Are Spine-Bearing Freshwater Gastropods Better Defended?
Abstract
:1. Introduction
2. Materials and Methods
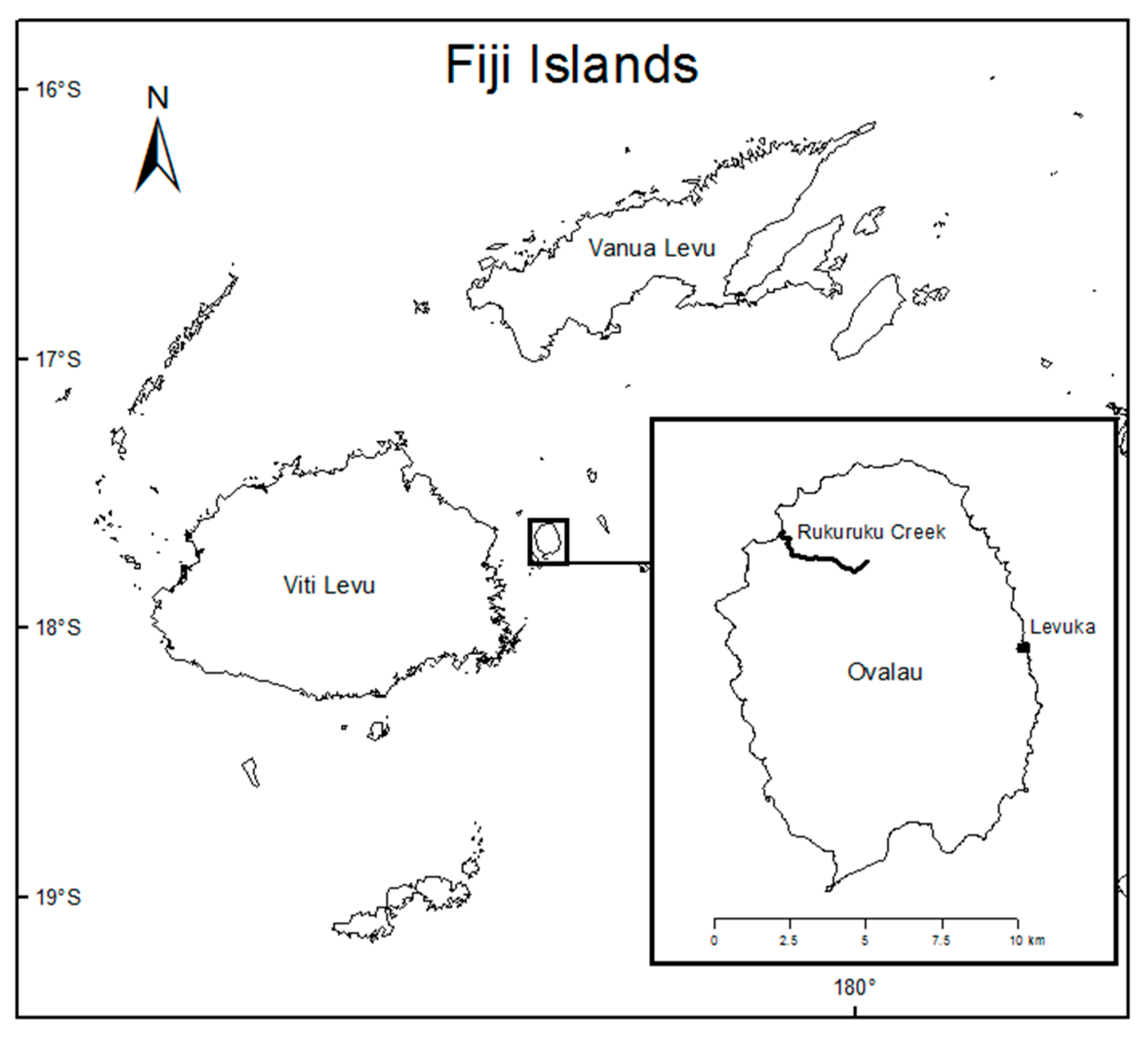
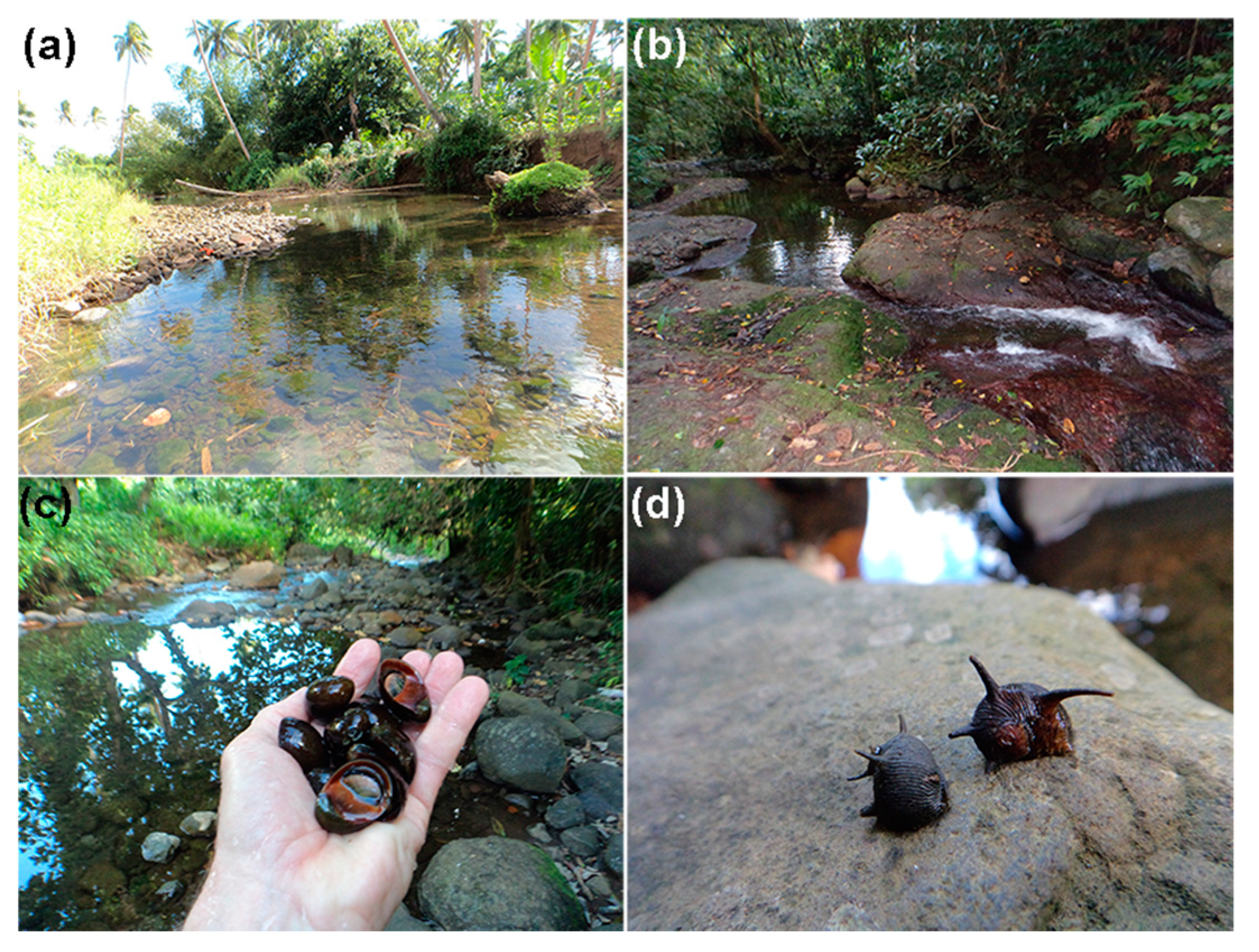
2.1. Study Location and Sampling
2.2. Data Analysis
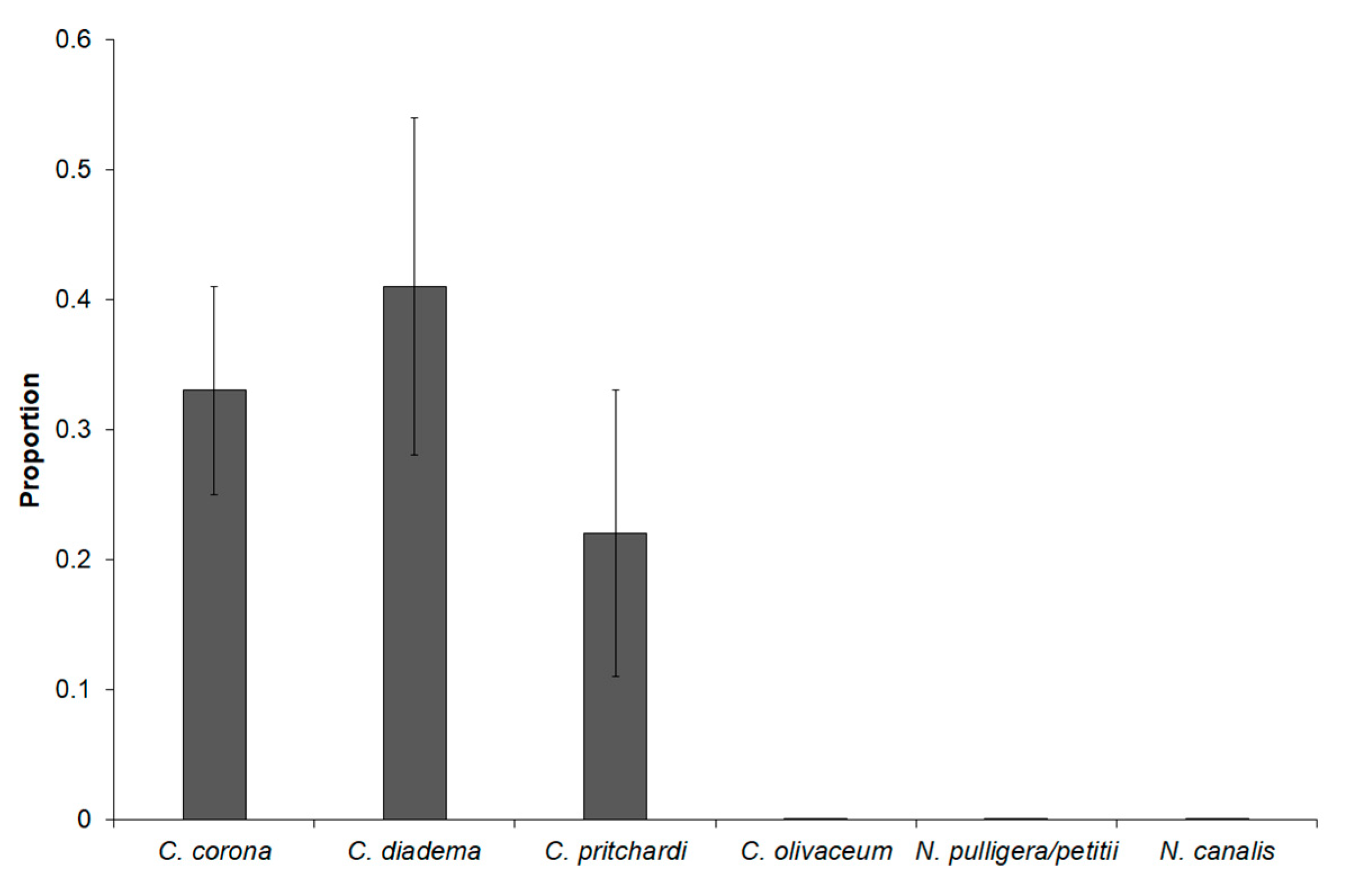
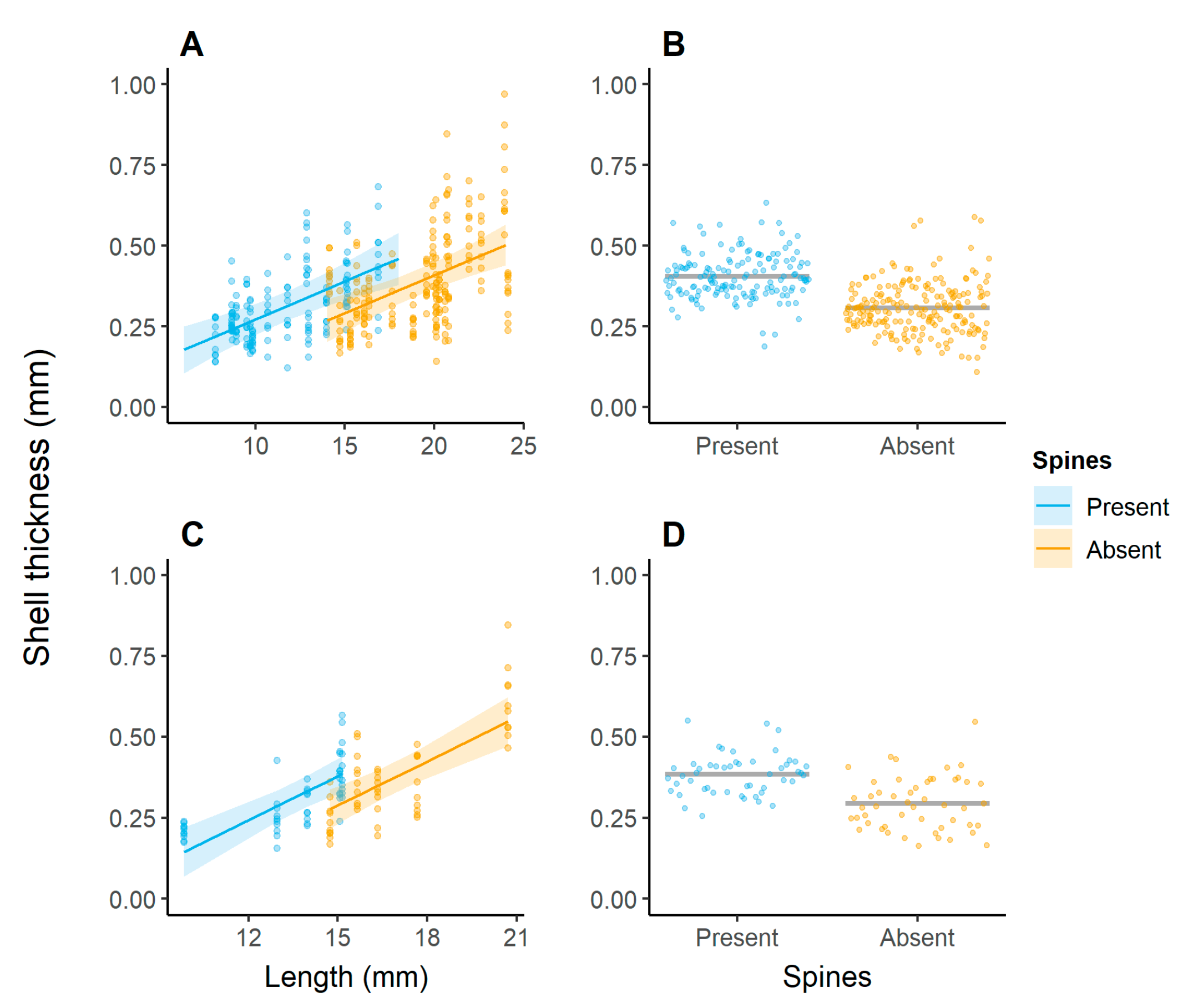
3. Results
4. Discussion
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A. Nonmarine molluscan fauna of Rukuruku Creek, Ovalau Island, Fiji, July 2014
References
- Sih, A.; Crowley, P.; McPeek, M.; Petranka, J.; Strohmeier, K. Predation, competition, and prey comm-unities: A review of field experiments. Ann. Rev. Ecol. Syst. 1985, 16, 269–311. [Google Scholar] [CrossRef]
- Vermeij, G.J. Evolution and Escalation: An Ecological History of Life; Princeton University Press: Princeton, NJ, USA, 1987. [Google Scholar]
- Inbar, M.; Lev-Yadun, S. Conspicuous and aposematic spines in the animal kingdom. Naturwissenschaften 2005, 92, 170–172. [Google Scholar] [CrossRef] [PubMed]
