Moving beyond Risk Quotients: Advancing Ecological Risk Assessment to Reflect Better, More Robust and Relevant Methods
Abstract
:1. Introduction
2. Background and Current Practices
2.1. Estimating Exposure Concentrations
2.2. Estimating Effect Concentrations
2.3. Levels of Concern
- 1.
- ASA: Do not base your conclusions solely on whether an association or effect was found to be “statistically significant” (i.e., the p-value passed some arbitrary threshold such as p < 0.05).
- 2.
- ASA: Do not believe that an association or effect exists just because it was statistically significant.
- 3.
- ASA: Do not believe that an association or effect is absent just because it was not statistically significant.
- 4.
- ASA: Do not believe that your p-value gives the probability that chance alone produced the observed association or effect or the probability that your test hypothesis is true.
- 5.
- ASA: Do not conclude anything about scientific or practical importance based on statistical significance (or lack thereof).
3. The Need for Better, More Robust and Relevant Methods
3.1. Mismatch between What ERA Measures and What It Endeavors to Protect
3.2. A Brief History of Population Modeling in the Context of ERA
3.3. Current State of the Science to Support ERA
4. Take Home Messages
- The environment is not deterministic, so we cannot rely solely on deterministic methods to understand its future;
- Higher-tier risk assessments should utilize the entire domain of data to transparently explain decisions based on variability and uncertainty within all available information;
- Incorporating species life histories as well as spatial and temporal considerations is critical to understanding risk;
- Holding on to “bright lines” (e.g., RQ-LOCs) for their ease of use is not an acceptable justification for putting the environment at risk by using outdated methods;
- Stakeholders will need to come to a consensus on how to interpret different ways of expressing risk that does not require risk managers to have to re-evaluate acceptability for every new ERA;
- The general USEPA ERA guidance [4] does not accommodate advances in the state of the science, therefore an update of this guidance is warranted.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Newman, M.C. Fundamentals of Ecotoxicology: The Science of Pollution, 5th ed.; CRC Press: Boca Raton, FL, USA, 2020; 708p. [Google Scholar]
- Chapman, P.M.; Fairbrother, A.; Brown, D. A critical evaluation of safety (uncertainty) factors for ecological risk assessment. Environ. Toxicol. Chem. 2009, 17, 99–108. [Google Scholar] [CrossRef]
- Suter, G.W. Ecological Risk Assessment, 2nd ed.; Taylor and Francis Group: Boca Raton, FL, USA, 2020; 674p. [Google Scholar]
- USEPA. EPA Guidelines for Ecological Risk Assessment; EPA/630/R-95/002F; US Environmental Protection Agency: Washington, DC, USA, 1998; 188p.
- USEPA. Overview of the Ecological Risk Assessment Process in the Office of Pesticide Programs, US Environmental Protection Agency; Office of Prevention, Pesticides and Toxic Substances, Office of Pesticide Programs: Washington, DC, USA, 2004; 99p.
- USEPA. Guidance for Assessing Pesticide risks to Bees; US Environmental Protection Agency. Office of Prevention, Pesticides and Toxic Substances, Office of Pesticide Programs: Washington, DC, USA, 2014; 59p. Available online: https://www.epa.gov/sites/default/files/2014-06/documents/pollinator_risk_assessment_guidance_06_19_14.pdf (accessed on 1 December 2021).
- USEPA. Revised Method for National Level Listed Species Biological Evaluations of Conventional Pesticides; US Environmental Protection Agency. Office of Prevention, Pesticides and Toxic Substances, Office of Pesticide Programs: Washington, DC, USA, 2020; 59p. Available online: https://www3.epa.gov/pesticides/nas/revised/revised-method-march2020.pdf (accessed on 1 December 2021).
