Mesoporous Adsorbents for Desulfurization of Model Diesel Fuel: Optimization, Kinetic, and Thermodynamic Studies
Abstract
:1. Introduction
2. Experimental
2.1. Materials and Chemicals
2.2. Preparation of Adsorbents
2.2.1. Synthesis of Mesoporous Al2O3 and Titanium Substituted Mesoporous Al2O3 Supports
2.2.2. Functionalization of Supports with Ethylenediamine
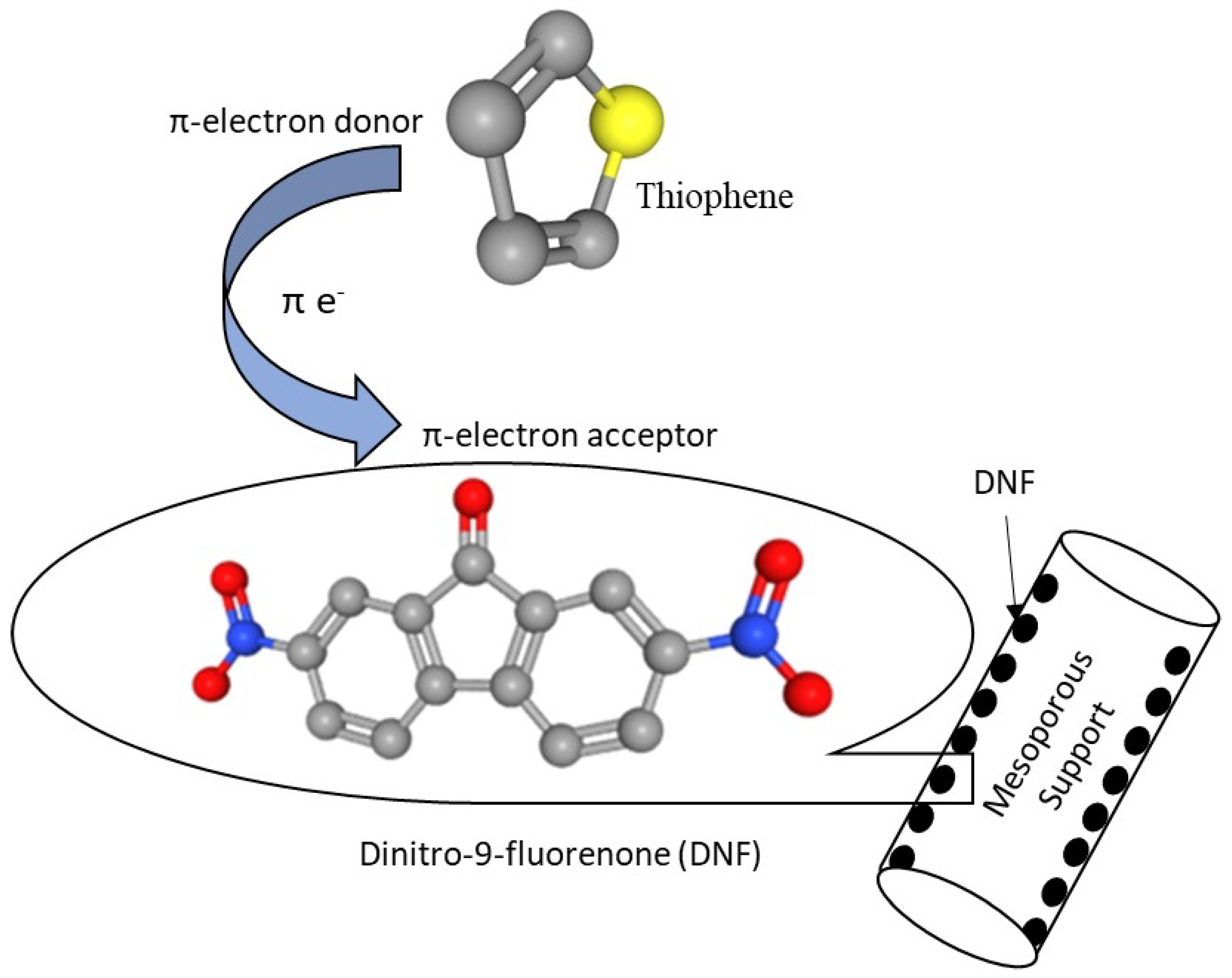
