Effect of Blending Dimethyl Carbonate and Ethanol with Gasoline on Combustion Characteristics
Abstract
:1. Introduction
2. Experiment
3. Results and Discussions
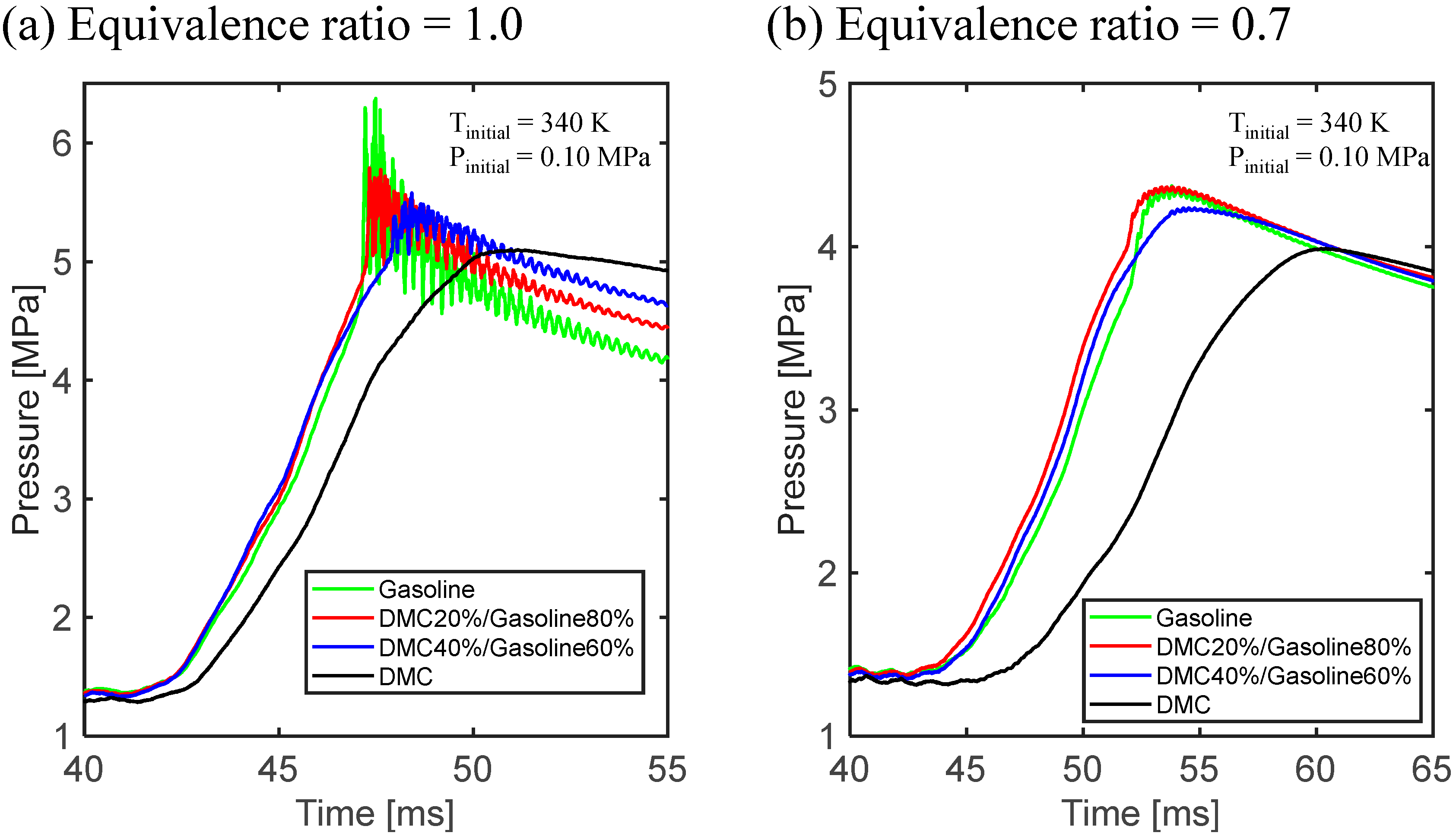
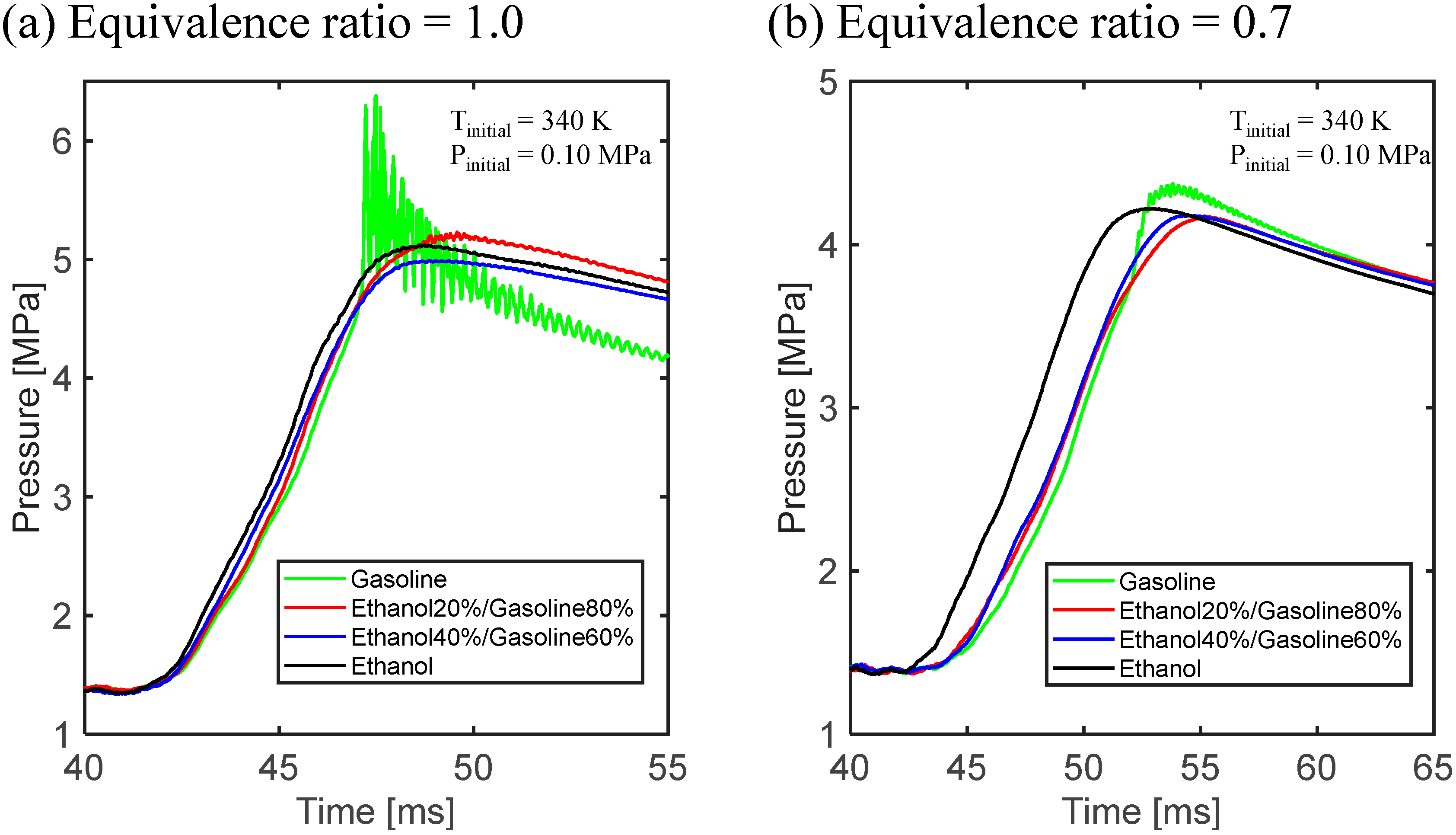
