Zinc and Iron Agronomic Biofortification of Brassicaceae Microgreens
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Site, Treatments, and Growing System
2.2. Planting Date, Seeding Density, Treatment Differentiation, and Harvest
2.3. Mineral Analysis
2.4. Statistical Analysis
3. Results and Discussion
3.1. Microgreens Biometric Response to Zinc (Zn) Enrichment
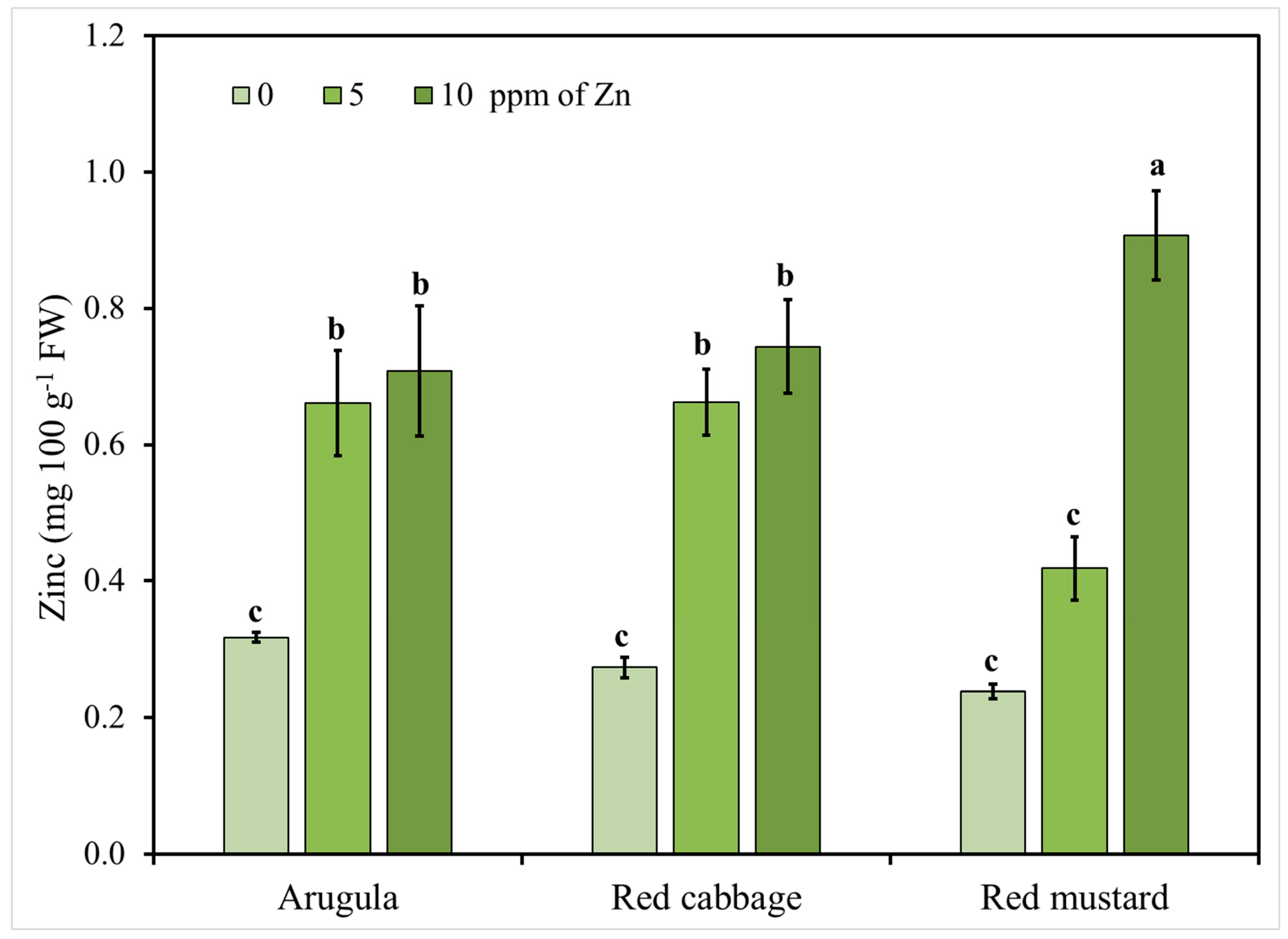
3.2. Microgreens Nutrient Accumulation Response to Zinc (Zn) Enrichment
3.3. Microgreens Biometric Response to Iron (Fe) Enrichment
3.4. Microgreens Nutrient Accumulation Response to Iron (Fe) Enrichment
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- McLean, E.; Cogswell, M.; Egli, I.; Wojdyla, D.; De Benoist, B. Worldwide prevalence of anaemia, WHO Vitamin and Mineral Nutrition Information System, 1993-2005. Public Health Nutr. 2009, 12, 444–454. [Google Scholar] [CrossRef] [PubMed]
- Wessells, K.R.; Brown, K.H. Estimating the Global Prevalence of Zinc Deficiency: Results Based on Zinc Availability in National Food Supplies and the Prevalence of Stunting. PLoS ONE 2012, 7, e50568. [Google Scholar] [CrossRef] [PubMed]
- Miller, B.D.D.; Welch, R.M. Food system strategies for preventing micronutrient malnutrition. Food Policy 2013, 42, 115–128. [Google Scholar] [CrossRef] [Green Version]
- Clemens, S. Zn and Fe biofortification: The right chemical environment for human bioavailability. Plant Sci. 2014, 225, 52–57. [Google Scholar] [CrossRef] [PubMed]
- Fraga, C.G. Relevance, essentiality and toxicity of trace elements in human health. Mol. Aspects Med. 2005, 26, 235–244. [Google Scholar] [CrossRef] [PubMed]
- Bharadva, K.; Mishra, S.; Tiwari, S.; Yadav, B.; Deshmukh, U.; Elizabeth, K.E.; Banapurmath, C.R. Prevention of Micronutrient Deficiencies in Young Children: Consensus Statement from Infant and Young Child Feeding Chapter of Indian Academy of Pediatrics. Indian Pediatr. 2019, 56, 577–586. [Google Scholar] [CrossRef]
- Black, R.E.; Allen, L.H.; Bhutta, Z.A.; Caulfield, L.E.; de Onis, M.; Ezzati, M.; Mathers, C.; Rivera, J. Maternal and child undernutrition: Global and regional exposures and health consequences. Lancet 2008, 371, 243–260. [Google Scholar] [CrossRef]
- Oteiza, P.I.; Mackenzie, G.G. Zinc, oxidant-triggered cell signaling, and human health. Mol. Aspects Med. 2005, 26, 245–255. [Google Scholar] [CrossRef]
- Johnson, F.; Giulivi, C. Superoxide dismutases and their impact upon human health. Mol. Aspects Med. 2005, 26, 340–352. [Google Scholar] [CrossRef]
- Hotz, C.; Brown, K.H. Contents International Zinc Nutrition Consultative Group (IZiNCG) Technical Document. Food Nutr. Bull. 2004, 25, S94–S200. [Google Scholar]
- Plum, L.M.; Rink, L.; Hajo, H. The essential toxin: Impact of zinc on human health. Int. J. Environ. Res. Public Health 2010, 7, 1342–1365. [Google Scholar] [CrossRef] [PubMed]
- Cakmak, I. Enrichment of cereal grains with zinc: Agronomic or genetic biofortification? Plant Soil 2008, 302, 1–17. [Google Scholar] [CrossRef]
- St. Clair, S.B.S.; Lynch, J.P. The opening of Pandora’s Box: Climate change impacts on soil fertility and crop nutrition in developing countries. Plant Soil 2010, 335, 101–115. [Google Scholar] [CrossRef]