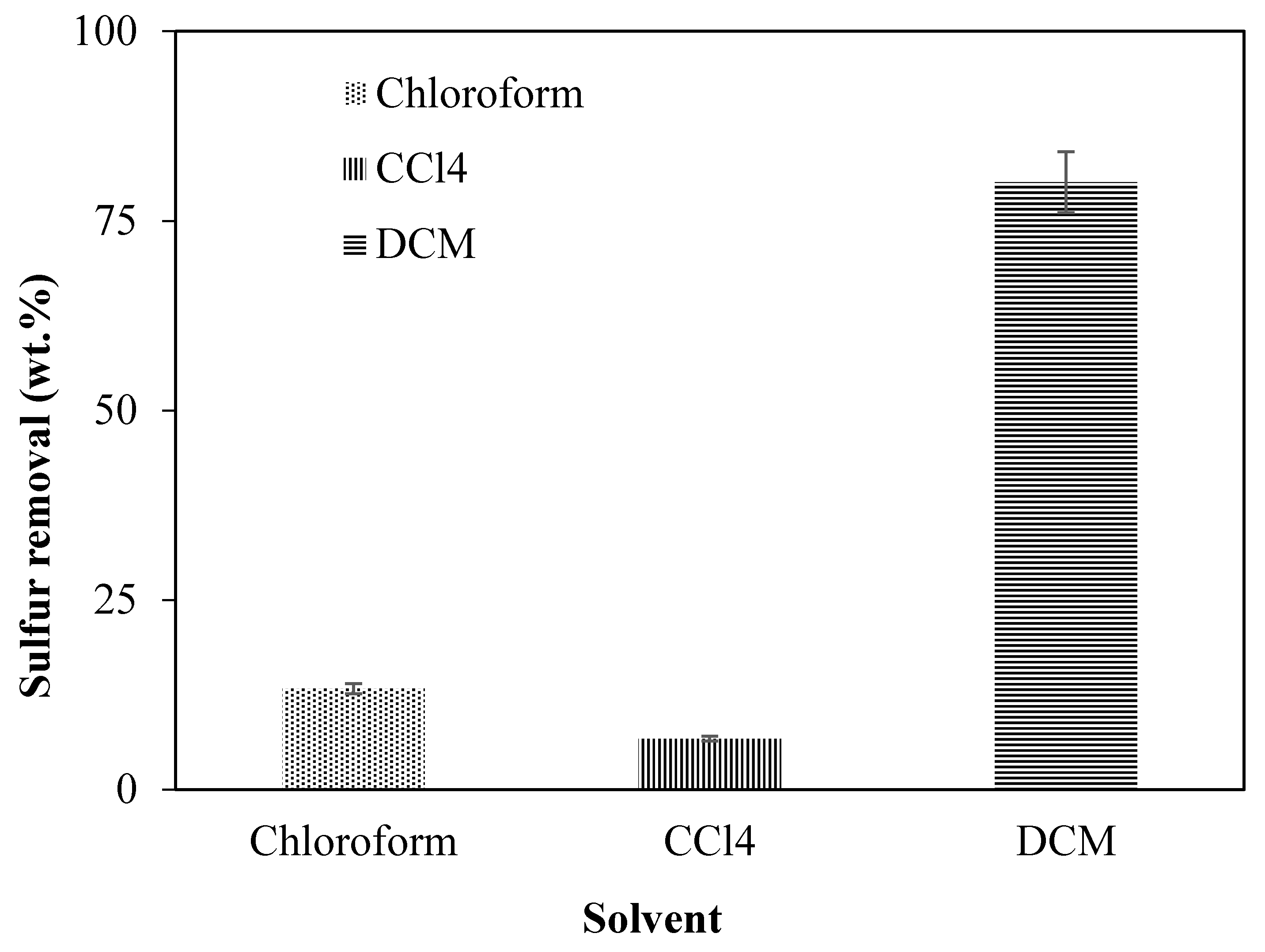
2.2.3. Incorporation of π-Acceptor on Supports
2.3. Process Parameter Optimization
2.4. Adsorption Kinetics
2.5. Adsorption Thermodynamics