- Grimm, V.; Railsback, S.F.; Vincenot, C.E.; Berger, U.; Gallagher, C.; Deangelis, D.L.; Edmonds, B.; Ge, J.; Giske, J.; Groeneveld, J.; et al. The ODD protocol for describing agent-based and other simulation models: A second update to improve clarity, replication, and structural realism. J. Artif. Soc. Soc. Simul. 2020, 23, 7. [Google Scholar] [CrossRef] [Green Version]
- Topping, C.J.; Høye, T.T.; Olesen, C.R. Opening the black box—Development, testing, and documentation of a mechanistically rich agent-based model. Ecol. Model. 2010, 221, 245–255. [Google Scholar] [CrossRef]
- Grimm, V.; Augusiak, J.; Focks, A.; Frank, B.M.; Gabsi, F.; Johnston, A.S.A.; Liu, C.; Martin, B.T.; Meli, M.; Radchuk, V.; et al. Towards better modelling and decision support: Documenting model development, testing, and analysis using trace. Ecol. Model 2014, 280, 129–139. [Google Scholar] [CrossRef]
- Raimondo, S.; Etterson, M.; Pollesch, N.; Garber, K.; Kanarek, A.; Lehmann, W.; Awkerman, J.A. A framework for linking population model development with ecological risk assessment objectives. Integr. Environ. Assess. Manag. 2018, 14, 369–380. [Google Scholar] [CrossRef]
- Schmolke, A.; Kapo, K.E.; Rueda-Cediel, P.; Thorbek, P.; Brain, R.; Forbes, V. Developing population models: A systematic approach for pesticide risk assessment using herbaceous plants as an example. Sci. Total Environ. 2017, 599–600, 1929–1938. [Google Scholar] [CrossRef]
- Accolla, C.; Vaugeois, M.; Grimm, V.; Moore, A.P.; Rueda-Cediel, P.; Schmolke, A.; Forbes, V.E. A Review of Key Features and Their Implementation in Unstructured, Structured, and Agent-Based Population Models for Ecological Risk Assessment. Integr. Environ. Assess. Manag. 2021, 17, 521–540. [Google Scholar] [CrossRef]
- Raimondo, S.; Schmolke, A.J.; Pollesch, N.; Accolla, C.; Galic, N.; Moore, A.; Vaugeois, M.; Rueda-Cediel, P.; Kanarek, A.; Awkerman, J.A.; et al. Pop-GUIDE: Population modeling Guidance, Use, Interpretation, and Development for Ecological Risk Assessment. Integr. Environ. Assess. Manag. 2021, 17, 767–784. [Google Scholar] [CrossRef]
- Forbes, V.E.; Calow, P.; Sibly, R.M. The extrapolation problem and how population modeling can help. Environ. Toxicol. Chem. 2008, 27, 1987–1994. [Google Scholar] [CrossRef] [Green Version]
- Young, D.F. U.S. Environmental Protection Agency Model for Estimating Pesticides in Surface Water. In Pesticides in Surface Water: Monitoring, Modeling, Risk Assessment, and Management; Goh, K.S., Gan, J., Young, D.F., Luo, Y., Eds.; American Chemical Society: Washington, DC, USA, 2019; Chapter 16; pp. 309–331. [Google Scholar]
- Hrovat, M.; Segner, H.; Jeram, S. Variability of in vivo fish acute toxicity data. Regul. Toxicol. Pharmacol. 2009, 54, 294–300. [Google Scholar] [CrossRef]
- Gergs, A.; Kulkarni, D.; Preuss, T.G. Body size-dependent toxicokinetics and toxicodynamics could explain intra- and interspecies variability in sensitivity. Environ. Poll. 2015, 206, 449–455. [Google Scholar] [CrossRef] [PubMed]
- Fox, D.R.; Landis, W.G. Don’t be fooled—A no observed-effect concentration is no substitute for a poor concentration-response experiment. Environ. Toxicol. Chem. 2016, 35, 2141–2148. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Green, J.W. Issues with using only regression models for ecotoxicity studies. Integr. Environ. Assess. Manag. 2016, 12, 198–199. [Google Scholar] [CrossRef] [PubMed]