- White, P.J.; Broadley, M.R. Biofortifying crops with essential mineral elements. Trends Plant Sci. 2005, 10, 586–593. [Google Scholar] [CrossRef] [PubMed]
- García-Casal, M.N. Planning and Implementing Food Fortification Programs to Combat Micronutrient Malnutrition: Iron. Food Nutr. Sci. 2014, 5, 880–888. [Google Scholar] [CrossRef] [Green Version]
- Murgia, I.; Arosio, P.; Tarantino, D.; Soave, C. Biofortification for combating “hidden hunger” for iron. Trends Plant Sci. 2012, 17, 47–55. [Google Scholar] [CrossRef]
- Palmgren, M.G.; Clemens, S.; Williams, L.E.; Krämer, U.; Borg, S.; Schjørring, J.K.; Sanders, D. Zinc biofortification of cereals: Problems and solutions. Trends Plant Sci. 2008, 13, 464–473. [Google Scholar] [CrossRef]
- White, P.J.; Broadley, M.R. Physiological limits to zinc biofortification of edible crops. Front. Plant Sci. 2011, 2, 1–11. [Google Scholar] [CrossRef]
- Dias, D.M.; de Castro Moreira, M.E.; Gomes, M.J.C.; Toledo, R.C.L.; Nutti, M.R.; Sant’Ana, H.M.P.; Martino, H.S.D. Rice and bean targets for biofortification combined with high carotenoid content crops regulate transcriptional mechanisms increasing iron bioavailability. Nutrients 2015, 7, 9683–9696. [Google Scholar] [CrossRef]
- Mukamuhirwa, F.; Tusiime, G.; Mukankusi, M.C. Inheritance of high iron and zinc concentration in selected bean varieties. Euphytica 2015, 205, 349–360. [Google Scholar] [CrossRef]
- Kumar, S.; Palve, A.; Joshi, C.; Srivastava, R.K.; Rukhsar. Crop biofortification for iron (Fe), zinc (Zn) and vitamin A with transgenic approaches. Heliyon 2019, 5, e01914. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Corguinha, A.P.B.; Carvalho, C.A.; de Souza, G.A.; de Carvalho, T.S.; Vieira, E.A.; Fialho, J.F.; Guilherme, L.R.G. Potential of cassava clones enriched with β-carotene and lycopene for zinc biofortification under different soil Zn conditions. J. Sci. Food Agric. 2019, 99, 666–674. [Google Scholar] [CrossRef] [PubMed]
- Stein, A.J.; Meenakshi, J.V.; Qaim, M.; Nestel, P.; Sachdev, H.P.S.; Bhutta, Z.A. Potential impacts of iron biofortification in India. Soc. Sci. Med. 2008, 66, 1797–1808. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zimmermann, M.B.; Hurrell, R.F. Improving iron, zinc and vitamin A nutrition through plant biotechnology. Curr. Opin. Biotechnol. 2002, 13, 142–145. [Google Scholar] [CrossRef]
- Bouis, H.E.; Saltzman, A. Improving nutrition through biofortification: A review of evidence from HarvestPlus, 2003 through 2016. Glob. Food Sec. 2017, 12, 49–58. [Google Scholar] [CrossRef]
- Sida-Arreola, J.P.; Sánchez-Chávez, E.; Ávila-Quezada, G.D.; Zamudio-Flores, P.B.; Acosta Muñíz, C.H. Iron biofortification and its impact on antioxidant system, yield and biomass in common bean. Plant, Soil Environ. 2015, 61, 573–576. [Google Scholar]
- Slamet-loedin, I.H.; Johnson-beebout, S.E.; Impa, S.; Tsakirpaloglou, N. Enriching rice with Zn and Fe while minimizing Cd risk. Front. Plant Sci. 2015, 6, 1–9. [Google Scholar] [CrossRef]
