Successive Solvent Extraction of Polyphenols and Flavonoids from Cistus creticus L. Leaves
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Plant Material and Handling
2.3. Preparation of Extracts
2.4. Determination of the Total Polyphenol Yield (YTP)
2.5. Determination of the Total Flavonoid Yield (YTFn)
2.6. Determination of the IC50 Value (Half Maximal Inhibitory Concentration) for DPPH Free Radical Scavenging
2.7. Determination of the Ferric Reducing Antioxidant Power (PR)
2.8. High-Performance Liquid Chromatography (HPLC-DAD)
2.9. Statistical Analysis
3. Results and Discussion
3.1. Extraction Yields and Antioxidant Activity of the Extracts
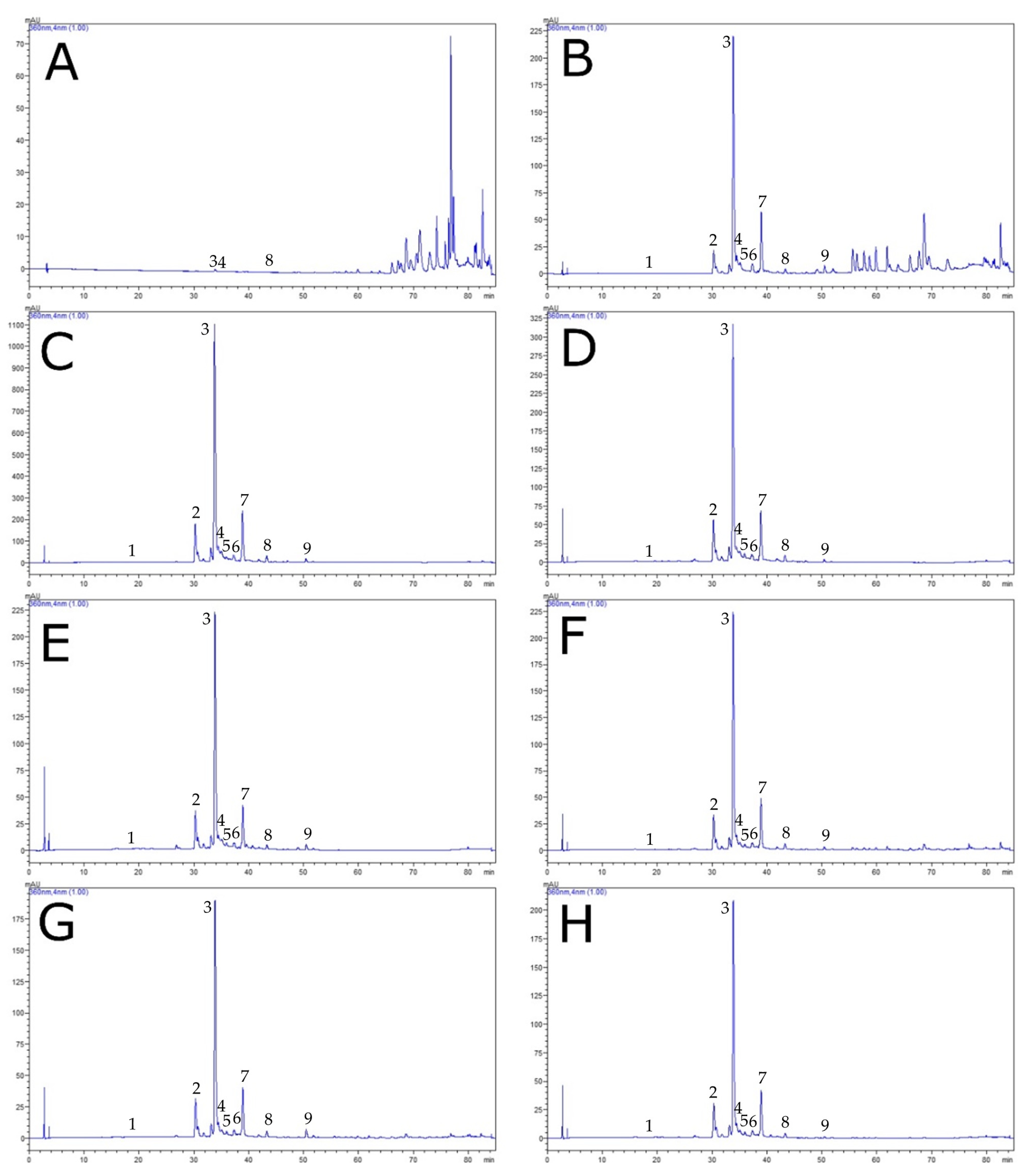
3.2. Polyphenolic Composition by HPLC
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Pizzino, G.; Irrera, N.; Cucinotta, M.; Pallio, G.; Mannino, F.; Arcoraci, V.; Squadrito, F.; Altavilla, D.; Bitto, A. Oxidative Stress: Harms and Benefits for Human Health. Oxid. Med. Cell. Longev. 2017, 2017, 8416763. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lobo, V.; Patil, A.; Phatak, A.; Chandra, N. Free radicals, antioxidants and functional foods: Impact on human health. Pharmacogn. Rev. 2010, 4, 118–126. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Redza-Dutordoir, M.; Averill-Bates, D.A. Activation of apoptosis signalling pathways by reactive oxygen species. Biochim. Biophys. Acta—Mol. Cell Res. 2016, 1863, 2977–2992. [Google Scholar] [CrossRef] [PubMed]
- Juan, C.A.; de la Lastra, J.M.P.; Plou, F.J.; Pérez-Lebeña, E. The chemistry of reactive oxygen species (Ros) revisited: Outlining their role in biological macromolecules (dna, lipids and proteins) and induced pathologies. Int. J. Mol. Sci. 2021, 22, 4642. [Google Scholar] [CrossRef]
- Poljsak, B.; Šuput, D.; Milisav, I. Achieving the balance between ROS and antioxidants: When to use the synthetic antioxidants. Oxid. Med. Cell. Longev. 2013, 2013, 956792. [Google Scholar] [CrossRef] [Green Version]
- Xu, D.P.; Li, Y.; Meng, X.; Zhou, T.; Zhou, Y.; Zheng, J.; Zhang, J.J.; Li, H. Bin Natural antioxidants in foods and medicinal plants: Extraction, assessment and resources. Int. J. Mol. Sci. 2017, 18, 96. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rathod, N.B.; Elabed, N.; Punia, S.; Ozogul, F.; Kim, S.-K.; Rocha, J.M. Recent Developments in Polyphenol Applications on Human Health: A Review with Current Knowledge. Plants 2023, 12, 1217. [Google Scholar] [CrossRef]