- Kooijman, S. An alternative for NOEC exists, but the standard model has to be abandoned first. Oikos 1996, 75, 310–316. [Google Scholar] [CrossRef]
- Laskowski, R. Some good reasons to ban the use of NOEC, LOEC, and related concepts in ecotoxicology. Oikos 1995, 73, 140–144. [Google Scholar] [CrossRef]
- Jager, T. Bad habits die hard: The NOEC’s persistence reflects poorly on ecotoxicology. Environ. Toxicol. Chem. 2012, 31, 228–229. [Google Scholar] [CrossRef]
- Landis, W.G.; Chapman, P.M. Well past time to stop using NOELs and LOELs. Integr. Environ. Assess. Manag. 2011, 7, vi–viii. [Google Scholar] [CrossRef]
- Warne, M.S.J.; van Dam, R. NOEC and LOEC data should no longer be generated or used. Australas. J. Ecotoxicol. 2008, 14, 1. [Google Scholar]
- Calow, P.; Sibly, R.M.; Forbes, V. Risk assessment on the basis of simplified life-history scenarios. Environ. Toxicol. Chem. 1997, 16, 1983–1989. [Google Scholar] [CrossRef]
- Barnthouse, L.W. The role of models in ecological risk assessment: A 1990’s perspective. Environ. Toxicol. Chem. 1992, 11, 1751–1760. [Google Scholar] [CrossRef]
- NRC. Assessing Risks to Endangered and Threatened Species from Pesticides; National Academies Press: Washington, DC, USA, 2013; 142p. [Google Scholar]
- Pastorok, R.A.; Bartell, S.M.; Ferson, S.; Ginzburg, L.R. Ecological Modeling in Risk Assessment: Chemical Effects on Populations, Ecosystems, and Landscapes; CRC Press: Boca Raton, FL, USA, 2001; 324p. [Google Scholar]
- Stark, J.D.; Banken, J.A.O. Importance of population structure at the time of toxicant exposure. Ecotoxicol. Environ. Saf. 1999, 42, 282–287. [Google Scholar] [CrossRef] [PubMed]
- Stark, J.D.; Banks, J.E.; Vargas, R. How risky is risk assessment: The role that life history strategies playi n susceptibility of species to stress. Proc. Natl. Acad. Sci. USA 2004, 101, 732–736. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thorbek, P.; Forbes, V.; Heimbach, F.; Hommen, U.; Thulke, H.-H.; Van den Brink, P.J.; Wogram, J.; Grimm, V. Ecological Models for Regulatory Risk Assessments of Pesticides: Developing a Strategy for the Future; Society of Environmental Toxicology and Chemistry (SETAC) and CRC Press: Boca Raton, FL, USA, 2010; 160p. [Google Scholar]
- Raimondo, S.; McKenney, C.L., Jr.; Barron, M.G. Application of perturbation simulations in population risk assessment for different life history strategies and elasticity patterns. Hum. Ecol. Risk Assess. 2006, 12, 983–999. [Google Scholar] [CrossRef]
- Barnthouse, L.W.; Suter, G.W., II; Rosen, A.E. Risks of toxic contaminants to exploited fish populations: Influence of life history, data uncertainty and exploitation intensity. Environ. Toxicol. 1990, 9, 297–311. [Google Scholar] [CrossRef]
- Mebane, C. Biological arguments for selecting effect sizes in ecotoxicological testing—A governmental perspective. Environ. Toxicol. Chem. 2016, 34, 2440–2442. [Google Scholar] [CrossRef]
- Wasserstein, R.L.; Schirm, A.L.; Lazar, N.A. Moving to a World Beyond “p < 0.05”. Am. Stat. 2019, 73, 1–19. [Google Scholar]
- Wasserstein, R.; Lazar, N. The ASA’s Statement on p-Values: Context, Process, and Purpose. Am. Stat. 2016, 70, 129–133. [Google Scholar] [CrossRef] [Green Version]
- USEPA. Risk Characterization Handbook; EPA/100-B-00-002; US Environmental Protection Agency: Washington, DC, USA, 2000; 189p.