- Hanley, M.E.; Lamont, B.B.; Fairbanks, M.M.; Rafferty, C.M. Plant structural traits and their role in anti-herbivore defence. Perspect. Plant Ecol. 2007, 8, 157–178. [Google Scholar] [CrossRef]
- Vermeij, G.J. Marine faunal dominance and molluscan shell form. Evolution 1974, 28, 656–664. [Google Scholar] [CrossRef] [PubMed]
- Vance, R.R. A mutualistic interaction between a sessile marine clam and its epibionts. Ecology 1978, 59, 679–685. [Google Scholar] [CrossRef]
- Palmer, A.R. Fish predation and the evolution of gastropod shell sculpture: Experimental and geographic evidence. Evolution 1979, 33, 697–713. [Google Scholar] [CrossRef] [PubMed]
- Miller, D.J.; LaBarbera, M. Effects of foliaceous varices on the mechanical properties of Chicoreus dilectus (Gastropoda: Muricidae). J. Zool. 1995, 236, 151–160. [Google Scholar] [CrossRef]
- West, K.; Cohen, A.; Baron, M. Morphology and behavior of crabs and gastropods from Lake Tanganyika, Africa: Implications for lacustrine predator-prey coevolution. Evolution 1991, 45, 589–607. [Google Scholar] [CrossRef]
- Feifarek, B.P. Spines and epibionts as antipredator defenses in the thorny oyster Spondylus americanus Hermann. J. Exp. Mar. Biol. Ecol. 1987, 105, 39–56. [Google Scholar] [CrossRef]