- Márquez-Quiroz, C.; De-La-cruz-Lázaro, E.; Osorio-Osorio, R.; Sánchez-Chávez, E. Biofortification of cowpea beans with iron: Iron’s influence on mineral content and yield. J. Soil Sci. Plant Nutr. 2015, 15, 839–847. [Google Scholar] [CrossRef]
- Wei, Y.; Shohag, M.J.I.; Wang, Y.; Lu, L.; Wu, C.; Yang, X. Effect of zinc sulfate fortification in germinated brown rice on seed zinc concentration, bioavailability, and seed germination. J. Agric. Food Chem. 2012, 60, 1871–1879. [Google Scholar] [CrossRef]
- Nilsson, J.; Olsson, K.; Engqvist, G.; Ekvall, J.; Olsson, M.; Nyman, M.; Åkesson, B. Variation in the content of glucosinolates, hydroxycinnamic acids, carotenoids, total antioxidant capacity and low-molecular-weight carbohydrates inBrassica vegetables. J. Sci. Food Agric. 2006, 86, 528–538. [Google Scholar] [CrossRef]
- Niyigaba, E.; Twizerimana, A.; Mugenzi, I.; Ngnadong, W.A.; Ye, Y.P.; Wu, B.M.; Hai, J.B. Winter wheat grain quality, zinc and iron concentration affected by a combined foliar spray of zinc and iron fertilizers. Agronomy 2019, 9, 250. [Google Scholar] [CrossRef]
- Ning, P.; Wang, S.; Fei, P.; Zhang, X.; Dong, J.; Shi, J.; Tian, X. Enhancing Zinc Accumulation and Bioavailability in Wheat Grains by Integrated Zinc and Pesticide Application. Agronomy 2019, 9, 530. [Google Scholar] [CrossRef]
- Dordas, C. Application of calcium and magnesium improves yield and essential oil yield of oregano (Origanum vulgare ssp. hirtum). Ital. J. Agron. 2008, 3, 417–418. [Google Scholar]
- Tzortzakis, N.G. Potassium and calcium enrichment alleviate salinity-induced stress in hydroponically grown endives. Hortic. Sci. 2010, 37, 155–162. [Google Scholar] [CrossRef] [Green Version]
- Chrysargyris, A.; Michailidi, E.; Tzortzakis, N. Physiological and biochemical responses of Lavandula angustifolia to salinity under mineral foliar application. Front. Plant Sci. 2018, 9, 1–23. [Google Scholar] [CrossRef]
- Sandberg, A.-S. Bioavailability of minerals in legumes. Br. J. Nutr. 2002, 88, 281–285. [Google Scholar] [CrossRef] [Green Version]
- Schlemmer, U.; Frølich, W.; Prieto, R.M.; Grases, F. Phytate in foods and significance for humans: Food sources, intake, processing, bioavailability, protective role and analysis. Mol. Nutr. Food Res. 2009, 53, 330–375. [Google Scholar] [CrossRef]
- Zou, T.; Xu, N.; Hu, G.; Pang, J.; Xu, H. Biofortification of soybean sprouts with zinc and bioaccessibility of zinc in the sprouts. J. Sci. Food Agric. 2014, 94, 3053–3060. [Google Scholar] [CrossRef]
- Lingyun, Y.; Jian, W.; Chenggang, W.; Shan, L.; Shidong, Z. Effect of Zinc Enrichment on Growth and Nutritional Quality in Pea Sprouts. J. Food Nutr. Res. 2016, 4, 100–107. [Google Scholar]
- Park, S.A.; Grusak, M.A.; Oh, M.M. Concentrations of minerals and phenolic compounds in three edible sprout species treated with iron-chelates during imbibition. Hortic. Environ. Biotechnol. 2014, 55, 471–478. [Google Scholar] [CrossRef]
- Przybysz, A.; Wrochna, M.; Małecka-Przybysz, M.; Gawrońska, H.; Gawroński, S.W. Vegetable sprouts enriched with iron: Effects on yield, ROS generation and antioxidative system. Sci. Hortic. 2016, 203, 110–117. [Google Scholar] [CrossRef]
