Sorafenib-Regorafenib Sequential Therapy in Japanese Patients with Unresectable Hepatocellular Carcinoma—Relative Dose Intensity and Post-Regorafenib Therapies in Real World Practice
Abstract
:1. Introduction
2. Results
2.1. Patients
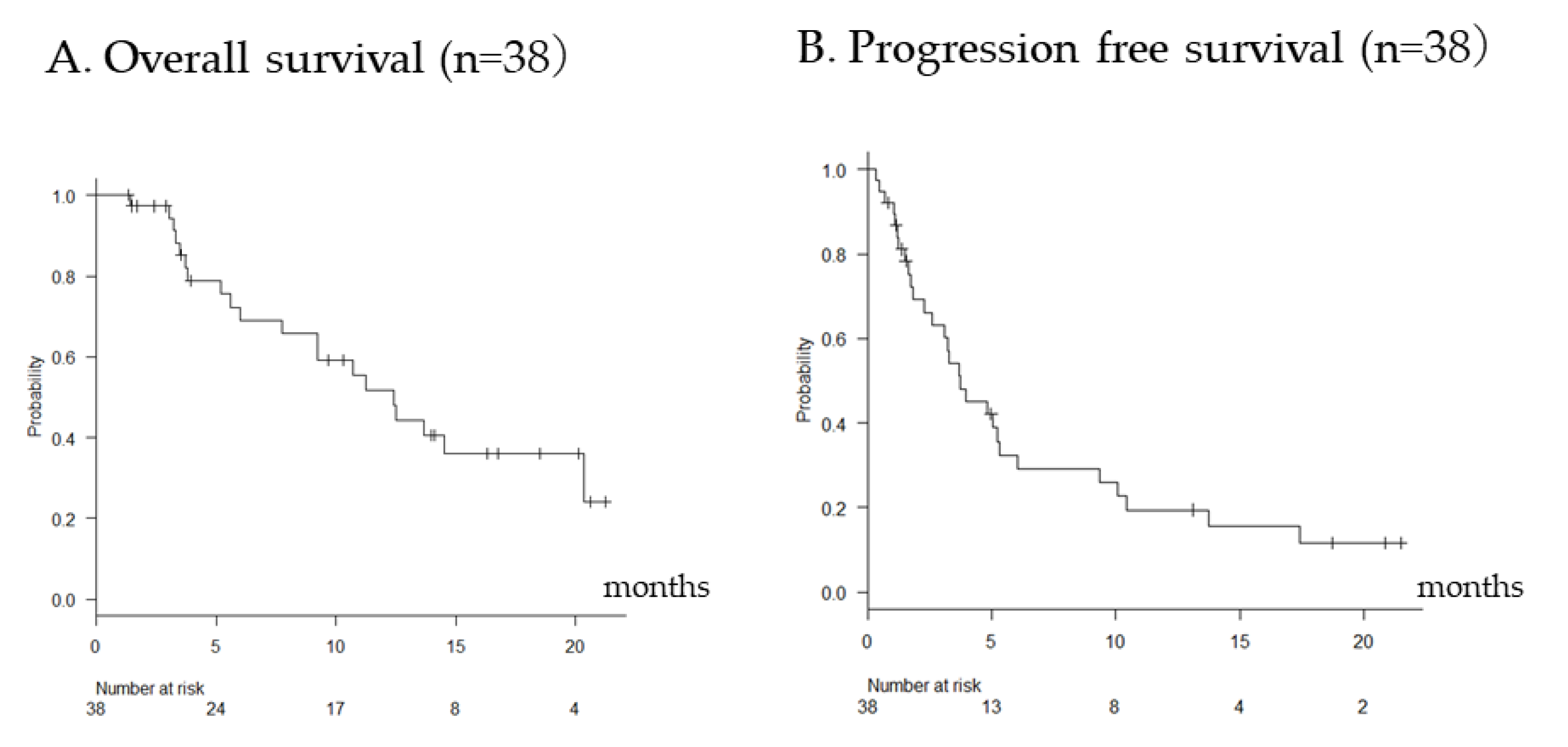
2.2. Overall Efficacy
2.3. Regorafenib Treatment Regimen and Relative Dose Intensity for the First Month
2.4. Adverse Events Associated with Regorafenib Therapy
2.5. Post-Regorafenib Therapies and Efficacy of Lenvatinib as a Third-Line Agent
2.6. Factors Associated with Favorable Prognosis in Regorafenib Therapy
3. Discussion
4. Methods
4.1. Patient and Clinical Parameters
4.2. Statistics
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ferlay, J.; Soerjomataram, I.; Dikshit, R.; Eser, S.; Mathers, C.; Rebelo, M.; Parkin, D.M.; Forman, D.; Bray, F. Cancer Incidence and Mortality Worldwide: Sources, Methods and Major Patterns in GLOBOCAN 2012. Int. J. Cancer 2015, 136, E359–E386. [Google Scholar] [CrossRef] [PubMed]
- Llovet, J.M.; Ricci, S.; Mazzaferro, V.; Hilgard, P.; Gane, E.; Blanc, J.F.; de Oliveira, A.C.; Santoro, A.; Raoul, J.L.; Forner, A.; et al. Sorafenib in Advanced Hepatocellular Carcinoma. N. Engl. J. Med. 2008, 359, 378–390. [Google Scholar] [CrossRef] [PubMed]
- Cheng, A.L.; Kang, Y.K.; Chen, Z.; Tsao, C.J.; Qin, S.; Kim, J.S.; Luo, R.; Feng, J.; Ye, S.; Yang, T.S. Efficacy and Safety of Sorafenib in Patients in the Asia-Pacific Region with Advanced Hepatocellular Carcinoma: A Phase III Randomised, Double-Blind, Placebo-Controlled Trial. Lancet Oncol. 2009, 10, 25–34. [Google Scholar] [CrossRef]
- Wilhelm, S.M.; Carter, C.; Tang, L.; Wilkie, D.; McNabola, A.; Rong, H.; Chen, C.; Zhang, X.; Vincent, P.; McHugh, M.; et al. BAY 43-9006 Exhibits Broad Spectrum Oral Antitumor Activity and Targets the RAF/MEK/ERK Pathway and Receptor Tyrosine Kinases Involved in Tumor Progression and Angiogenesis. Cancer Res. 2004, 64, 7099–7109. [Google Scholar] [CrossRef] [PubMed]
- Wilhelm, S.M.; Dumas, J.; Adnane, L.; Lynch, M.; Carter, C.A.; Schütz, G.; Thierauch, K.H.; Zopf, D. Regorafenib (BAY 73-4506): A New Oral Multikinase Inhibitor of Angiogenic, Stromal and Oncogenic Receptor Tyrosine Kinases with Potent Preclinical Antitumor Activity. Int. J. Cancer 2011, 129, 245–255. [Google Scholar] [CrossRef] [PubMed]