- Ankley, G.T.; Bennett, R.S.; Erickson, R.J.; Hoff, D.J.; Hornung, M.W.; Johnson, R.D.; Mount, D.R.; Nichols, J.W.; Russom, C.L.; Schmieder, P.K.; et al. Adverse outcome pathways: A conceptual framework to support ecotoxicology research and risk assessment. Environ. Toxicol. Chem. 2010, 29, 730–741. [Google Scholar] [CrossRef]
- Conolly, R.B.; Ankley, G.T.; Cheng, W.; Mayo, M.L.; Miller, D.H.; Perkins, E.J.; Villeneuve, D.L.; Watanabe, K.H. Quantitative adverse outcome pathways and their application to predictivity toxicology. Environ. Sci. Technol. 2017, 51, 4661–4672. [Google Scholar] [CrossRef]
- Forbes, V.E.; Calow, P. Promises and problems for the new paradigm for risk assessment and an alternative approach involving predictive systems models. Environ. Toxicol. Chem. 2012, 31, 2663–2671. [Google Scholar] [CrossRef]
- Malthus, T.R. An. Essay on the Principle of Population; J. Johnson: London, UK, 1798. [Google Scholar]
- Petersen, C.G.J. The yearly immigration of young plaice to the Limfjord from the German Sea. Report of the Danish Biological Station. Ichthyol. Res. 1896, 6, 1–48. [Google Scholar]
- Angelini, R.; Moloney, C.L. Fisheries, ecology, and modelling: An historical perspective. Pan-Am. J. Aquat. Sci. 2007, 2, 75–85. [Google Scholar]
- Caswell, H. Matrix Population Models: Construction, Analysis, and Interpretation, 2nd ed.; Sinauer Associates: Sunderland, MA, USA, 2001; 752p. [Google Scholar]
- Hanksi, I. Metapopulation Ecology; Oxford Series in Ecology and Evolution; Oxford University Press: New York, NY, USA, 1999; 328p. [Google Scholar]
- Railsback, S.F.; Grimm, V. Agent-Based and Individual-Based Modeling: A Practical Introduction; Princeton University Press: Princeton, NJ, USA, 2012; 360p. [Google Scholar]
- O’Neill, R.V.; Gardner, R.H.; Barnthouse, L.W.; Suter, G.W.; Hildebrand, S.G.; Gehrs, C.W. Ecosystem risk analysis: A new methodology. Environ. Toxicol. Chem. 1982, 1, 167–177. [Google Scholar] [CrossRef]
- Suter, G.W.; Barnthouse, L.W.; Breck, J.E.; Gardner, R.H.; O’Neill, R.V. Extrapolating from the laboratory to the field: How uncertain are you? In Aquatic Toxicology and Hazard Assessment: Seventh Symposium; ASTM STP 854; Cardwell, R.D., Purdy, R., Bahner, R.C., Eds.; ASTM International: Philadelphia, PA, USA, 1985; pp. 400–413. [Google Scholar]
- Barnthouse, L.W.; Munns, W.R., Jr.; Sorensen, M.T. Population-Level Ecological Risk Assessment; Taylor and Francis Group: New York, NY, USA, 2007; 376p. [Google Scholar]
- Forbes, V.E.; Galic, N.; Schmolke, A.; Vavra, J.; Pastorok, R.; Thorbek, P. Assessing the risks of pesticides to threatened and endangered species using population modeling: A critical review and recommendations for future work. Environ. Toxicol. Chem. 2016, 35, 1904–1913. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hommen, U.; Forbes, V.E.; Grimm, V.; Preuss, T.; Thorbek, P.; Ducrot, V. How to use mechanistic effect models in risk assessment of pesticides: Case studies and recommendations from the SETAC workshop MODELINK. Integr. Environ. Assess. Manag. 2015, 12, 21–31. [Google Scholar] [CrossRef] [Green Version]
- USEPA. Ecological Modeling in Risk Assessment: Chemical Effects on Populations, Ecosystems, and Landscapes; EPA/100/R-09/006; USEPA: Washington, DC, USA, 2009; 71p.
- Grimm, V.; Ashauer, R.; Forbes, V.; Hommen, U.; Preuss, T.G.; Schmidt, A.; van den Brink, P.J.; Wogram, J.; Thorbek, P. CREAM: A European project on mechanistic effect models for ecological risk assessment of chemicals. Environ. Sci. Pollut. Res. 2009, 16, 614–617. [Google Scholar] [CrossRef] [Green Version]
- USEPA. Biological Evaluation Chapters for Chlorpyrifos ESA Assessment. Available online: https://www.epa.gov/endangered-species/biological-evaluation-chapters-chlorpyrifos-esa-assessment#chapter%201%202016a (accessed on 1 December 2021).
- USEPA. Biological Evaluation Chapters for Diazinon ESA Assessment. Available online: https://www.epa.gov/endangered-species/biological-evaluation-chapters-chlorpyrifos-esa-assessment#chapter%201%202016b (accessed on 1 December 2021).
- USEPA. Biological Evaluation Chapters for Malathion ESA Assessment. Available online: https://www.epa.gov/endangered-species/biological-evaluation-chapters-chlorpyrifos-esa-assessment#chapter%201%202016c (accessed on 1 December 2021).