- Vermeij, G.J. Gastropod skeletal defences: Land, freshwater, and sea compared. Vita Malacol. 2015, 13, 1–25. [Google Scholar]
- Vermeij, G.J.; Covich, A.P. Coevolution of freshwater gastropods and their predators. Am. Nat. 1978, 112, 833–843. [Google Scholar] [CrossRef]
- Haynes, A. An evaluation of members of the genera Clithon Montfort, 1810 and Neritina Lamarck 1816 (Gastropoda: Neritidae). Moll. Res. 2005, 25, 75–84. [Google Scholar]
- Eichhorst, T.E. Neritidae of the World; ConchBooks: Hackenheim, Germany, 2016; Volume 1–2, p. 1366. [Google Scholar]
- Ponder, W. Superorder Neritopsina. In Mollusca: The Southern Synthesis. Fauna of Australia; Beesley, P.L., Ross, G.J., Wells, A., Eds.; CSIRO Publishing: Melbourne, Australia, 1998; Volume 5, pp. 693–694. [Google Scholar]
- Scott, B.J.; Kenny, R. Superfamily Neritoidea. In Mollusca: The Southern Synthesis. Fauna of Australia; Beesley, P.L., Ross, G.J., Wells, A., Eds.; CSIRO Publishing: Melbourne, Australia, 1998; Volume 5, pp. 694–702. [Google Scholar]