- Abou-Elkacem, L.; Arns, S.; Brix, G.; Gremse, F.; Zopf, D.; Kiessling, F.; Lederle, W. Regorafenib Inhibits Growth, Angiogenesis, and Metastasis in a Highly Aggressive, Orthotopic Colon Cancer Model. Mol. Cancer Ther. 2013, 12, 1322–1331. [Google Scholar] [CrossRef]
- Bruix, J.; Qin, S.; Merle, P.; Granito, A.; Huang, Y.H.; Bodoky, G.; Pracht, M.; Yokosuka, O.; Rosmorduc, O.; Breder, V.; et al. Regorafenib for Patients with Hepatocellular Carcinoma Who Progressed on Sorafenib Treatment (RESORCE): A Randomised, Double-Blind, Placebo-Controlled, Phase 3 Trial. Lancet 2017, 389, 56–66. [Google Scholar] [CrossRef]
- Abou-Alfa, G.K.; Meyer, T.; Cheng, A.L.; El-Khoueiry, A.B.; Rimassa, L.; Ryoo, B.Y.; Cicin, I.; Merle, P.; Chen, Y.; Park, J.W.; et al. Cabozantinib in Patients with Advanced and Progressing Hepatocellular Carcinoma. N. Engl. J. Med. 2018, 379, 54–63. [Google Scholar] [CrossRef]
- El-Khoueiry, A.B.; Sangro, B.; Yau, T.; Crocenzi, T.S.; Kudo, M.; Hsu, C.; Kim, T.Y.; Choo, S.P.; Trojan, J.; Meyer, T.; et al. Nivolumab in Patients with Advanced Hepatocellular Carcinoma (CheckMate 040): An Open-Label, Non-Comparative, Phase 1/2 Dose Escalation and Expansion Trial. Lancet 2017, 389, 2492–2502. [Google Scholar] [CrossRef]
- Zhu, A.X.; Finn, R.S.; Edeline, J.; Cattan, S.; Ogasawara, S.; Palmer, D.; Verslype, C.; Zagonel, V.; Fartoux, L.; Vogel, A.; et al. Pembrolizumab in Patients with Advanced Hepatocellular Carcinoma Previously Treated with Sorafenib (KEYNOTE-224): A Non-Randomised, Open-Label Phase 2 Trial. Lancet Oncol. 2018, 19, 940–952. [Google Scholar] [CrossRef]
- Park, J.O.; Ryoo, B.Y.; Yen, C.J.; Kudo, M.; Yang, L.; Abada, P.B.; Cheng, R.; Orlando, M.; Zhu, A.X.; Okusaka, T. Second-Line Ramucirumab Therapy for Advanced Hepatocellular Carcinoma (REACH): An East Asian and non-East Asian Subgroup Analysis. Oncotarget 2016, 7, 75482–75491. [Google Scholar] [CrossRef] [PubMed]
- Zhu, A.X.; Kang, Y.K.; Yen, C.J.; Finn, R.S.; Galle, P.R.; Llovet, J.M.; Assenat, E.; Brandi, G.; Pracht, M.; Lim, H.Y.; et al. Ramucirumab in Advanced Hepatocellular Carcinoma and Elevated Alpha-Fetoprotein following Sorafenib (REACH-2): A Randomised, Double-Blind, Placebocontrolled Phase 3 Trial. Lancet Oncol. 2019, 20, 282–296. [Google Scholar] [CrossRef]
- Kudo, M.; Finn, R.S.; Qin, S.; Han, K.H.; Ikeda, K.; Piscaglia, F.; Baron, A.; Park, J.W.; Han, G.; Jassem, J.; et al. Lenvatinib Versus Sorafenib in First-Line Treatment of Patients with Unresectable Hepatocellular Carcinoma: A Randomised Phase 3 Non-Inferiority Trial. Lancet 2018, 391, 1163–1173. [Google Scholar] [CrossRef]