- EFSA. Scientific Opinion on good modelling practice in the context of mechanistic effect models for risk assessment of plant protection products. European Food Safety Authority. EFSA J. 2014, 12, 3589. [Google Scholar]
- EFSA. Statement on the suitability of the BEEHAVE model for its potential use in a regulatory context and for the risk assessment of multiple stressors in honeybees at the landscape level. European Food Safety Authority. EFSA J. 2015, 13, 4125. [Google Scholar]
- Etterson, M.E.; Garber, K.; Odenkirchen, E. Mechanistic modeling of insecticide risks to breeding birds in North American agroecosystems. PLoS ONE 2017, 12, e0176998. [Google Scholar] [CrossRef] [Green Version]
- Pollesch, N.L.; Flynn, K.M.; Kadlec, S.M.; Swintek, J.A.; Raimondo, S.; Etterson, M.A. Developing integral projection models for ecotoxicology. Ecol. Model. 2022, 464, 109813. [Google Scholar] [CrossRef]
- Awkerman, J.A.; Raimondo, S. Simulated developmental and reproductive impacts on amphibian populations and implications for assessing long-term effects. Ecotoxicol. Environ. Saf. 2018, 149, 233–240. [Google Scholar] [CrossRef] [PubMed]
- Miller, D.H.; Etterson, M.; Oliver, L.; Paulukonis, E.; Pollesch, N.; Purucker, T.; Rogers, D.C.; Sinnathamby, S.; Raimondo, S. Investigating vernal pool fairy shrimp exposure to organophosphate pesticides: Implications for population-level risk assessment. Ecologies, 2022; submitted for publication. [Google Scholar]
- Forbes, V.E.; Sibly, R.M.; Calow, P. Toxicant impacts on density-limited populations: A critical review of theory, practice, and results. Ecol. Appl. 2001, 11, 1249–1257. [Google Scholar] [CrossRef]
- Etterson, M.E.; Ankley, G.T. Endogenous lifecycle models for chemical risk assessment. Environ. Sci. Technol. 2021, 55, 15596–15608. [Google Scholar] [CrossRef] [PubMed]
- Vaugeois, M.; Venturelli, P.A.; Hummel, S.L.; Forbes, V.E. Population modeling to inform management and recovery efforts for lake sturgeon, Acipenser fulvescens. Integr. Environ. Assess. Manag. 2022. [Google Scholar] [CrossRef] [PubMed]
- Rowe, C.L. “The calamity of so long life”: Life histories, contaminants and potential emerging threats to long-lived vertebrates. BioScience 2008, 58, 623–631. [Google Scholar] [CrossRef]
- Etterson, M.E. Realism, Conservatism, and Tiered Ecological Risk Assessment. Ecologies, 2022; in press. [Google Scholar]