- Alara, O.R.; Abdurahman, N.H.; Ukaegbu, C.I. Extraction of phenolic compounds: A review. Curr. Res. Food Sci. 2021, 4, 200–214. [Google Scholar] [CrossRef] [PubMed]
- Brglez Mojzer, E.; Knez Hrnčič, M.; Škerget, M.; Knez, Ž.; Bren, U. Polyphenols: Extraction Methods, Antioxidative Action, Bioavailability and Anticarcinogenic Effects. Molecules 2016, 21, 901. [Google Scholar] [CrossRef]
- Athanasiadis, V.; Chatzimitakos, T.; Kotsou, K.; Palaiogiannis, D.; Bozinou, E.; Lalas, S.I. Optimization of the Extraction Parameters for the Isolation of Bioactive Compounds from Orange Peel Waste. Sustainability 2022, 14, 13926. [Google Scholar] [CrossRef]
- Chatzimitakos, T.; Athanasiadis, V.; Kotsou, K.; Palaiogiannis, D.; Bozinou, E.; Lalas, S.I. Optimized Isolation Procedure for the Extraction of Bioactive Compounds from Spent Coffee Grounds. Appl. Sci. 2023, 13, 2819. [Google Scholar] [CrossRef]
- Allcca-Alca, E.E.; León-Calvo, N.C.; Luque-Vilca, O.M.; Martínez-Cifuentes, M.; Pérez-Correa, J.R.; Mariotti-Celis, M.S.; Huamán-Castilla, N.L. Hot pressurized liquid extraction of polyphenols from the skin and seeds of Vitis vinifera L. Cv. negra criolla pomace a peruvian native pisco industry waste. Agronomy 2021, 11, 866. [Google Scholar] [CrossRef]
- Sosa Ferrera, Z.; Padrón Sanz, C.; Mahugo Santana, C.; Santana Rodríguez, J.J. The use of micellar systems in the extraction and pre-concentration of organic pollutants in environmental samples. TrAC—Trends Anal. Chem. 2004, 23, 469–479. [Google Scholar] [CrossRef]
- Ajila, C.M.; Brar, S.K.; Verma, M.; Tyagi, R.D.; Godbout, S.; Valéro, J.R. Extraction and Analysis of Polyphenols: Recent trends. Crit. Rev. Biotechnol. 2011, 31, 227–249. [Google Scholar] [CrossRef] [PubMed]
- Palaiogiannis, D.; Athanasiadis, V.; Bozinou, E.; Chatzimitakos, T.; Makris, D.P.; Lalas, S.I. Extraction of Polyphenolic and Volatile Compounds from Cistus creticus Using Deep Eutectic Solvents and Pulsed Electric Fields. Compounds 2022, 2, 311–320. [Google Scholar] [CrossRef]
- Ettoumi, F.E.; Zhang, R.; Belwal, T.; Javed, M.; Xu, Y.; Li, L.; Weide, L.; Luo, Z. Generation and characterization of nanobubbles in ionic liquid for a green extraction of polyphenols from Carya cathayensis Sarg. Food Chem. 2022, 369, 130932. [Google Scholar] [CrossRef]