- Afify, A.E.M.M.R.; El-Beltagi, H.S.; El-Salam, S.M.A.; Omran, A.A. Bioavailability of iron, zinc, phytate and phytase activity during soaking and germination of white sorghum varieties. PLoS ONE 2011, 6, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Liang, J.; Han, B.Z.; Nout, M.J.R.; Hamer, R.J. Effect of soaking and phytase treatment on phytic acid, calcium, iron and zinc in rice fractions. Food Chem. 2009, 115, 789–794. [Google Scholar] [CrossRef]
- Frei, M.; Tetteh, R.N.; Razafindrazaka, A.L.; Fuh, M.A.; Wu, L.B.; Becker, M. Responses of rice to chronic and acute iron toxicity: Genotypic differences and biofortification aspects. Plant Soil 2016, 408, 149–161. [Google Scholar] [CrossRef]
- Tomasi, N.; Pinton, R.; Dalla Costa, L.; Cortella, G.; Terzano, R.; Mimmo, T.; Scampicchio, M.; Cesco, S. New “solutions” for floating cultivation system of ready-to-eat salad: A review. Trends Food Sci. Technol. 2014, 46, 267–276. [Google Scholar] [CrossRef]
- Di Gioia, F.; Avato, P.; Serio, F.; Argentieri, M.P. Glucosinolate profile of Eruca sativa, Diplotaxis tenuifolia and Diplotaxis erucoides grown in soil and soilless systems. J. Food Compos. Anal. 2018, 69, 197–204. [Google Scholar] [CrossRef]
- Petropoulos, S.A.; Fernandes, Â.; Calhelha, R.C.; Di Gioia, F.; Kolovou, P.; Barros, L.; Ferreira, I.C.F.R. Chemical composition and bioactive properties of Cichorium spinosum L. in relation to nitrate/ammonium nitrogen ratio. J. Sci. Food Agric. 2019. [Google Scholar] [CrossRef]
- Rouphael, Y.; Kyriacou, M.C. Enhancing quality of fresh vegetables through salinity eustress and biofortification applications facilitated by soilless cultivation. Front. Plant Sci. 2018, 9, 1–6. [Google Scholar] [CrossRef]
- Raviv, M.; Lieth, J.H. Significance of Soilless Culture in Agriculture. In Soilless Culture Theory and Practice; Raviv, M., Lieth, J.H., Eds.; Elsevier: Amsterdam, The Nederlands, 2007; pp. 1–11. [Google Scholar]
- Orsini, F.; Kahane, R.; Nono-Womdim, R.; Gianquinto, G. Urban agriculture in the developing world: A review. Agron. Sustain. Dev. 2013, 33, 695–720. [Google Scholar] [CrossRef]
- Bonasia, A.; Lazzizera, C.; Elia, A.; Conversa, G. Nutritional, biophysical and physiological characteristics of wild rocket genotypes as affected by soilless cultivation system, salinity level of nutrient solution and growing period. Front. Plant Sci. 2017, 8, 15. [Google Scholar] [CrossRef]
- Xiao, Z.; Lester, G.E.; Luo, Y.; Wang, Q. Assessment of vitamin and carotenoid concentrations of emerging food products: Edible microgreens. J. Agric. Food Chem. 2012, 60, 7644–7651. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Xiao, Z.; Lin, L.-Z.; Lester, G.E.; Wang, Q.; Harnly, J.M.; Chen, P. Profiling polyphenols in five Brassica species microgreens by UHPLC-PDA-ESI/HRMS(n). J. Agric. Food Chem. 2013, 61, 10960–10970. [Google Scholar] [CrossRef] [PubMed]
- Di Gioia, F.; Santamaria, P. Microgreens - Novel Fresh and Functional Food to Explore All the Value of Biodiversity; Eco-logica Srl: Bari, Italy, 2015; ISBN 9788890928932. [Google Scholar]