- Garber, K.; DeGrandi-Hoffman, G.; Curry, R.; Minucci, J.M.; Dawson, D.; Douglass, C.; Milone, J.; Purucker, S.T. Simulating the effects of pesticides on honey bee (Apis mellifera L.) colonies with BeePop+. Ecologies, 2022; submitted for publication. [Google Scholar]
- Accolla, C.; Schmolke, A.; Jacobson, A.; Roy, C.; Forbes, V.E.; Brain, R.; Galic, N. Modeling pesticide effects on multiple threatened and endangered Cyprinid fish species: The role of life-history traits and ecology. Ecologies, 2022; in press. [Google Scholar]
- Awkerman, J.A.; Greenberg, C.H. Projected climate and hydroregime variability constrain ephemeral wetland-dependent amphibian populations in simulations of southern toads. Ecologies, 2022; submitted for publication. [Google Scholar]
- Hudina, S.; Maguire, I.; Dragičević, P.; Galić, N. Evaluating the efficacy of approaches to control invasive populations: A conceptual model development for the signal crayfish. Ecologies 2022, 3, 78–95. [Google Scholar] [CrossRef]
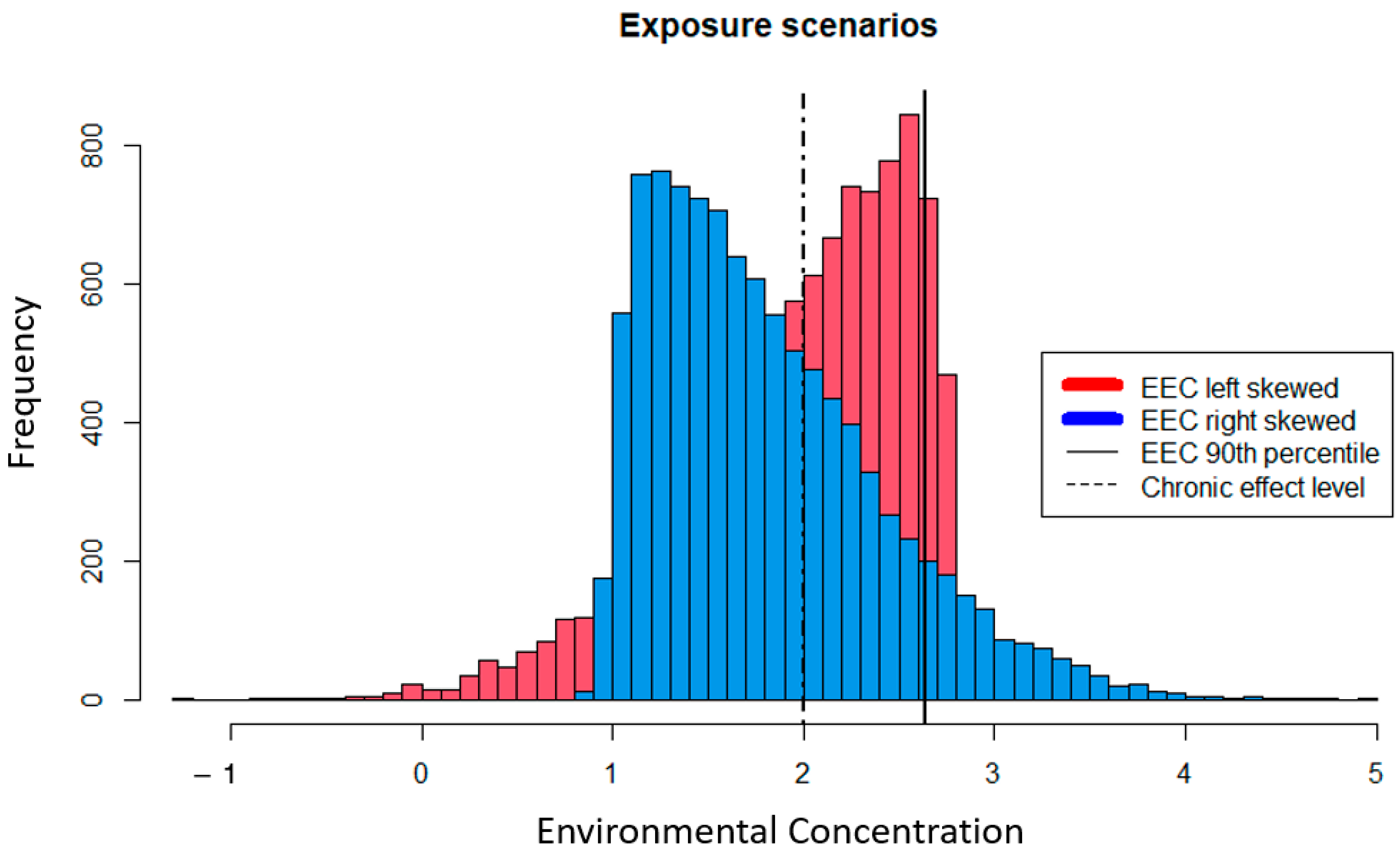
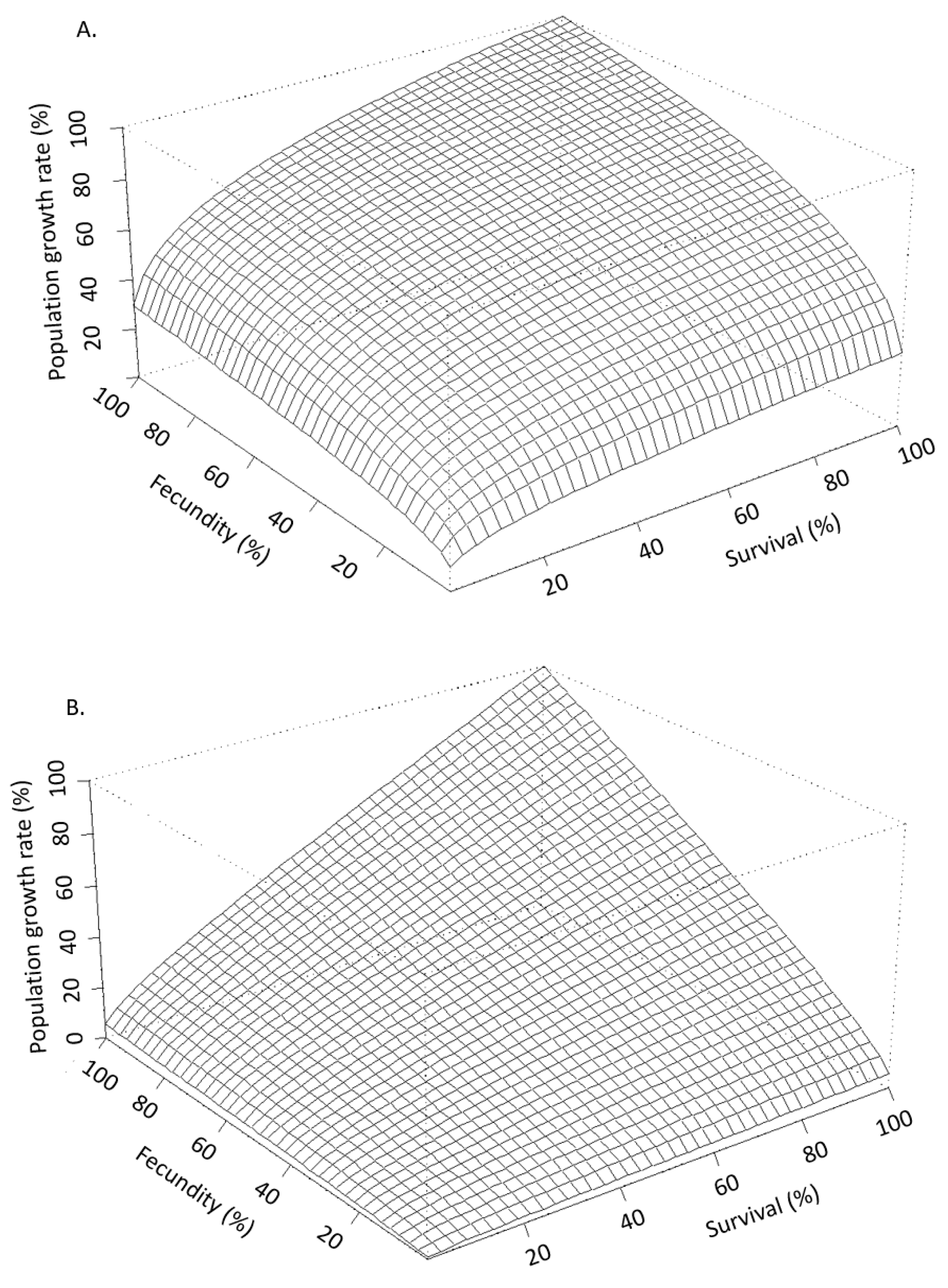
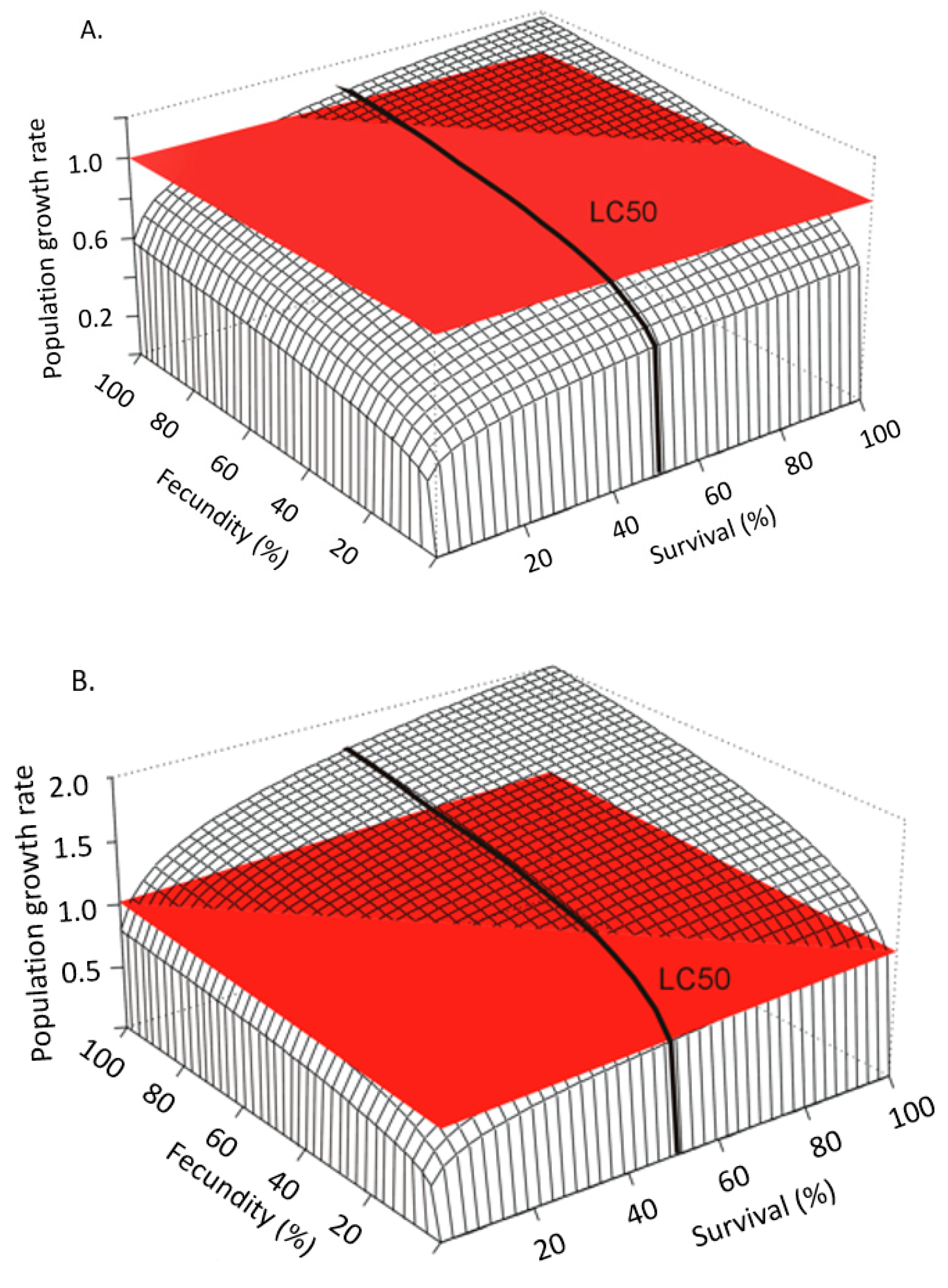
| Exposure Duration | Taxa | LOC (RQ > Limit) | Interpretation |
|---|---|---|---|