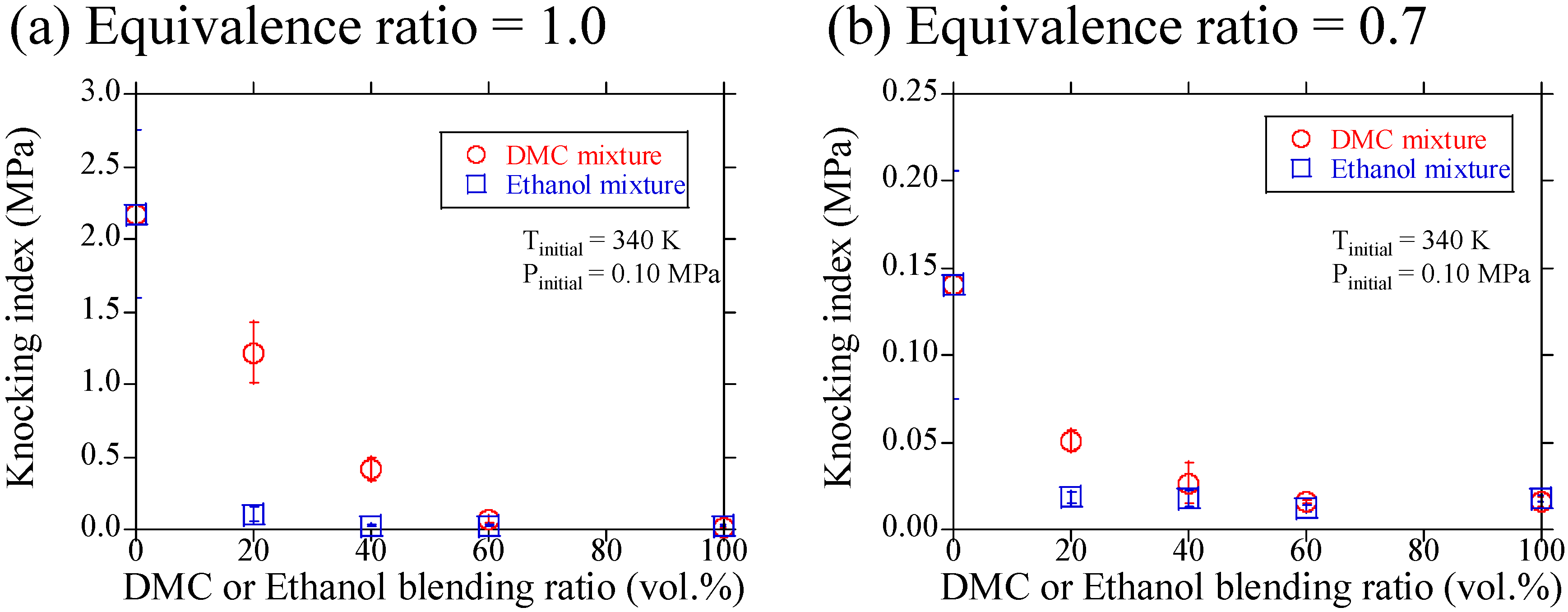
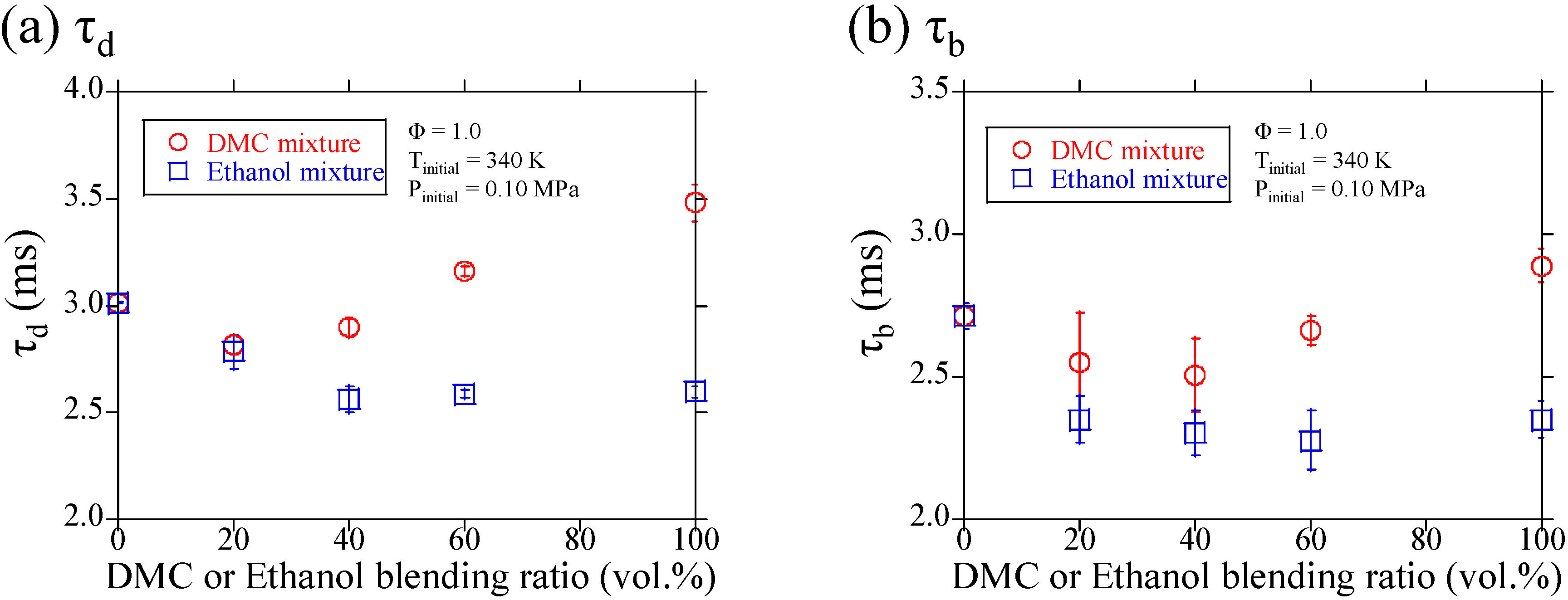
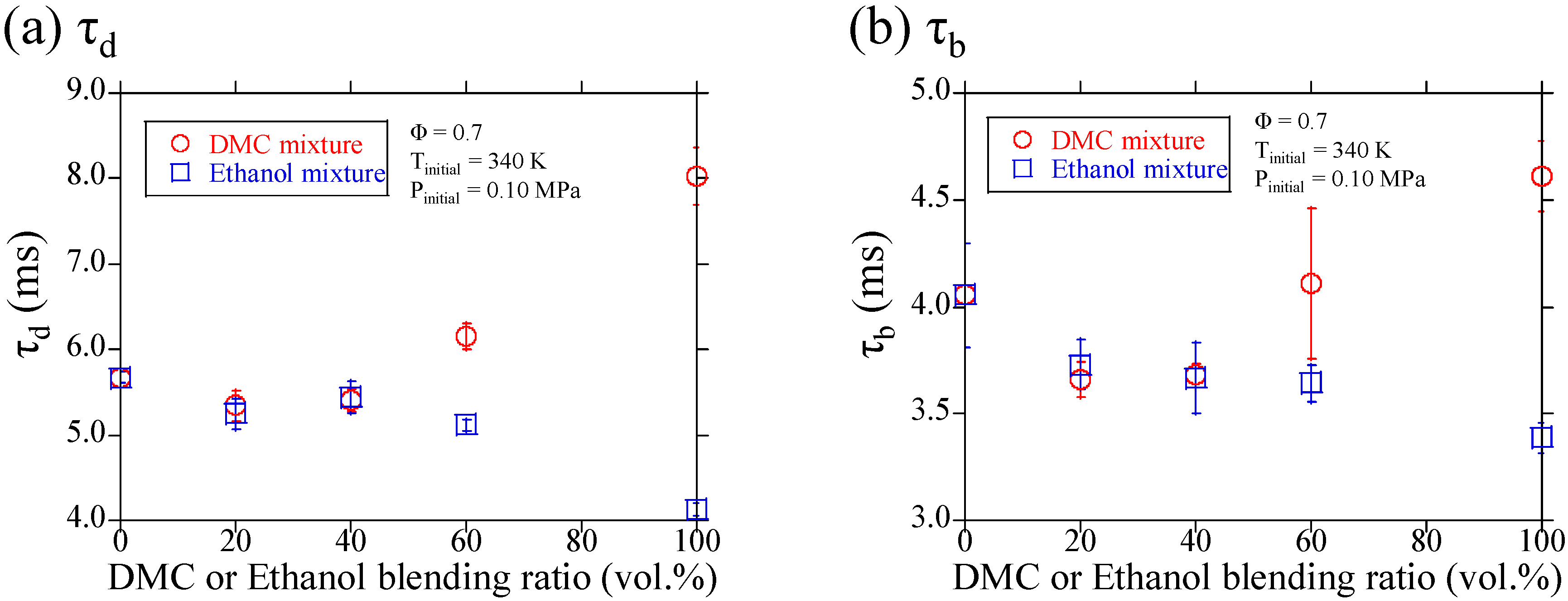