- Ebert, A.W.; Wu, T.H.; Yang, R.Y. Amaranth sprouts and microgreens - A homestead vegetable production option to enhance food and nutrition security in the rural-urban continuum. In Proceedings of the Regional Symposium on Sustaining Small-Scale Vegetable Production and Marketing Systems for Food and Nutrition Security (SEAVEG2014), Bangkok, Thailand, 25–27 February 2014; pp. 233–244. [Google Scholar]
- Xiao, Z.; Codling, E.E.; Luo, Y.; Nou, X.; Lester, G.E.; Wang, Q. Microgreens of Brassicaceae: Mineral composition and content of 30 varieties. J. Food Compos. Anal. 2016, 49, 87–93. [Google Scholar] [CrossRef] [Green Version]
- Kyriacou, M.C.; Rouphael, Y.; Di Gioia, F.; Kyratzis, A.; Serio, F.; Renna, M.; De Pascale, S.; Santamaria, P. Micro-scale vegetable production and the rise of microgreens. Trends Food Sci. Technol. 2016, 57, 103–115. [Google Scholar] [CrossRef]
- Di Gioia, F.; Renna, M.; Santamaria, P. Sprouts, Microgreens and “Baby Leaf” Vegetables. In Minimally Processed Refrigerated Fruits and Vegetables. Food Engineering Series; Yildiz, F., Wiley, R., Eds.; Springer: Boston, MA, USA, 2017; pp. 403–432. [Google Scholar]
- Di Gioia, F.; De Bellis, P.; Mininni, C.; Santamaria, P.; Serio, F. Physicochemical, agronomical and microbiological evaluation of alternative growing media for the production of rapini (Brassica rapa L.) microgreens. J. Sci. Food Agric. 2017, 97, 1212–1219. [Google Scholar] [CrossRef] [PubMed]
- Kyriacou, M.C.; El-nakhel, C.; Graziani, G.; Pannico, A.; Soteriou, G.A.; Giordano, M.; Ritieni, A.; Pascale, S. De Functional quality in novel food sources: Genotypic variation in the nutritive and phytochemical composition of thirteen microgreens species. Food Chem. 2019, 277, 107–118. [Google Scholar] [CrossRef]
- Renna, M.; Di Gioia, F.; Leoni, B.; Mininni, C.; Santamaria, P. Culinary Assessment of Self-Produced Microgreens as Basic Ingredients in Sweet and Savory Dishes. J. Culin. Sci. Technol. 2017, 15, 126–142. [Google Scholar] [CrossRef]
- Petropoulos, S.; Di Gioia, F.; Ntatsi, G. Vegetable Organosulfur Compounds and their Health Promoting Effects. Curr. Pharm. Des. 2017, 23, 2850–2875. [Google Scholar] [CrossRef]
- Anderson, D.L.; Henderson, L.J. Comparing Sealed Chamber Digestion with Other Digestion Methods Used for Plant Tissue Analysis. Agron. J. 1988, 80, 549. [Google Scholar] [CrossRef]
- Ozores-Hampton, M.; Di Gioia, F.; Sato, S.; Simonne, E.; Morgan, K. Effects of nitrogen rates on nitrogen, phosphorous, and potassium partitioning, accumulation, and use efficiency in seepage-irrigated fresh market tomatoes. HortScience 2015, 50, 1636–1643. [Google Scholar] [CrossRef]
- Hanlon, E.A.; Gonzalez, J.S.; Bartos, J.M. Mehlich 1 Extractable P, Ca, Mg, Mn, Cu and Zn; University of Florida: Gainesville, FL, USA, 1997. [Google Scholar]
- Plank, C.O. Plant analysis reference procedures for the southern region of the United States. South Coop. Ser. Bull. 1992, 368, 68. [Google Scholar]
- Munter, R.C.; Halverson, T.L.; Anderson, R.D. Quality assurance for plant tissue analysis by ICP-AES. Commun. Soil Sci. Plant Anal. 1984, 15, 1285–1322. [Google Scholar] [CrossRef]