| Acute | Aquatics | 0.5 | High acute risk |
| 0.1 | Risks may be mitigated through restricted use | ||
| 0.05 | Eendangered species may be affected acutely | ||
| Mammals and birds | 0.5 | High acute risk | |
| 0.2 | Risks may be mitigated through restricted use | ||
| 0.1 | Endangered species may be affected acutely | ||
| Bees | 0.4 | Acute risk | |
| Chronic | All taxa | 1 | Presumption of chronic risk |
| Endangered species | 1 | May be affected chronically |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Raimondo, S.; Forbes, V.E. Moving beyond Risk Quotients: Advancing Ecological Risk Assessment to Reflect Better, More Robust and Relevant Methods. Ecologies 2022, 3, 145-160. https://doi.org/10.3390/ecologies3020012
Raimondo S, Forbes VE. Moving beyond Risk Quotients: Advancing Ecological Risk Assessment to Reflect Better, More Robust and Relevant Methods. Ecologies. 2022; 3(2):145-160. https://doi.org/10.3390/ecologies3020012
Chicago/Turabian StyleRaimondo, Sandy, and Valery E. Forbes. 2022. "Moving beyond Risk Quotients: Advancing Ecological Risk Assessment to Reflect Better, More Robust and Relevant Methods" Ecologies 3, no. 2: 145-160. https://doi.org/10.3390/ecologies3020012