- Athanasiadis, V.; Pappas, V.M.; Palaiogiannis, D.; Chatzimitakos, T.; Bozinou, E.; Makris, D.P.; Lalas, S.I. Pulsed Electric Field-Based Extraction of Total Polyphenols from Sideritis raiseri Using Hydroethanolic Mixtures. Oxygen 2022, 2, 91–98. [Google Scholar] [CrossRef]
- Supriatno, S.; Lelifajri, L. Effect of sequential extraction on total phenolic content (TPC) and antioxidant activities (AA) of Luffa acutangula Linnaeus dried pulps. AIP Conf. Proc. 2018, 2002, 020062. [Google Scholar] [CrossRef]
- Nawaz, H.; Aslam, M.; Tul Muntaha, S.; Haq Nawaz, C. Effect of Solvent Polarity and Extraction Method on Phytochemical Composition and Antioxidant Potential of Corn Silk. Free. Radic. Antioxid. 2019, 9, 5–11. [Google Scholar] [CrossRef] [Green Version]
- Zalegh, I.; Akssira, M.; Bourhia, M.; Mellouki, F.; Rhallabi, N.; Salamatullah, A.M.; Alkaltham, M.S.; Khalil Alyahya, H.; Mhand, R.A. A Review on Cistus sp.: Phytochemical and Antimicrobial Activities. Plants 2021, 10, 1214. [Google Scholar] [CrossRef]
- Tomou, E.M.; Lytra, K.; Rallis, S.; Tzakos, A.G.; Skaltsa, H. An updated review of genus Cistus L. since 2014: Traditional uses, phytochemistry, and pharmacological properties. Phytochem. Rev. 2022, 21, 2049–2087. [Google Scholar] [CrossRef]
- Álvarez-Martínez, F.J.; Borrás-Rocher, F.; Micol, V.; Barrajón-Catalán, E. Artificial Intelligence Applied to Improve Scientific Reviews: The Antibacterial Activity of Cistus Plants as Proof of Concept. Antibiotics 2023, 12, 327. [Google Scholar] [CrossRef] [PubMed]
- Viapiana, A.; Konopacka, A.; Waleron, K.; Wesolowski, M. Cistus incanus L. commercial products as a good source of polyphenols in human diet. Ind. Crops Prod. 2017, 107, 297–304. [Google Scholar] [CrossRef]
- Lakka, A.; Grigorakis, S.; Karageorgou, I.; Batra, G.; Kaltsa, O.; Bozinou, E.; Lalas, S.; Makris, D.P. Saffron processing wastes as a bioresource of high-value added compounds: Development of a green extraction process for polyphenol recovery using a natural deep eutectic solvent. Antioxidants 2019, 8, 586. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kaczorová, D.; Karalija, E.; Dahija, S.; Bešta-Gajević, R.; Parić, A.; Ćavar Zeljković, S. Influence of extraction solvent on the phenolic profile and bioactivity of two achillea species. Molecules 2021, 26, 1601. [Google Scholar] [CrossRef]