- Ramezani, M.; Seghatoleslami, M.; Mousavi, G.; Sayyari-Zahan, M.H. Effect of salinity and foliar application of iron and zinc on yield and water use efficiency of Ajowan (Carum copticum). Int. J. Agric. Crop Sci. 2012, 4, 421–426. [Google Scholar]
- Moghimipour, Z.; Sourestani, M.M.; Ansari, N.A.; Ramezani, Z. The effect of foliar application of zinc on essential oil content and composition of holy basil [Ocimum sanctum] at first and second harvests. J. Essent. Oil-Bear. Plants 2017, 20, 449–458. [Google Scholar] [CrossRef]
- Hassanpouraghdam, M.; Gohari, G.; Tabatabaei, S.; Dadpour, M.; Shirdel, M. NaCl salinity and Zn foliar application influence essential oil composition of basil (Ocimum basilicum L.). Acta Agric. Slov. 2011, 97, 4–9. [Google Scholar] [CrossRef]
- Ghorbanpour, M.; Asgari Lajayer, H.; Hadian, J. Influence of copper and zinc on growth, metal accumulation and chemical composition of essential oils in sweet basil (Ocimum basilicum L.). J. Med. Plants 2016, 15, 132–144. [Google Scholar]
- Ahl, H.A.H.S.; Omer, E.A. Effect of spraying with zinc and / or iron on growth and chemical composition of coriander (Coriandrum sativum L.) harvested at three stages of development. J. Med. Food Plants 2009, 1, 30–46. [Google Scholar]
- Hanif, M.A.; Nawaz, H.; Ayub, M.A.; Tabassum, N.; Kanwal, N.; Rashid, N.; Saleem, M.; Ahmad, M. Evaluation of the effects of Zinc on the chemical composition and biological activity of basil essential oil by using Raman spectroscopy. Ind. Crops Prod. 2017, 96, 91–101. [Google Scholar] [CrossRef]
- Rietra, R.P.J.J.; Heinen, M.; Dimkpa, C.O.; Bindraban, P.S. Effects of nutrient antagonism and synergism on yield and fertilizer use efficiency. Commun. Soil Sci. Plant Anal. 2017, 48, 1895–1920. [Google Scholar] [CrossRef]
- Gupta, N.; Ram, H.; Kumar, B. Mechanism of Zinc absorption in plants: Uptake, transport, translocation and accumulation. Rev. Environ. Sci. Biotechnol. 2016, 15, 89–109. [Google Scholar] [CrossRef]
- Ricachenevsky, F.K.; de Araújo Junior, A.T.; Fett, J.P.; Sperotto, R.A. You shall not pass: Root vacuoles as a symplastic checkpoint for metal translocation to shoots and possible application to grain nutritional quality. Front. Plant Sci. 2018, 9, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Institute of Medicine Dietary Reference Intakes for Vitamin A, Vitamin K, Arsenic, Boron, Chromium, Copper, Iodine, Iron, Manganese, Molybdenum, Nickel, Silicon, Vanadium, and Zinc; The National Academies Press: Washington, DC, USA, 2001; ISBN 0309072794.
- De Dorlodot, S.; Lutts, S.; Bertin, P. Effects of ferrous iron toxicity on the growth and mineral composition of an interspecific rice. J. Plant Nutr. 2005, 28, 1–20. [Google Scholar] [CrossRef]
- Giordano, M.; El-Nakhel, C.; Pannico, A.; Kyriacou, M.C.; Stazi, S.R.; De Pascale, S.; Rouphael, Y. Iron biofortification of red and green pigmented lettuce in closed soilless cultivation impacts crop performance and modulates mineral and bioactive composition. Agronomy 2019, 9, 290. [Google Scholar] [CrossRef]
- Cecílio Filho, A.B.; Mendoza Cortez, J.W.; de Sordi, D.; Urrestarazu, M. Common Chicory Performance as Influenced by Iron Concentration in the Nutrient Solution. J. Plant Nutr. 2015, 38, 1489–1494. [Google Scholar] [CrossRef]