- Teufel, M.; Seidel, H.; Köchert, K.; Meinhardt, G.; Finn, R.S.; Llovet, J.M.; Bruix, J. Biomarkers Associated with Response to Regorafenib in Patients with Hepatocellular Carcinoma. Gastroenterology 2019, 156, 1731–1741. [Google Scholar] [CrossRef] [PubMed]
- Lencioni, R.; Llovet, J.M. Modified RECIST (mRECIST) Assessment for Hepatocellular Carcinoma. Semin. Liver Dis. 2010, 30, 52–60. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Epelbaum, R.; Haim, N.; Ben-Shahar, M.; Ron, Y.; Cohen, Y. Dose Intensity Analysis for CHOP Chemotherapy in Diffuse Aggressive Large Cell Lymphoma. Isr. J. Med. Sci. 1988, 24, 533–538. [Google Scholar]
- Blayney, D.W.; LeBlanc, M.L.; Grogan, T.; Gaynor, E.R.; Chapman, R.A.; Spiridonidis, C.H.; Taylor, S.A.; Bearman, S.I.; Miller, T.P.; Fisher, R.; et al. Dose-Intense Chemotherapy Every 2 Weeks with Dose-Intense Cyclophosphamide, Doxorubicin, Vincristine and Prednisone may Improve Survival in Intermediate- and High-Grade Lymphoma: A Phase II Study of the Southwest Oncology Group (SWOG 9349). J. Clin. Oncol. 2003, 21, 2466–2473. [Google Scholar] [CrossRef]
- Kawashima, A.; Takayama, H.; Arai, Y.; Tanigawa, G.; Nin, M.; Kajikawa, J.; Imazu, T.; Kinoshita, T.; Yasunaga, Y.; Inoue, H.; et al. One-Month Relative dose Intensity of not Less Than 50% Predicts Favourable Progression-Free Survival in Sorafenib Therapy for Advanced Renal Cell Carcinoma in Japanese Patients. Eur. J. Cancer 2011, 47, 1521–1526. [Google Scholar] [CrossRef]
- Yoo, C.; Park, J.W.; Kim, Y.J.; Kim, D.Y.; Yu, S.J.; Lim, T.S.; Lee, S.J.; Ryoo, B.Y.; Lim, H.Y. Multicenter Retrospective Analysis of the Safety and Efficacy of Regorafenib after Progression on Sorafenib in Korean Patients with Hepatocellular Carcinoma. Investig. New Drugs 2019, 37, 567–572. [Google Scholar] [CrossRef]
- Ogasawara, S.; Ooka, Y.; Itokawa, N.; Inoue, M.; Okabe, S.; Seki, A.; Haga, Y.; Obu, M.; Atsukawa, M.; Itobayashi, E.; et al. Sequential Therapy with Sorafenib and Regorafenib for Advanced Hepatocellular Carcinoma: A Multicenter Retrospective Study in Japan. Investig. New Drugs 2019. [Google Scholar] [CrossRef]
- Kuzuya, T.; Ishigami, M.; Ito, T.; Ishizu, Y.; Honda, T.; Ishikawa, T.; Hirooka, Y.; Fujishiro, M. Clinical Characteristics and Outcomes of Candidates for Second-Line Therapy, Including Regorafenib and Ramucirumab, for Advanced Hepatocellular Carcinoma after Sorafenib Treatment. Hepatol. Res. 2019. [Google Scholar] [CrossRef] [PubMed]