- Haynes, A. Notes on the stream neritids (Gastropoda; Prosobranchia) of Oceania. Micronesica 1988, 21, 93–102. [Google Scholar]
- Davis, A.R.; Ponder, W.F. Biogeographic conundrum: Why so few stream nerite species (Gastropoda: Neritidae) in Australia? Freshw. Biol. 2019, 64, 2084–2088. [Google Scholar] [CrossRef] [Green Version]
- McDowall, R.M. On amphidromy, a distinct form of diadromy in aquatic organisms. Fish Fish. 2007, 8, 1–13. [Google Scholar] [CrossRef]
- Crandall, E.D.; Taffel, J.R.; Barber, P.H. High gene flow due to pelagic larval dispersal among South Pacific archipelagos in two amphidromous gastropods (Neritomorpha: Neritidae). Heredity 2010, 104, 563–572. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McDowall, R.M. Diadromy in Fishes: Migrations between Marine and Freshwater Environments; Croom Helm: London, UK, 1988. [Google Scholar]
- Cook, B.D.; Page, T.J.; Hughes, J.M. Phylogeography of related diadromous species in continental and island settings, and a comparison of their potential and realized dispersal patterns. J. Biogeogr. 2012, 39, 421–430. [Google Scholar] [CrossRef]
- Abdou, A.; Keith, P.; Galzin, R. Freshwater neritids (Mollusca: Gastropoda) of tropical islands: Amphidromy as a life cycle, a review. Rev. D’ecol. 2015, 70, 387–397. [Google Scholar]
- Kano, Y. Hitchhiking behaviour in the obligatory upstream migration of amphidromous snails. Biol. Lett. 2009, 5, 465–468. [Google Scholar] [CrossRef] [Green Version]
- Harrison, X.A.; Donaldson, L.; Correa-Cano, M.E.; Evans, J.; Fisher, D.N.; Goodwin, C.E.D.; Robinson, B.S.; Hodgson, D.J.; Inger, R. A brief introduction to mixed effects modelling and multi-model inference in ecology. Peer J. 2018, 6, e4794. [Google Scholar] [CrossRef] [Green Version]
- Bates, D.; Mächler, M.; Bolker, B.; Walker, S. Fitting Linear Mixed-Effects Models Using lme4. J. Stat. Softw. 2015, 67, 1–48. [Google Scholar] [CrossRef]
- R Core Team. R: A language and environment for statistical computing. In R Foundation for Statistical Computing; R Core Team: Vienna, Austria, 2020; Available online: https://www.R-project.org/ (accessed on 25 May 2020).