- Assimakopoulou, A. Effect of iron supply and nitrogen form on growth, nutritional status and ferric reducing activity of spinach in nutrient solution culture. Sci. Hortic. 2006, 110, 21–29. [Google Scholar] [CrossRef]
- Hernández-Castro, E.; Trejo-Téllez, L.I.; Gómez-Merino, F.C.; Rodríguez-Mendoza, M.N.; Sánchez-García, P.; Robledo-Paz, A. Bioaccumulation of iron, selenium, nitrate, and proteins in chard shoots. J. Soil Sci. Plant Nutr. 2015, 15, 694–710. [Google Scholar] [CrossRef]
- Di Gioia, F.; Gonnella, M.; Santamaria, P. Contribution of Leafy Vegetables to Dietary Nitrate Intake and Regulations. In Nitrate in Leafy Vegetables: Toxicity and Safety Measures; Umar, S., Naser, A., Nafees, A., Eds.; I.K. International Publishing House: New Delhi, India, 2013; pp. 1–16. [Google Scholar]
- Greer, F.R.; Shannon, M. Infant methemoglobinemia: The role of dietary nitrate in food and water. Pediatrics 2005, 116, 784–786. [Google Scholar] [CrossRef]
- Di Gioia, F.; Gonnella, M.; Buono, V.; Ayala, O.; Santamaria, P. Agronomic, physiological and quality response of romaine and red oak-leaf lettuce to nitrogen input. Ital. J. Agron. 2017, 12, 47–58. [Google Scholar] [CrossRef]
- Di Gioia, F.; Gonnella, M.; Buono, V.; Ayala, O.; Cacchiarelli, J.; Santamaria, P. Calcium cyanamide effects on nitrogen use efficiency, yield, nitrates, and dry matter content of lettuce. Agron. J. 2017, 109, 354–362. [Google Scholar] [CrossRef]
- Colonna, E.; Rouphael, Y.; Barbieri, G.; De Pascale, S. Nutritional quality of ten leafy vegetables harvested at two light intensities. Food Chem. 2016, 199, 702–710. [Google Scholar] [CrossRef]



| Treatments | Shoot Population Density | Fresh | Shoot Fresh Weight | Dry |
|---|---|---|---|---|
| Yield | Matter | |||
| (shoot m−2) | (g m−2) | (mg shoot−1) | (g 100 g−1 FW) | |
| Zn (mg L−1) | ||||
| 0 | 22,060 | 1718 | 87.81 | 6.36 |
| 5 | 23,384 | 1716 | 83.29 | 6.42 |
| 10 | 21,363 | 1794 | 85.83 | 6.25 |
| 20 | 23,089 | 1679 | 80.13 | 6.56 |
| Specie (S) | ||||
| Arugula | 25,697 a | 1558 b | 55.32 c | 6.37 b |
| Red cabbage | 19,125 c | 1786 a | 105.08 a | 6.95 a |
| Red mustard | 22,599 b | 1837 a | 92.40 b | 5.87 c |
| p value | ||||
| Zn | 0.31 | 0.57 | 0.80 | 0.45 |
| S | 0.0001 | 0.02 | 0.0001 | 0.0003 |
| Zn × S | 0.71 | 0.19 | 0.13 | 0.45 |
| Treatments | Ca | K | Mg | P | Cu | Fe | Zn |
|---|---|---|---|---|---|---|---|
| Zn (mg L−1) | (mg 100 g−1 FW) | ||||||
| 0 | 124.78 b | 361.22 | 43.78 b | 64.89 | 0.05 b | 0.52 a | 0.28 b |
| 5 | 123.11 b | 332.33 | 43.67 b | 64.22 | 0.05 b | 0.44 ab | 0.58 b |
| 10 | 135.00 a | 348.44 | 46.44 ab | 64.56 | 0.05 b | 0.44 ab | 0.79 b |
| 20 | 142.22 a | 355.56 | 49.22 a | 67.44 | 0.07 a | 0.35 b | 6.94 a |
| Species (S) | |||||||
| Arugula | 113.75 b | 374.08 a | 36.75 b | 65.17 b | 0.07 a | 0.42 b | 1.91 |
| Red cabbage | 171.00 a | 301.50 b | 62.25 a | 69.33 a | 0.04 b | 0.53 a | 2.44 |
| Red mustard | 109.08 b | 372.58 a | 38.33 b | 61.33 c | 0.06 a | 0.35 c | 2.08 |
| p value | |||||||
| Zn | 0.01 | 0.72 | 0.01 | 0.38 | 0.02 | 0.01 | 0.0001 |
| S | 0.0001 | 0.003 | 0.0001 | 0.00 | 0.00 | 0.0001 | 0.06 |