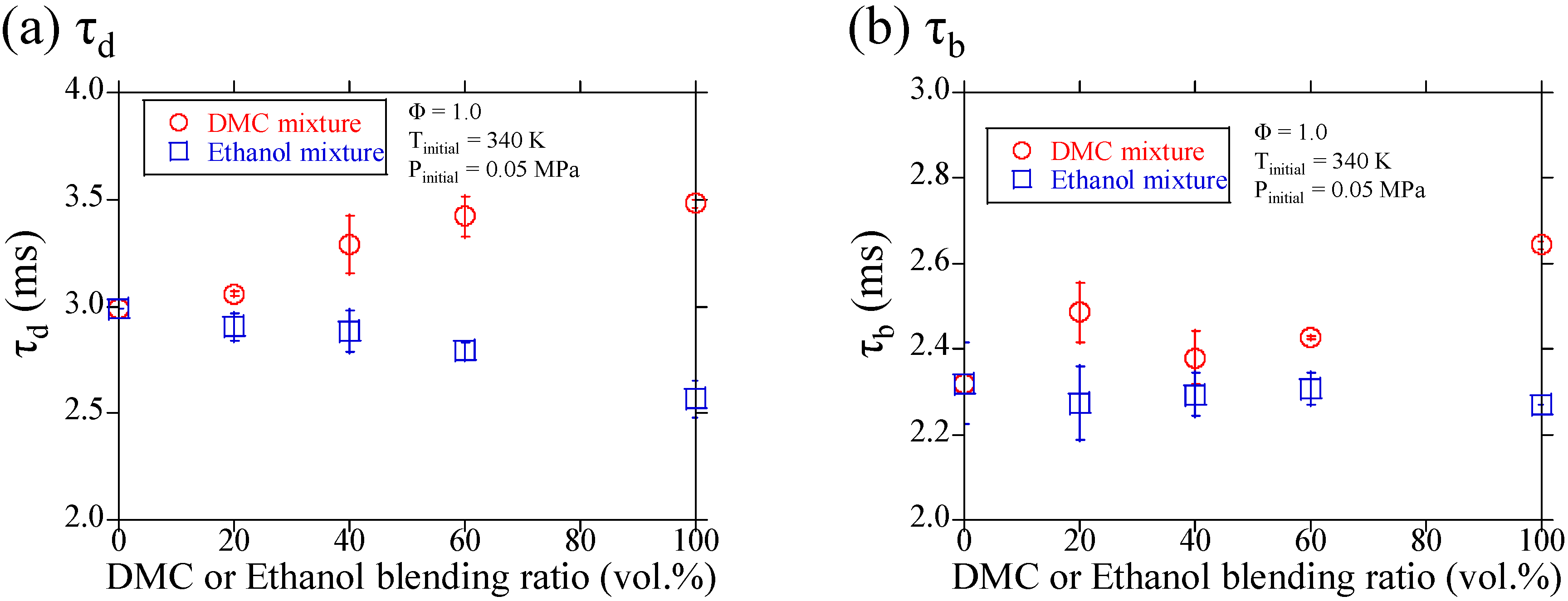
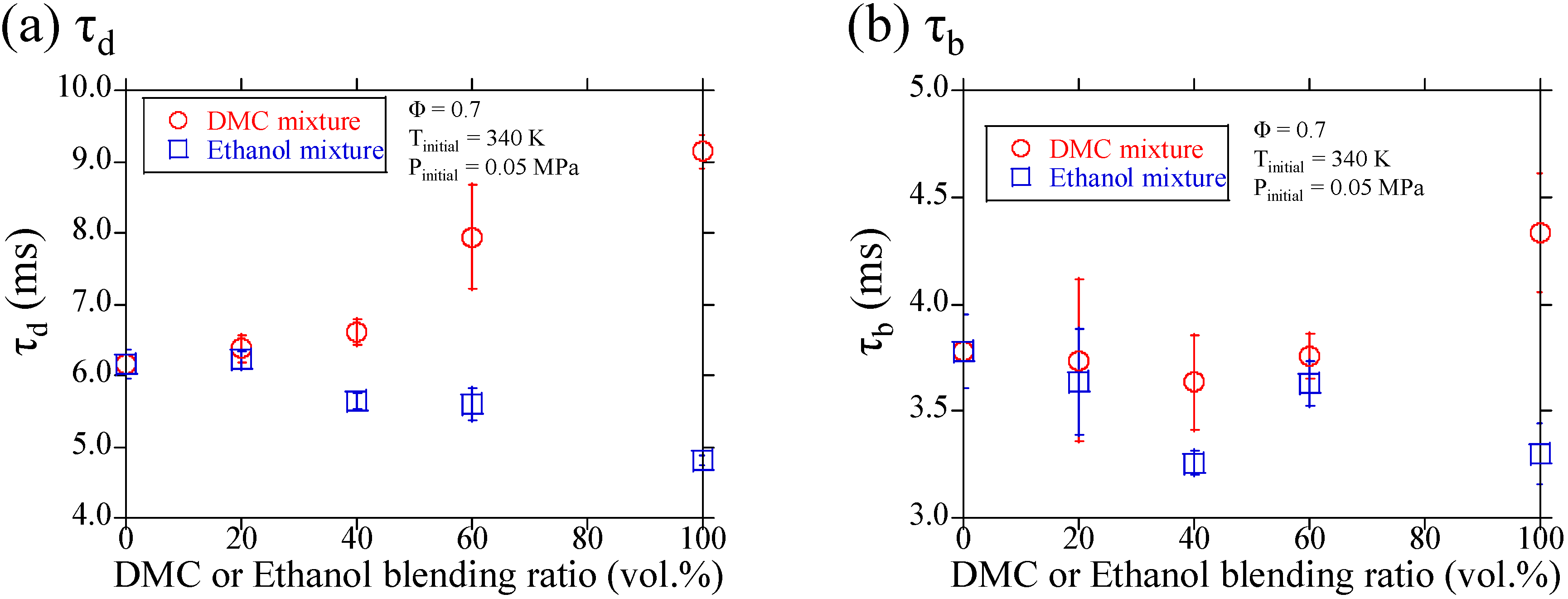
3.1. Experimental Results
3.2. Discussions
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- BP Energy Outlook, 2020 ed.; BP: London, UK, 2020.