- Kuznetsova, A.; Brockhoff, P.B.; Christensen, R.H.B. lmerTest Package: Tests in Linear Mixed Effects Models. J. Stat. Softw. 2017, 82, 1–26. [Google Scholar] [CrossRef] [Green Version]
- Wickham, H. ggplot2: Elegant Graphics for Data Analysis; Springer: New York, NY, USA, 2016; Available online: https://ggplot2.tidyverse.org (accessed on 25 May 2020).
- Breheny, P.; Burchett, W. Visualization of Regression Models Using visreg. R J. 2017, 9, 56–71. [Google Scholar] [CrossRef]
- Lüdecke, D. ggeffects: Tidy Data Frames of Marginal Effects from Regression Models. J. Open Source Softw. 2018, 3, 772. [Google Scholar] [CrossRef] [Green Version]
- Kohn, A.J. Anti-predator defences of shelled gastropods. In Functional Morphology of the Invertebrate Skeleton; Savazzi, E., Ed.; John Wiley and Sons: Chichester, UK, 1999; pp. 169–181. [Google Scholar]
- Vermeij, G.J. A Natural History of Shells; Princeton University Press: Princeton, NJ, USA, 1993. [Google Scholar]
- Reynolds, W.W.; Reynolds, L.J. Zoogeography and the predator-prey ‘arms race’: A comparison of Eriphia and Nerita species from three faunal regions. Hydrobiologia 1977, 56, 63–67. [Google Scholar] [CrossRef]
- Boseto, D.; Jenkins, A.P. A Checklist of Freshwater and Brackish Water Fishes of the Fiji Islands. Wetlands International-Oceania Suva 2006. Available online: https://www.sprep.org/att/irc/ecopies/countries/fiji/141.pdf. (accessed on 1 September 2020).
- Fishes of Australia. Available online: https://fishesofaustralia.net.au/home/species/704#moreinfo (accessed on 20 May 2020).
- Copeland, L.K.; Boseto, D.T.; Jenkins, A.P. Freshwater ichthyofauna of the Pacific-Asia biodiversity transect (PABITRA) gateway in Viti Levu, Fiji. Pac. Conserv. Biol. 2016, 22, 236–241. [Google Scholar] [CrossRef]
- Schneider, D.W.; Lyons, J. Dynamics of upstream migration in two species of tropical freshwater snails. J. N. Am. Benthol. Soc. 1993, 12, 3–16. [Google Scholar] [CrossRef]
- Blanco, J.F.; Scatena, F. Floods, habitat hydraulics and up stream migration of Neritina virginea (Gastropoda: Neritidae) in Northeastern Puerto Rico. Caribb. J. Sci. 2005, 41, 55–71. [Google Scholar]
- Coley, P.D. Effects of plant growth rate and leaf lifetime on the amount and type of anti-herbivore defense. Oecologia 1988, 74, 531–536. [Google Scholar] [CrossRef]
- Holomuzki, J.R.; Biggs, B.J.F. Habitat-specific variation and performance trade-offs in shell armature of New Zealand mudsnails. Ecology 2006, 87, 1038–1047. [Google Scholar] [CrossRef]
- Vermeij, G.J. Gastropod shell form, breakage and repair in relation to predation by the crab Calappa. Malacologia 1982, 23, 1–12. [Google Scholar]
- Leonard, G.H.; Bertness, M.D.; Yund, P.O. Crab predation, waterborne cues, and inducible defenses in the blue mussel, Mytilus Edulis. Ecology 1999, 80, 1–14. [Google Scholar] [CrossRef]