- Hiraoka, A.; Kumada, T.; Kariyama, K.; Takaguchi, K.; Atsukawa, M.; Itobayashi, E.; Tsuji, K.; Tajiri, K.; Hirooka, M.; Shimada, N.; et al. Clinical Features of Lenvatinib for Unresectable Hepatocellular Carcinoma in Real-World Conditions: Multicenter Analysis. Cancer Med. 2019, 8, 137–146. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, K.; Kawakami, K.; Yokokawa, T.; Aoyama, T.; Suzuki, K.; Wakatsuki, T.; Suenaga, M.; Sato, H.; Sugiyama, E.; Yamaguchi, K.; et al. Association of Hand-Foot Skin Reaction with Regorafenib Efficacy in the Treatment of Metastatic Colorectal Cancer. Oncology 2019, 96, 200–206. [Google Scholar] [CrossRef] [PubMed]
- Riechelmann, R.P.; Leite, L.S.; Bariani, G.M.; Glasberg, J.; Rivelli, T.G.; da Fonseca, L.G.; Nebuloni, D.R.; Braghiroli, M.I.; Queiroz, M.A.; Isejima, A.M.; et al. Regorafenib in Patients with Antiangiogenic-Naïve and Chemotherapy-Refractory Advanced Colorectal Cancer: Results from a Phase 2b Trial. Oncologist 2019, 24, 1180–1187. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi, K.; Komatsu, Y.; Satoh, T.; Uetake, H.; Yoshino, T.; Nishida, T.; Yamazaki, N.; Takikawa, H.; Morimoto, T.; Chosa, M.; et al. Large-Scale, Prospective Observational Study of Regorafenib in Japanese Patients with Metastatic Colorectal Cancer in a Real-World Clinical Setting. Oncologist 2019, 24, e450–e457. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bruix, J.; Merle, P.; Granito, A.; Huang, Y.H.; Bodoky, G.; Yokosuka, O.; Rosmorduc, O.; Breder, V.V.; Gerolami, R.; Masi, G.; et al. Hand-foot Skin Reaction (HFSR) and Overall Survival (OS) in the Phase 3 RESORCE Trial of Regorafenib for Treatment of Hepatocellular Carcinoma (HCC) Progressing on Sorafenib. J. Clin. Oncol. 2018, 36 (Suppl. S4), 412. [Google Scholar] [CrossRef]
- Lee, J.H.; Chung, Y.H.; Kim, J.A.; Shim, J.H.; Lee, D.; Lee, H.C.; Shin, E.S.; Yoon, J.H.; Kim, B.I.; Bae, S.H.; et al. Genetic Predisposition of Hand-Foot Skin Reaction after Sorafenib Therapy in Patients with Hepatocellular Carcinoma. Cancer 2013, 119, 136–142. [Google Scholar] [CrossRef] [PubMed]
- Gerisch, M.; Hafner, F.T.; Lang, D.; Radtke, M.; Diefenbach, K.; Cleton, A.; Lettieri, J. Mass Balance, Metabolic Disposition, and Pharmacokinetics of a Single Oral Dose of Regorafenib in Healthy Human Subjects. Cancer Chemother. Pharmacol. 2018, 81, 195–206. [Google Scholar] [CrossRef] [PubMed]
