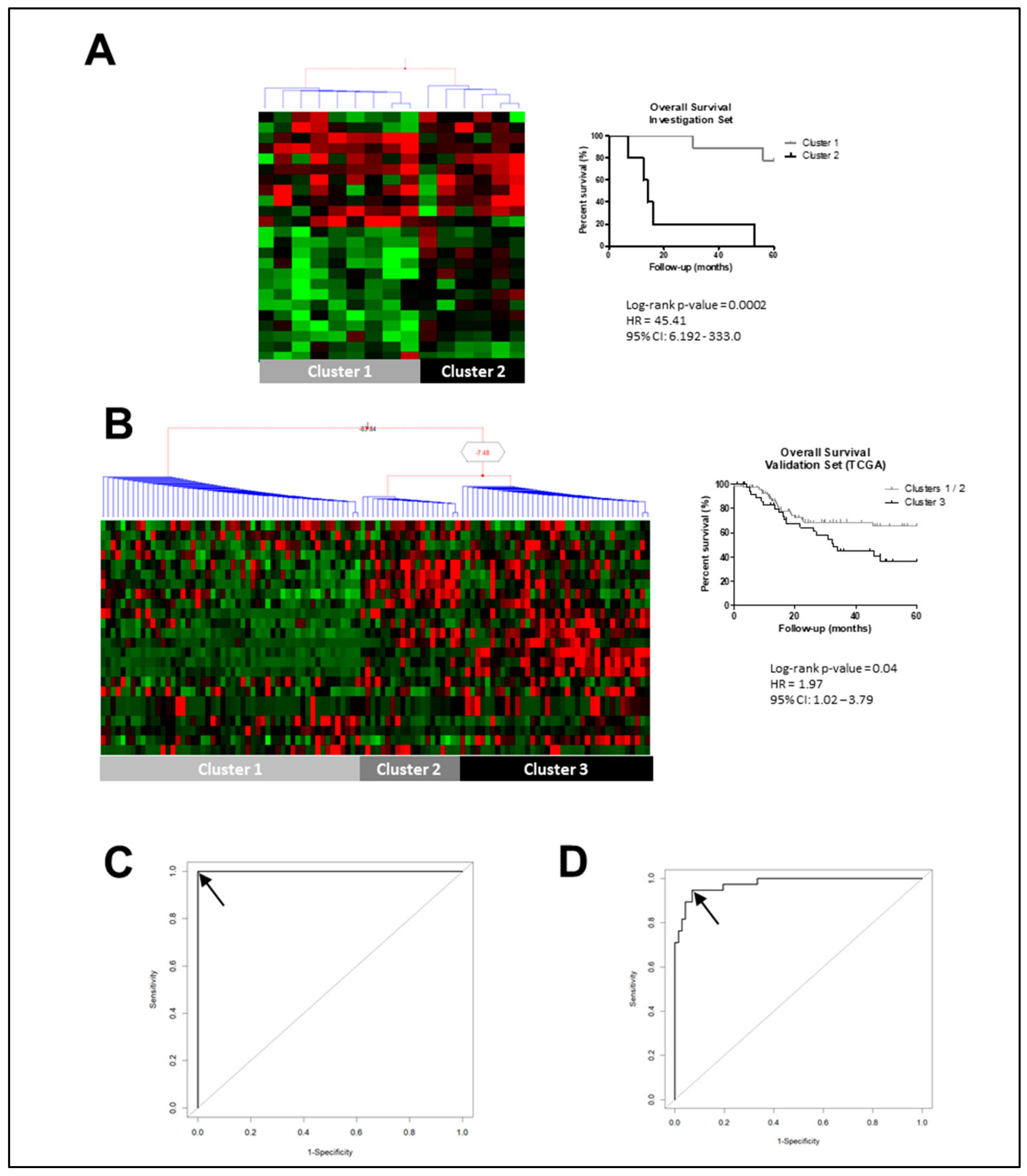

| Gene Symbol | Official Full Name | Log-Rank p-Value |
|---|---|---|
| ACOX1 | acyl-CoA oxidase 1 | 0.007 |
| ACVR1 | activin A receptor type 1 | 0.007 |
| ADH7 | alcohol dehydrogenase 7 | 0.005 |
| AGFG2 | ArfGAP with FG repeats 2 | 0.010 |
| ALCAM | activated leukocyte cell adhesion molecule | 0.007 |
| BTBD11 | BTB domain containing 11 | 0.003 |
| C12orf75 | chromosome 12 open reading frame 75 | 0.010 |
| CDK14 | cyclin dependent kinase 14 | 0.045 |
| CYP2C19 | cytochrome P450 family 2 subfamily C member 19 | 0.005 |
| GBP6 | guanylate binding protein family member 6 | 0.045 |
| GLTP | glycolipid transfer protein | 0.045 |
| GNG4 | G protein subunit gamma 4 | 0.010 |
| LOX | lysyl oxidase | 0.045 |
| LYPD6B | LY6/PLAUR domain containing 6B | 0.013 |
| ME1 | malic enzyme 1 | 0.045 |
| NPEPPS | aminopeptidase puromycin sensitive | 0.045 |
| ODC1 | ornithine decarboxylase 1 | 0.003 |
| PMM1 | Phosphomannomutase 1 | 0.016 |
| PTGR1 | prostaglandin reductase 1 | 0.000 |
| SERPINA3 | serpin family A member 3 | 0.045 |
| ST3GAL4 | ST3 beta-galactoside alpha-2,3-sialyltransferase 4 | 0.045 |
| TPD52L1 | tumor protein D52-like 1 | 0.045 |
| ZDHHC13 | zinc finger DHHC-type containing 13 | 0.010 |
| ZNF750 | zinc finger protein 750 | 0.045 |
| Feature | Categories | Univariate Analysis | Multivariate Analysis | ||||
|---|---|---|---|---|---|---|---|
| HR | (95% CI) | p Value | HR | (95% CI) | p Value | ||
| LSCC TCGA Provisional Data (n = 110) | |||||||
| Age at diagnosis (years) | >62 vs. <62 | 1.19 | (0.67–2.12) | 0.54 | |||
| Tumor Stage | III-IV vs. I-II | 0.74 | (0.31–1.77) | 0.51 | |||
| Tumor Differentiation | G3 vs. G2 vs. G1 | 0.69 | (0.43–1.12) | 0.14 | 0.98 | (0.34–2.82) | 0.980 |
| Perineural Invasion | Yes vs. No | 3.97 | (1.67–9.47) | 0.001 | 2.50 | (0.92–6.73) | 0.069 |
| Surgical Margins | Positive/Close vs. Negative | 4.20 | (1.79–9.83) | 0.0009 | 4.11 | (1.75–9.66) | 0.001 |
| ACOX1 | High vs. Low | 1.04 | (0.59–1.85) | 0.86 | |||
| ACVR1 | High vs. Low | 1.26 | (0.70–2.25) | 0.43 | |||
| ADH7 | High vs. Low | 1.05 | (0.59–1.87) | 0.84 | |||
| AGFG2 | High vs. Low | 0.75 | (0.42–1.33) | 0.33 | |||
| ALCAM | High vs. Low | 2.05 | (1.13–3.69) | 0.01 | 2.74 | (1.26–5.97) | 0.010 |