- Ramirez, A.; Sarathy, S.M.; Gascon, J. CO2 derived E-fuels: Research trends, misconceptions, and future directions. Trends Chem. 2020, 2, 785–795. [Google Scholar] [CrossRef]
- Jeswani, H.K.; Chilvers, A.; Azapagic, A. Environmental sustainability of biofuels: A review. Proc. R. Soc. A 2020, 476, 20200351. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Fortes, M.; Schöneberger, J.C.; Boulamanti, A.; Tzimas, E. Methanol synthesis using captured CO2 as raw material: Techno-economic and environmental assessment. Appl. Energy 2016, 161, 718–732. [Google Scholar] [CrossRef]
- Artz, J.; Müller, T.E.; Thenert, K.; Kleinekorte, J.; Meys, R.; Sternberg, A.; Bardow, A.; Leitner, W. Sustainable Conversion of Carbon Dioxide: An Integrated Review of Catalysis and Life Cycle Assessment. Chem. Rev. 2018, 118, 434–504. [Google Scholar] [CrossRef]
- Zabed, H.; Sahu, J.N.; Boyce, A.N.; Faruq, G. Fuel ethanol production from lignocellulosic biomass: An overview on feedstocks and technological approaches. Renew. Sustain. Energy Rev. 2016, 66, 751–774. [Google Scholar] [CrossRef]
- Saeidi, S.; Najari, S.; Hessel, V.; Wilson, K.; Keil, F.J.; Concepción, P.; Suib, S.L.; Rodrigues, A.E. Recent advances in CO2 hydrogenation to value-added products—Current challenges and future directions. Prog. Energy Combust. Sci. 2021, 85, 100905. [Google Scholar] [CrossRef]
- Abdalla, A.O.G.; Liu, D. Dimethyl Carbonate as a Promising Oxygenated Fuel for Combustion: A Review. Energies 2018, 11, 1552. [Google Scholar] [CrossRef]
- Manzetti, S.; Andersen, O. A review of emission products from bioethanol and its blends with gasoline. Background for new guidelines for emission control. Fuel 2015, 140, 293–301. [Google Scholar] [CrossRef]
- Badia, J.H.; Badia, J.H.; Ramírez, E.; Bringué, R.; Cunill, F.; Delgado, J. New Octane Booster Molecules for Modern Gasoline Composition. Energy Fuels 2021, 35, 10949–10997. [Google Scholar] [CrossRef]
- van Lipzig, J.P.J.; Nilsson, E.J.K.; de Goey, L.P.H.; Konnov, A.A. Laminar burning velocities of n-heptane, iso-octane, ethanol and their binary and tertiary mixtures. Fuel 2011, 90, 2773–2781. [Google Scholar] [CrossRef]
- Awad, O.I.; Mamat, R.; Ibrahim, T.K.; Hammid, A.T.; Yusri, I.M.; Hamidi, M.A.; Humada, A.M.; Yusop, A.F. Overview of the oxygenated fuels in spark ignition engine: Environmental and performance. Renew. Sustain. Energy Rev. 2018, 91, 394–408. [Google Scholar] [CrossRef]
- Wen, L.-B.; Xin, C.-Y.; Yang, S.-C. The effect of adding dimethyl carbonate (DMC) and ethanol to unleaded gasoline on exhaust emission. Appl. Energy 2010, 87, 115–121. [Google Scholar] [CrossRef]
- Schifter, I.; González, U.; Díaz, L.; Sánchez-Reyna, G.; Mejía-Centeno, I.; González-Macías, C. Comparison of performance and emissions for gasoline-oxygenated blends up to 20 percent oxygen and implications for combustion on a spark-ignited engine. Fuel 2017, 208, 673–681. [Google Scholar] [CrossRef]
- Christensen, E.; Yanowitz, J.; Ratcliff, M.; McCormick, R.L. Renewable Oxygenate Blending Effects on Gasoline Properties. Energy Fuels 2011, 25, 4723–4733. [Google Scholar] [CrossRef]
- Broustail, G.; Seers, P.; Halter, F.; Moréac, G.; Mounaim-Rousselle, C. Experimental determination of laminar burning velocity for butanol and ethanol iso-octane blends. Fuel 2011, 90, 1–6. [Google Scholar] [CrossRef]
- Bogin, G.E., Jr.; Luecke, J.; Ratcliff, M.A.; Osecky, E.; Zigler, B.T. Effects of iso-octane/ethanol blend ratios on the observance of negative temperature coefficient behavior within the Ignition Quality Tester. Fuel 2016, 186, 82–90. [Google Scholar] [CrossRef]