- Galanakis, C.M.; Goulas, V.; Tsakona, S.; Manganaris, G.A.; Gekas, V. A knowledge base for the recovery of natural phenols with different solvents. Int. J. Food Prop. 2013, 16, 382–396. [Google Scholar] [CrossRef] [Green Version]
- Gori, A.; Boucherle, B.; Rey, A.; Rome, M.; Fuzzati, N.; Peuchmaur, M. Development of an innovative maceration technique to optimize extraction and phase partition of natural products. Fitoterapia 2021, 148, 104798. [Google Scholar] [CrossRef]
- Ait Lahcen, S.; El Hattabi, L.; Benkaddour, R.; Chahboun, N.; Ghanmi, M.; Satrani, B.; Tabyaoui, M.; Zarrouk, A. Chemical composition, antioxidant, antimicrobial and antifungal activity of Moroccan Cistus creticus leaves. Chem. Data Collect. 2020, 26, 100346. [Google Scholar] [CrossRef]
- Gaweł-Bęben, K.; Kukula-Koch, W.; Hoian, U.; Czop, M.; Strzępek-Gomółka, M.; Antosiewicz, B. Characterization of Cistus × incanus L. and Cistus ladanifer L. extracts as potential multifunctional antioxidant ingredients for skin protecting cosmetics. Antioxidants 2020, 9, 202. [Google Scholar] [CrossRef] [Green Version]
- Dimcheva, V.; Karsheva, M. Cistus incanus from strandja mountain as a source of bioactive antioxidants. Plants 2018, 7, 8. [Google Scholar] [CrossRef] [Green Version]
- Carev, I.; Maravić, A.; Ilić, N.; Čulić, V.Č.; Politeo, O.; Zorić, Z.; Radan, M. UPLC-MS/MS phytochemical analysis of two Croatian Cistus species and their biological activity. Life 2020, 10, 112. [Google Scholar] [CrossRef]
- Gori, A.; Nascimento, L.B.; Ferrini, F.; Centritto, M.; Brunetti, C. Seasonal and diurnal variation in leaf phenolics of three medicinal mediterranean wild species: What is the best harvesting moment to obtain the richest and the most antioxidant extracts? Molecules 2020, 25, 956. [Google Scholar] [CrossRef] [Green Version]
- Bautista, I.; Boscaiu, M.; Lidón, A.; Llinares, J.V.; Lull, C.; Donat, M.P.; Mayoral, O.; Vicente, O. Environmentally induced changes in antioxidant phenolic compounds levels in wild plants. Acta Physiol. Plant. 2016, 38, 9. [Google Scholar] [CrossRef]
- Kotsou, K.; Chatzimitakos, T.; Athanasiadis, V.; Bozinou, E.; Adamaki-Sotiraki, C.; Rumbos, C.I.; Athanassiou, C.G.; Lalas, S.I. Waste Orange Peels as a Feed Additive for the Enhancement of the Nutritional Value of Tenebrio molitor. Foods 2023, 12, 783. [Google Scholar] [CrossRef] [PubMed]