| BTBD11 | High vs. Low | 1.44 | (0.81–2.54) | 0.20 | 2.26 | (0.72–7.06) | 0.158 |
| C12ORF75 | High vs. Low | 1.04 | (0.59–1.86) | 0.87 | |||
| CDK14 | High vs. Low | 0.92 | (0.51–1.66) | 0.78 | |||
| CYP2C19 | High vs. Low | 1.23 | (0.70–2.18) | 0.45 | |||
| GBP6 | High vs. Low | 1.09 | (0.61–1.94) | 0.75 | |||
| GLTP | High vs. Low | 0.90 | (0.51–1.61) | 0.74 | |||
| GNG4 | High vs. Low | 1.44 | (0.81–2.54) | 0.21 | |||
| LOX | High vs. Low | 1.81 | (1.01–3.24) | 0.04 | 1.99 | (0.66–6.02) | 0.218 |
| LYPD6B | High vs. Low | 0.65 | (0.36–1.18) | 0.16 | 0.60 | (0.18–1.95) | 0.402 |
| ME1 | High vs. Low | 1.14 | (0.64–2.02) | 0.64 | |||
| NPEPPS | High vs. Low | 1.16 | (0.65–2.05) | 0.60 | |||
| ODC1 | High vs. Low | 1.38 | (0.78–2.46) | 0.26 | |||
| PMM1 | High vs. Low | 1.21 | (0.68–2.15) | 0.49 | |||
| PTGR1 | High vs. Low | 1.31 | (0.74–2.33) | 0.34 | |||
| SERPINA3 | High vs. Low | 1.44 | (0.39–1.23) | 0.21 | |||
| ST3GLA4 | High vs. Low | 0.80 | (0.45–1.43) | 0.46 | |||
| TPD52L1 | High vs. Low | 0.85 | (0.48–1.51) | 0.59 | |||
| ZDHHC13 | High vs. Low | 0.97 | (0.55–1.71) | 0.91 | |||
| ZNF750 | High vs. Low | 0.90 | (0.51–1.60) | 0.73 | |||
| Feature | Brazilian Samples | Investigation Set | Validation Set | ||||||
|---|---|---|---|---|---|---|---|---|---|
| (n = 44) | Brazilian Transcriptome (n = 14) | TCGA Data (n = 110) | p-Value # | p-Value $ | |||||
| n | % | n | % | n | (%) | ||||
| Age (years) | Median | 62.5 | 58 | 62 | 0.81 | 0.43 | |||
| Range | 44-88 | 45–77 | 38–83 | ||||||
| Gender | Male | 42 | 95.45% | 13 | 92.86% | 91 | 82.73% | 0.46 | 0.04 |
| Female | 2 | 4.55% | 1 | 7.14% | 19 | 17.27% | |||
| NA | 0 | 0 | 0.00% | 0 | 0.00% | ||||
| Tumor Differentiation | Well | 6 | 13.64% | 1 | 7.14% | 7 | 6.36% | 0.25 | 0.03 |
| Moderate | 34 | 77.27% | 12 | 85.71% | 70 | 63.64% | |||
| Poor | 4 | 9.09% | 1 | 7.14% | 29 | 26.36% | |||
| NA | 0 | 0 | 0.00% | 4 | 3.64% | ||||
| Tumor Stage | I | 3 | 6.82% | 1 | 7.14% | 2 | 1.82% | 0.62 | 1.00 |
| II | 1 | 2.27% | 1 | 7.14% | 9 | 8.18% | |||
| III | 7 | 15.91% | 2 | 14.29% | 19 | 17.27% | |||
| IV | 31 | 70.45% | 9 | 64.29% | 80 | 72.73% | |||
| NA | 2 | 4.55% | 1 | 7.14% | 0 | 0.00% | |||
| Lymph node metastasis | No | 16 | 36.36% | 3 | 21.43% | 39 | 35.45% | 0.35 | 0.70 |
| Yes | 26 | 59.09% | 9 | 64.29% | 52 | 47.27% | |||