- Barraza-Botet, C.L.; Wooldridge, M.S. Combustion chemistry of iso-octane/ethanol blends: Effects on ignition and reaction pathways, Combust. Flame 2018, 188, 324–336. [Google Scholar] [CrossRef]
- Foong, T.M.; Morganti, K.J.; Brear, M.J.; da Silva, G.; Yang, Y.; Dryer, F.L. The octane numbers of ethanol blended with gasoline and its surrogates. Fuel 2014, 115, 727–739. [Google Scholar] [CrossRef]
- Schifter, I.; González, U.; González-Macías, C. Effects of ethanol, ethyl-tert-butyl ether and dimethyl-carbonate blends with gasoline on SI engine. Fuel 2016, 183, 253–261. [Google Scholar] [CrossRef]
- Chan, J.H.; Tsolakis, A.; Herreros, J.M.; Kallis, K.X.; Hergueta, C.; Sittichompoo, S.; Bogarra, M. Combustion, gaseous emissions and PM characteristics of Di-Methyl Carbonate (DMC)-gasoline blend on gasoline Direct Injection (GDI) engine. Fuel 2020, 263, 116742. [Google Scholar] [CrossRef]
- Wagner, C.; Keskin, M.-T.; Pitsch, H.; Grill, M.; Bargende, M.; Cai, L. Potential Analysis and Virtual Development of SI Engines Operated with Synthetic Fuel DMC+. SAE Technical Paper 2020-01-0342. 2020. Available online: https://www.sae.org/publications/technical-papers/content/2020-01-0342/ (accessed on 23 October 2023).
- Chen, G.; Yu, W.; Fu, J.; Huang, J.M.Z.; Yang, J.; Wang, Z.; Jin, H.; Qi, F. Experimental and modeling study of the effects of adding oxygenated fuels to premixed n-heptane flames. Combust. Flame 2012, 159, 2324–2335. [Google Scholar] [CrossRef]
- Gao, Z.; Hu, E.; Xu, Z.; Huang, S.; Ku, J.; Huang, Z. Measurements and kinetic study on the ignition delay time of dimethyl carbonate/n-heptane/oxygen/argon mixtures. Combust. Sci. Technol. 2018, 190, 933–948. [Google Scholar] [CrossRef]
- Tan, Y.R.; Salamanca, M.; Bai, J.; Akroyd, J.; Kraft, M. Structural effects of C3 oxygenated fuels on soot formation in ethylene coflow diffusion flames. Combust. Flame 2021, 232, 111512. [Google Scholar] [CrossRef]
- Pacheco, M.A.; Marshall, C.L. Review of Dimethyl Carbonate (DMC) Manufacture and Its Characteristics as a Fuel Additive. Energy Fuels 1997, 11, 2–29. [Google Scholar] [CrossRef]
- Takahashi, E.; Nagano, Y.; Kitagawa, T.; Nishioka, M.; Nakamura, T.; Nakano, M. Demonstration of knock intensity mitigation through dielectric barrier discharge reformation in an RCEM. Combust. Flame 2020, 216, 185–193. [Google Scholar] [CrossRef]
- Takahashi, E.; Kuramochi, A.; Nishioka, M. Turbulent flame propagation enhancement by application of dielectric barrier discharge to fuel-air mixtures. Combust. Flame 2018, 192, 401–409. [Google Scholar] [CrossRef]
- Takahashi, E.; Sakamoto, S.; Imamura, O.; Ohkuma, Y.; Yamasaki, H.; Furutani, H.; Akihama, K. Fundamental characteristics of laser breakdown assisted long distance discharge ignition. J. Phys. D Appl. Phys. 2019, 52, 485501. [Google Scholar] [CrossRef]
- Takahashi, E.; Kato, S. Laser ablation ignition of flammable gas. Jpn. J. Appl. Phys. 2021, 60, 047001. [Google Scholar] [CrossRef]
- Vipavanich, C.; Chuepeng, S.; Skullong, S. Heat release analysis and thermal efficiency of a single cylinder diesel dual fuel engine with gasoline port injection. Case Stud. Therm. Eng. 2018, 12, 143–148. [Google Scholar] [CrossRef]
- Heywood, J.B. Internal Combustion Engine Fundamentals; McGraw-Hill Education: New York, NY, USA, 1988; pp. 372–373,382. [Google Scholar]
- Han, W.-Q.; Yao, C.-D. Research on high cetane and high octane number fuels and the mechanism for their common oxidation and auto-ignition. Fuel 2015, 150, 29–40. [Google Scholar] [CrossRef]