- Panche, A.N.; Diwan, A.D.; Chandra, S.R. Flavonoids: An overview. J. Nutr. Sci. 2016, 5, e47. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grigonis, D.; Venskutonis, P.R.; Sivik, B.; Sandahl, M.; Eskilsson, C.S. Comparison of different extraction techniques for isolation of antioxidants from sweet grass (Hierochloë odorata). J. Supercrit. Fluids 2005, 33, 223–233. [Google Scholar] [CrossRef]
- Lukas, B.; Bragagna, L.; Starzyk, K.; Labedz, K.; Stolze, K.; Novak, J. Polyphenol diversity and antioxidant activity of european Cistus creticus L. (Cistaceae) compared to six further, partly sympatric Cistus species. Plants 2021, 10, 615. [Google Scholar] [CrossRef]
| Solvent | YTP (mg GAE/g) 1 | YTFn (mg QE/g) 2 | AAR IC50 (μg/mL) | PR (μmol AAE/g) 3 | % Extraction Yield |
|---|---|---|---|---|---|
| Hexane | 14.88 ± 0.88 e,* | 2.53 ± 0.07 d | 14,913.15 ± 879.88 a | 63.75 ± 3.06 e | 4.35 |
| Ethyl acetate | 24.26 ± 0.85 d | 9.5 ± 0.43 b | 2505.97 ± 102.74 b | 177.65 ± 6.04 d | 2.87 |
| Acetone | 64.37 ± 2.45 c | 28.03 ± 1.74 a | 486.04 ± 23.33 c | 792.65 ± 19.82 c | 4.40 |
| Ethanol | 95.33 ± 5.91 a | 7.58 ± 0.54 c | 350.99 ± 23.87 d | 1103.11 ± 38.61 a | 12.63 |
| Water | 79.46 ± 2.22 b | 3.72 ± 0.24 d | 417.69 ± 27.57 c | 929.5 ± 18.59 b | 11.98 |
| Solvent | YTP (mg GAE/g) 1 | YTFn (mg QE/g) 2 | AAR IC50 (μg/mL) | PR (μmol AAE/g) 3 | % Extraction Yield |
|---|---|---|---|---|---|
| Ethanol | 89.01 ± 6.23 a,b,* | 20.03 ± 1.5 a | 358.14 ± 25.43 b | 1116.18 ± 74.78 a | 25.89 |
| 50% v/v Ethanol: water | 96.51 ± 5.79 a | 10.24 ± 0.71 b | 341.18 ± 15.01 b | 1237.15 ± 30.93 a | 45.17 |
| Water | 84.80 ± 5.43 b | 9.92 ± 0.25 b | 394.18 ± 8.67 a | 1094.96 ± 78.84 b | 38.37 |
| Identified Polyphenol (mg/g) | Solvent | ||||
|---|---|---|---|---|---|
| Hexane | Ethyl Acetate | Acetone | Ethanol | Water | |
| Luteolin glucoside derivative | nd ** | 0.059 ± 0.002 c,* | 0.075 ± 0.003 b | 0.08 ± 0.005 b | 0.112 ± 0.006 a |
| Luteolin 7-(2″-p-coumaroylglucoside) | nd | 0.202 ± 0.008 b | 0.393 ± 0.027 a | 0.132 ± 0.004 d | 0.173 ± 0.012 c |
| 1_Myricetin glucoside | 0.038 ± 0.002 e | 0.347 ± 0.019 d | 2.635 ± 0.184 a | 0.841 ± 0.057 b | 0.543 ± 0.04 c |
| Myricetin rhamnoside | 0.046 ± 0.003 d | 3.806 ± 0.217 c | 19.626 ± 0.942 a | 5.511 ± 0.165 b | 3.715 ± 0.256 c |
| 1_Quercetin glucoside derivative | nd | 0.123 ± 0.004 b | 0.428 ± 0.016 a | 0.065 ± 0.002 c | 0.046 ± 0.003 d |