| Zn × S | 0.25 | 0.37 | 0.12 | 0.03 | 0.52 | 0.23 | 0.02 |
| Species (S) | Fe (mg L−1) | Shoot Population Density | Fresh Yield | Shoot Fresh Weight | Dry Matter |
|---|---|---|---|---|---|
| (shoot m−2) | (g m−2) | (mg shoot−1) | (g 100 g−1 FW) | ||
| Arugula | 0 | 29,582 a | 2125 a | 86.0 de | 5.61 e |
| 10 | 28,775 a | 1728 b | 66.6 ef | 6.37 e | |
| 20 | 24,968 b | 2039 ab | 80.9 de | 5.59 e | |
| 40 | 13,520 f | 503 d | 43.1 g | 9.56 c | |
| Red cabbage | 0 | 17,778 def | 2142 a | 140.0 a | 6.69 e |
| 10 | 16,861 ef | 1842 ab | 120.7 b | 7.33 de | |
| 20 | 16,961 ef | 1451 c | 95.8 cd | 8.53 cd | |
| 40 | 5740 g | 400 d | 91.9 cd | 19.32 a | |
| Red mustard | 0 | 22,738 bc | 1810 ab | 94.4 cd | 6.56 e |
| 10 | 22,033 bcd | 2171 a | 112.0 bc | 5.76 e | |
| 20 | 20,113 cde | 1875 ab | 101.3 bcd | 6.13 e | |
| 40 | 4484 g | 234 d | 50.3 fg | 14.10 b | |
| p value | |||||
| Fe | 0.0001 | 0.0001 | 0.0001 | 0.0001 | |
| S | 0.0001 | 0.0001 | 0.0001 | 0.0001 | |
| Fe × S | 0.10 | 0.001 | 0.004 | 0.0001 |
| Species (S) | Fe (mg L−1) | Ca | K | Mg | P | Cu | Fe | Zn |
|---|---|---|---|---|---|---|---|---|
| (mg 100 g−1 FW) | ||||||||
| Arugula | 0 | 119.41 d | 398.16 bc | 38.16 f | 56.80 d | 0.06 c | 0.49 d | 0.28 e |
| 10 | 124.73 d | 344.53 cde | 43.26 ef | 52.37 d | 0.07 c | 0.83 d | 0.41 e | |
| 20 | 119.40 d | 452.23 b | 41.26 ef | 58.21 d | 0.06 c | 0.81 d | 0.56 de | |
| 40 | 150.21 d | 427.07 bc | 56.99 e | 120.81 c | 0.13 bc | 11.07 c | 0.92 c | |
| Red cabbage | 0 | 184.02 c | 297.77 de | 69.52 d | 54.83 d | 0.10 c | 0.77 d | 0.32 e |
| 10 | 197.29 c | 307.91 de | 79.91 cd | 60.41 d | 0.09 c | 1.74 d | 0.55 de | |
| 20 | 207.57 c | 260.71 e | 86.14 bc | 64.07 d | 0.14 bc | 2.90 d | 1.21 b | |
| 40 | 326.83 a | 422.61 bc | 134.51 a | 202.09 a | 0.42 a | 44.85 a | 2.53 a | |
| Red mustard | 0 | 128.81 d | 384.48 bcd | 49.39 ef | 48.10 d | 0.06 c | 0.49 d | 0.25 e |
| 10 | 130.87 d | 475.06 b | 47.55 ef | 52.85 d | 0.07 c | 1.20 d | 0.41 e | |
| 20 | 134.58d | 465.28 b | 51.05 ef | 52.71 d | 0.07 c | 1.64 d | 0.85 cd | |
| 40 | 239.70 b | 566.39 a | 93.68 b | 161.28 b | 0.18 b | 32.33 b | 1.38 b | |
| p value | ||||||||
| Fe | 0.0001 | 0.01 | 0.0001 | 0.0001 | 0.0001 | 0.0001 | 0.0001 | |
| S | 0.0001 | 0.0001 | 0.0001 | 0.0001 | 0.0001 | 0.0001 | 0.0001 | |
| Fe × S | 0.0001 | 0.01 | 0.0004 | 0.0001 | 0.0001 | 0.0001 | 0.0001 | |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Di Gioia, F.; Petropoulos, S.A.; Ozores-Hampton, M.; Morgan, K.; Rosskopf, E.N. Zinc and Iron Agronomic Biofortification of Brassicaceae Microgreens. Agronomy 2019, 9, 677. https://doi.org/10.3390/agronomy9110677
Di Gioia F, Petropoulos SA, Ozores-Hampton M, Morgan K, Rosskopf EN. Zinc and Iron Agronomic Biofortification of Brassicaceae Microgreens. Agronomy. 2019; 9(11):677. https://doi.org/10.3390/agronomy9110677
Chicago/Turabian StyleDi Gioia, Francesco, Spyridon A. Petropoulos, Monica Ozores-Hampton, Kelly Morgan, and Erin N. Rosskopf. 2019. "Zinc and Iron Agronomic Biofortification of Brassicaceae Microgreens" Agronomy 9, no. 11: 677. https://doi.org/10.3390/agronomy9110677