- Schilthuizen, M. Sexual selection on land snail shell ornamentation: A hypothesis that may explain shell diversity. BMC Evol. Biol. 2003, 3, 13–19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Glazier, D.S.; Clusella-Trullas, S.; Terblanche, J.S. Sexual dimorphism and physiological correlates of horn length in a South African isopod crustacean. J. Zool. 2016, 300, 99–110. [Google Scholar] [CrossRef] [Green Version]
- Jenkins, A.P.; Jupiter, S.D.; Qauqau, I.; Atherton, J. The importance of ecosystem-based management for conserving aquatic migratory pathways on tropical high islands: A case study from Fiji. Aquat. Conserv. Mar. Freshw. Ecosyst. 2010, 20, 224–238. [Google Scholar] [CrossRef]

| Distance from Entrance (m) | ||||
|---|---|---|---|---|
| 100 | 600 | 1000 | 2000 | |
| C. corona |  | |||
| C. diadema |  | |||
| C. pritchardi |  |  |  | |
| C. pritchardi * |  |  | ||
| C. olivaceum |  |  |  | |
| N. pulligera/petitii |  |  |  |  |
| N. porcata |  |  | ||
| N. canalis |  |  | ||
| Ne. macgillivrayi |  | |||
 ≤ 0.10,
≤ 0.10,  = 0.10–0.25,
= 0.10–0.25,  ≥ 0.26;
≥ 0.26;  = spinose individuals;
= spinose individuals;  = nonspinose individuals.
= nonspinose individuals.| Species | Shell Length (Max SL) mm | Max. No. of Spines | Max. Length of Spines | % Shells Damaged | n |
|---|---|---|---|---|---|
| Clithon corona | 7.9–16.9 (27) | 6 | 5.0 mm | 0 | 17 |
| Clithon diadema | 3.8–10.4 (16) | 4 | 4.5 mm | 0 | 54 |
| Clithon pritchardi * | 9.8–22.0 (24) | 4 | 5.8 mm | 5.8 | 17 |
| Clithon pritchardi | 14.7–26.0 (24) | 0 | 4.0 | 25 | |
| Clithon olivaceum | 14.1–24.0 (40) | 0 | 6.8 | 44 | |
| Neritina pulligera/petitii | 17.0–37.0 (43) | 0 | 6.4 | 31 | |
| Neritina canalis | 20.1–26.0 (25) | 0 | 0 | 8 |
| Sum of Squares | Numerator DF | Denominator DF | f (Value) | p | |
|---|---|---|---|---|---|
| (A) All taxa | |||||
| Spines | 0.023 | 1 | 9.14 | 3.75 | 0.084 |
| Length | 0.125 | 1 | 14.94 | 19.94 | <0.001 |
| (B) C. pritchardi | |||||
| Spines | 0.022 | 1 | 6.99 | 3.84 | 0.091 |
| Length | 0.167 | 1 | 6.99 | 28.90 | 0.001 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Davis, A.R.; Rees, M.J.; Rashni, B.; Haynes, A. Are Spine-Bearing Freshwater Gastropods Better Defended? Ecologies 2020, 1, 3-13. https://doi.org/10.3390/ecologies1010002
Davis AR, Rees MJ, Rashni B, Haynes A. Are Spine-Bearing Freshwater Gastropods Better Defended? Ecologies. 2020; 1(1):3-13. https://doi.org/10.3390/ecologies1010002
Chicago/Turabian StyleDavis, Andrew R., Matthew J. Rees, Bindiya Rashni, and Alison Haynes. 2020. "Are Spine-Bearing Freshwater Gastropods Better Defended?" Ecologies 1, no. 1: 3-13. https://doi.org/10.3390/ecologies1010002
APA StyleDavis, A. R., Rees, M. J., Rashni, B., & Haynes, A. (2020). Are Spine-Bearing Freshwater Gastropods Better Defended? Ecologies, 1(1), 3-13. https://doi.org/10.3390/ecologies1010002