| Rutin | nd | 0.097 ± 0.006 b | 0.349 ± 0.012 a | 0.1 ± 0.006 b | 0.067 ± 0.004 c |
| 2_Quercetin glucoside derivative | nd | 0.232 ± 0.008 b | 0.724 ± 0.049 a | 0.204 ± 0.008 b | 0.136 ± 0.009 c |
| Quercetin rhamnoside derivative | 0.006 ± 0 e | 1.438 ± 0.098 c | 6.227 ± 0.137 a | 1.771 ± 0.113 b | 1.05 ± 0.007 d |
| 2_Myricetin glucoside | nd | 0.108 ± 0.004 c | 0.518 ± 0.038 a | 0.18 ± 0.005 b | 0.104 ± 0 c |
| Total extraction yield | 0.09 ± 0.005 d | 6.411 ± 0.365 c | 30.975 ± 1.408 a | 8.886 ± 0.365 b | 5.946 ± 0.338 c |
| Identified Polyphenol (mg/g) | Solvent | ||
|---|---|---|---|
| Ethanol | 50% v/v Ethanol: Water | Water | |
| Luteolin glucoside derivative | 0.075 ± 0.002 b,* | 0.089 ± 0.004 a | 0.084 ± 0.002 a |
| Luteolin 7-(2″-p-coumaroylglucoside) | 0.108 ± 0.006 c | 0.353 ± 0.013 a | 0.187 ± 0.011 b |
| 1_Myricetin glucoside | 1.236 ± 0.065 a | 1.168 ± 0.084 a | 1.16 ± 0.079 a |
| Myricetin rhamnoside | 7.52 ± 0.376 a | 7.032 ± 0.527 a,b | 6.39 ± 0.147 b |
| 1_Quercetin glucoside derivative | 0.081 ± 0.006 b | 0.1 ± 0.006 a | 0.068 ± 0.005 c |
| Rutin | 0.123 ± 0.007 a | 0.126 ± 0.009 a | 0.105 ± 0.006 b |
| 2_Quercetin glucoside derivative | 0.267 ± 0.015 a,b | 0.29 ± 0.006 a | 0.264 ± 0.015 b |
| Quercetin rhamnoside derivative | 2.165 ± 0.076 b | 2.473 ± 0.121 a | 2.041 ± 0.114 b |
| 2_Myricetin glucoside | 0.219 ± 0.006 a | 0.198 ± 0.005 b | 0.157 ± 0.009 c |
| Total extraction yield | 11.793 ± 0.561 a | 11.829 ± 0.776 a | 10.457 ± 0.388 b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Palaiogiannis, D.; Chatzimitakos, T.; Athanasiadis, V.; Bozinou, E.; Makris, D.P.; Lalas, S.I. Successive Solvent Extraction of Polyphenols and Flavonoids from Cistus creticus L. Leaves. Oxygen 2023, 3, 274-286. https://doi.org/10.3390/oxygen3030018
Palaiogiannis D, Chatzimitakos T, Athanasiadis V, Bozinou E, Makris DP, Lalas SI. Successive Solvent Extraction of Polyphenols and Flavonoids from Cistus creticus L. Leaves. Oxygen. 2023; 3(3):274-286. https://doi.org/10.3390/oxygen3030018
Chicago/Turabian StylePalaiogiannis, Dimitrios, Theodoros Chatzimitakos, Vassilis Athanasiadis, Eleni Bozinou, Dimitris P. Makris, and Stavros I. Lalas. 2023. "Successive Solvent Extraction of Polyphenols and Flavonoids from Cistus creticus L. Leaves" Oxygen 3, no. 3: 274-286. https://doi.org/10.3390/oxygen3030018