- Boot, M.D.; Tian, M.; Hensen, E.J.M.; Sarathy, S.M. Impact of fuel molecular structure on auto-ignition behavior—Design rules for future high performance gasolines. Prog. Energy Combust. Sci. 2017, 60, 1–25. [Google Scholar] [CrossRef]
- Yang, B.; Sun, W.; Moshammer, K.; Hansen, N. Review of the Influence of Oxygenated Additives on the Combustion Chemistry of Hydrocarbons. Energy Fuels 2021, 35, 13550–13568. [Google Scholar] [CrossRef]
- Kanayama, K.; Takahashi, S.; Morikura, S.; Nakamura, H.; Tezuka, T.; Maruta, K. Study on oxidation and pyrolysis of carbonate esters using a micro flow reactor with a controlled temperature profile. Part I: Reactivities of dimethyl carbonate, ethyl methyl carbonate and diethyl carbonate. Combust. Flame 2022, 237, 111810. [Google Scholar] [CrossRef]
- Marinov, N.M. A Detailed Chemical Kinetic Model for High Temperature Ethanol Oxidation. Int. J. Chem. Kinet. 1999, 31, 183–220. [Google Scholar] [CrossRef]
- Glaude, P.A.; Pitz, W.J.; Thomson, M.J. Chemical kinetic modeling of dimethyl carbonate in an opposed-flow diffusion flame. Proc. Combust. Inst. 2005, 30, 1111–1118. [Google Scholar] [CrossRef]
- Rau, F.; Hartl, S.; Voss, S.; Still, M.; Hasse, C.; Trimis, D. Laminar burning velocity measurements using the Heat Flux method and numerical predictions of iso-octane/ethanol blends for different preheat temperatures. Fuel 2015, 140, 10–16. [Google Scholar] [CrossRef]
- Mannaa, O.A.; Mansour, M.S.; Roberts, W.L.; Chung, S.H. Laminar Burning Velocities of Fuels for Advanced Combustion Engines (FACE) Gasoline and Gasoline Surrogates with and without Ethanol Blending Associated with Octane Rating. Combust. Sci. Technol. 2016, 188, 692–706. [Google Scholar] [CrossRef]
- Burluka, A.A.; Gaughan, R.G.; Griffiths, J.F.; Mandilas, C.; Sheppard, C.G.W.; Woolley, R. Turbulent burning rates of gasoline components, Part 1—Effect of fuel structure of C6 hydrocarbons. Fuel 2016, 167, 347–356. [Google Scholar] [CrossRef]
- Li, Z.; Han, W.; Liu, D.; Chen, Z. Laminar flame propagation and ignition properties of premixed iso-octane/air with hydrogen addition. Fuel 2015, 158, 443–450. [Google Scholar] [CrossRef]
- Baloo, M.; Dariani, B.M.; Akhlaghi, M.; Chitsaz, I. Effect of iso-octane/methane blend on laminar burning velocity and flame instability. Fuel 2015, 144, 264–273. [Google Scholar] [CrossRef]
- Petrakides, S.; Chen, R.; Gao, D.; Wei, H. Experimental Investigation on the Laminar Burning Velocities and Markstein Lengths of Methane and PRF95 Dual Fuels. Energy Fuels 2016, 30, 6777–6789. [Google Scholar] [CrossRef]

| Bore and stroke | 100 mm and 120 mm |
| Corresponding piston speed | 1200 rpm |
| Compression ratio | 7.5 |
| Piston compression velocity | 5 m/s |
| Piston shape | Flat top |
| Fuel | Gasoline/DMC (DMC: 0, 20, 40, 60, 100 vol.%) Gasoline/ethanol (ethanol: 0, 20, 40, 60, 100 vol.%) |
| Equivalence ratio | 0.7 and 1.0 |
| Initial temperature of chamber | 340 K |
| Initial pressure of chamber | 0.05 and 0.10 MPa |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Suzuki, S.; Takahashi, E.; Oguma, M.; Akihama, K. Effect of Blending Dimethyl Carbonate and Ethanol with Gasoline on Combustion Characteristics. Fuels 2023, 4, 441-453. https://doi.org/10.3390/fuels4040027
Suzuki S, Takahashi E, Oguma M, Akihama K. Effect of Blending Dimethyl Carbonate and Ethanol with Gasoline on Combustion Characteristics. Fuels. 2023; 4(4):441-453. https://doi.org/10.3390/fuels4040027
Chicago/Turabian StyleSuzuki, Shunsuke, Eiichi Takahashi, Mitsuharu Oguma, and Kazuhiro Akihama. 2023. "Effect of Blending Dimethyl Carbonate and Ethanol with Gasoline on Combustion Characteristics" Fuels 4, no. 4: 441-453. https://doi.org/10.3390/fuels4040027