- Johnson, P.J.; Berhane, S.; Kagebayashi, C.; Satomura, S.; Teng, M.; Reeves, H.L.; O’Beirne, J.; Fox, R.; Skowronska, A.; Palmer, D.; et al. Assessment of Liver Function in Patients with Hepatocellular Carcinoma: A New Evidence-Based Approach-the ALBI Grade. J. Clin. Oncol. 2015, 33, 550–558. [Google Scholar] [CrossRef] [PubMed]

| Factor | N = 38 |
|---|---|
| Age (years), median (range) | 75 (31–88) |
| Sex: Male/Female (%) | 32 (84)/6 (16) |
| Body weight (kg): median (range) | 57.9 (30.0–84.0) |
| Etiology HBV/HCV/Alcohol/Others (%) | 7 (18)/16 (43)/8 (21)/7 (18) |
| Child–Pugh A/B/C (%) | 33 (87)/5 (13)/0 (0) |
| Pretreatment ALBI score: median (range) | −2.33 (−1.37 to −3.76) |
| ECOG PS 0/1/2 (%) | 17 (45)/21 (55)/0 (0) |
| BCLC stage A/B/C (%) | 0 (0)/17 (45)/21 (55) |
| Major portal invasion Yes/No (%) | 3 (8)/35 (92) |
| Baseline AFP concentration (ng/mL), median (range) | 174.2 (2.6–448,620) |
| Baseline AFP < 400 ng/mL Yes/No (%) | 22 (58)/16 (42) |
| Clinical course second-line/third-line (%) | 35(92)/3 (8) |
| Sorafenib duration (months): median (range) | 4.8 (0.3–62.6) |
| Final dose of sorafenib > 400 mg Yes/No (%) | 10 (26)/28 (74) |
| 1M-RDI Cutoff Point (%) | Patient Number | OS | ||
|---|---|---|---|---|
| HR | 95% CI | p | ||
| <100 versus 100 | 35/3 | 0.87 | 0.20–3.77 | 0.85 |
| <75 versus ≥75 | 23/15 | 0.61 | 0.25–1.50 | 0.28 |
| <50 versus ≥50 | 14/24 | 0.19 | 0.08–0.48 | 0.0004 |
| <25 versus ≥25 | 3/35 | 0.06 | 0.01–0.28 | 0.0002 |
| Factor | 1M-RDI ≥ 50%, (n = 24) | 1M-RDI < 50%, (n = 14) | p-Value |
|---|---|---|---|
| Age (median) * | 70 (31–86) | 77 (58–88) | 0.05 |
| Gender (male, %) | 20 (83) | 12 (85) | 1.00 |
| BW (median, kg) * | 58.3 (46.4–69.5) | 52.7 (30.0–84.0) | 0.20 |
| Albumin (median, g/dL) * | 3.5 (2.5–5.3) | 3.1 (2.7–4.4) | 0.06 |
| T-Bil (median, mg/dL) * | 0.6 (0.2–2.2) | 0.8 (0.2–1.6) | 0.24 |
| PT (median, %) * | 97 (75–117) | 88 (73–113) | 0.10 |
| ALBI score (median) * | −2.4 (−3.76 to −1.41) | −2.0 (−3.03 to −1.37) | 0.01 |
| Pretreatment AFP *, (median, ng/mL) | 155 (2.6–118,126) | 783 (3.3–448,620) | 0.54 |
| Pretreatment PIVKA-II *, (median, mAU/mL) | 306 (2.2–144,669) | 5516 (11.1–668,014) | 0.008 |
| Extrahepatic metastasis, (yes, %) | 2 (8.3) | 1 (7.1) | 1.00 |