| NA | 2 | 4.55% | 2 | 14.29% | 19 | 17.27% | |||
| Perineural Invasion | Negative | 29 | 65.91% | 7 | 50.00% | 45 | 40.91% | 1.00 | 1.00 |
| Positive | 15 | 34.09% | 4 | 28.57% | 24 | 21.82% | |||
| NA | 2 | 4.55% | 3 | 21.43% | 41 | 37.27% | |||
| Involved Surgical Margin | Negative | 33 | 75.0% | 11 | 78.57% | 81 | 73.64% | 0.43 | 0.31 |
| Positive/close | 9 | 20.45% | 3 | 21.43% | 13 | 11.82% | |||
| NA | 2 | 4.55% | 0 | 0.00% | 16 | 14.55% | |||
| Tobacco Smoking | Current/Former | 36 | 81.82% | 10 | 71.43% | 101 | 91.82% | 0.18 | 0.17 |
| No | 5 | 11.36% | 2 | 14.29% | 6 | 5.45% | |||
| NA | 3 | 6.82% | 2 | 14.29% | 4 | 3.64% | |||
| Alcohol Consumption | Current/Former | 31 | 70.45% | 6 | 42.86% | 39 | 35.45% | 0.09 | 1.00 |
| No | 5 | 11.36% | 4 | 28.57% | 7 | 6.36% | |||
| NA | 8 | 18.19% | 6 | 42.86% | 71 | 64.55% | |||
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nicolau-Neto, P.; de Souza-Santos, P.T.; Severo Ramundo, M.; Valverde, P.; Martins, I.; Costa Santos, I.; Dias, F.; de Almeida Simão, T.; Ribeiro Pinto, L.F. Transcriptome Analysis Identifies ALCAM Overexpression as a Prognosis Biomarker in Laryngeal Squamous Cell Carcinoma. Cancers 2020, 12, 470. https://doi.org/10.3390/cancers12020470
Nicolau-Neto P, de Souza-Santos PT, Severo Ramundo M, Valverde P, Martins I, Costa Santos I, Dias F, de Almeida Simão T, Ribeiro Pinto LF. Transcriptome Analysis Identifies ALCAM Overexpression as a Prognosis Biomarker in Laryngeal Squamous Cell Carcinoma. Cancers. 2020; 12(2):470. https://doi.org/10.3390/cancers12020470
Chicago/Turabian StyleNicolau-Neto, Pedro, Paulo Thiago de Souza-Santos, Mariana Severo Ramundo, Priscila Valverde, Ivanir Martins, Izabella Costa Santos, Fernando Dias, Tatiana de Almeida Simão, and Luis Felipe Ribeiro Pinto. 2020. "Transcriptome Analysis Identifies ALCAM Overexpression as a Prognosis Biomarker in Laryngeal Squamous Cell Carcinoma" Cancers 12, no. 2: 470. https://doi.org/10.3390/cancers12020470
APA StyleNicolau-Neto, P., de Souza-Santos, P. T., Severo Ramundo, M., Valverde, P., Martins, I., Costa Santos, I., Dias, F., de Almeida Simão, T., & Ribeiro Pinto, L. F. (2020). Transcriptome Analysis Identifies ALCAM Overexpression as a Prognosis Biomarker in Laryngeal Squamous Cell Carcinoma. Cancers, 12(2), 470. https://doi.org/10.3390/cancers12020470