| Sorafenib duration *, (median, months) | 5.7 (0.2–62.6) | 4.1 (0.6–33.3) | 0.33 |
| Factor | Any n (%) | Grade ≥ 3 n (%) |
|---|---|---|
| HFSR | 25 (65.8) | 2 (5.3) |
| Hypertension | 18 (47.4) | 3 (7.9) |
| Diarrhea | 21 (55.3) | 4 (10.5) |
| Decreased appetite | 24 (63.2) | 3 (7.9) |
| Fatigue | 28 (73.7) | 1 (2.6) |
| Decreased body weight | 16 (42.1) | 0 (0) |
| Increased AST | 20 (52.6) | 2 (5.3) |
| Increased ALT | 19 (50.0) | 2 (5.3) |
| Increased T-Bil | 11 (28.9) | 0 (0) |
| Univariate | Multivariate | |||
|---|---|---|---|---|
| Factor | p-Value | HR | 95% CI | p-Value |
| Age (years) | 0.10 | |||
| Body weight (kg) | 0.25 | |||
| Performance Status > 0 | 0.005 | 2.9 | 0.79–10.9 | 0.11 |
| Pretreatment ALBI score | 0.09 | |||
| Pretreatment ALT (IU/mL) | 0.49 | |||
| Pretreatment AST (IU/mL) | 0.25 | |||
| Pretreatment Alb (g/dL) | 0.26 | |||
| Pretreatment AFP (ng/mL) | 0.05 | |||
| Pretreatment PIVKA-II (mAU/mL) | 0.08 | |||
| Extrahepatic metastasis | 0.60 | |||
| Major portal invasion | 0.25 | |||
| Sorafenib duration (months) | 0.08 | |||
| HFSR during regorafenib | <0.0001 | 0.03 | 0.008–0.16 | <0.0001 |
| 1M-RDI ≥ 50% of regorafenib | 0.0004 | 0.18 | 0.06–0.55 | 0.003 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, W.; Tsuchiya, K.; Kurosaki, M.; Yasui, Y.; Inada, K.; Kirino, S.; Yamashita, K.; Sekiguchi, S.; Hayakawa, Y.; Osawa, L.; et al. Sorafenib-Regorafenib Sequential Therapy in Japanese Patients with Unresectable Hepatocellular Carcinoma—Relative Dose Intensity and Post-Regorafenib Therapies in Real World Practice. Cancers 2019, 11, 1517. https://doi.org/10.3390/cancers11101517
Wang W, Tsuchiya K, Kurosaki M, Yasui Y, Inada K, Kirino S, Yamashita K, Sekiguchi S, Hayakawa Y, Osawa L, et al. Sorafenib-Regorafenib Sequential Therapy in Japanese Patients with Unresectable Hepatocellular Carcinoma—Relative Dose Intensity and Post-Regorafenib Therapies in Real World Practice. Cancers. 2019; 11(10):1517. https://doi.org/10.3390/cancers11101517
Chicago/Turabian StyleWang, Wan, Kaoru Tsuchiya, Masayuki Kurosaki, Yutaka Yasui, Kento Inada, Sakura Kirino, Koji Yamashita, Shuhei Sekiguchi, Yuka Hayakawa, Leona Osawa, and et al. 2019. "Sorafenib-Regorafenib Sequential Therapy in Japanese Patients with Unresectable Hepatocellular Carcinoma—Relative Dose Intensity and Post-Regorafenib Therapies in Real World Practice" Cancers 11, no. 10: 1517. https://doi.org/10.3390/cancers11101517