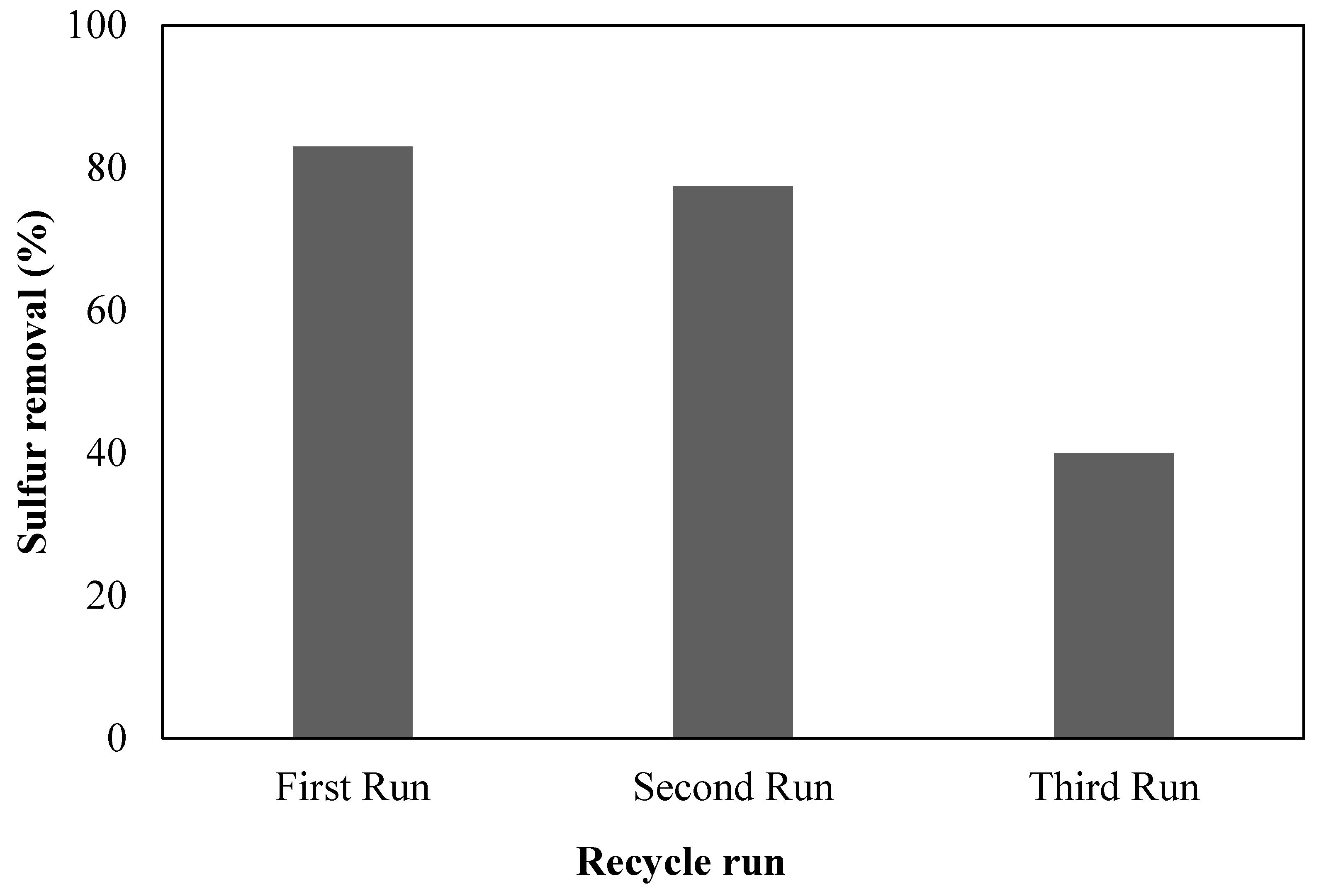
2.6. Regeneration and Reusability Studies
3. Result and Discussion
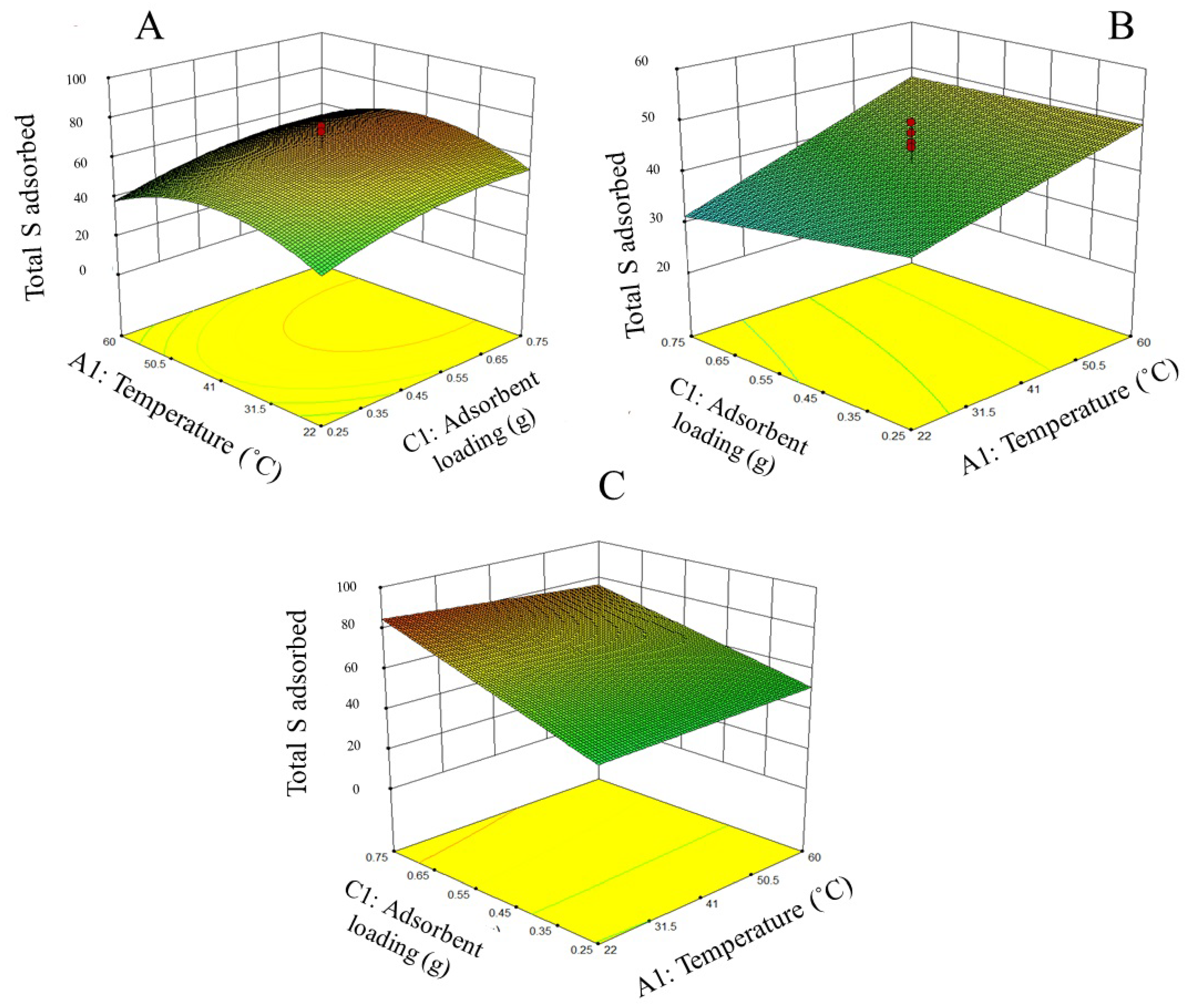
3.1. Adsorption Parameters Optimization
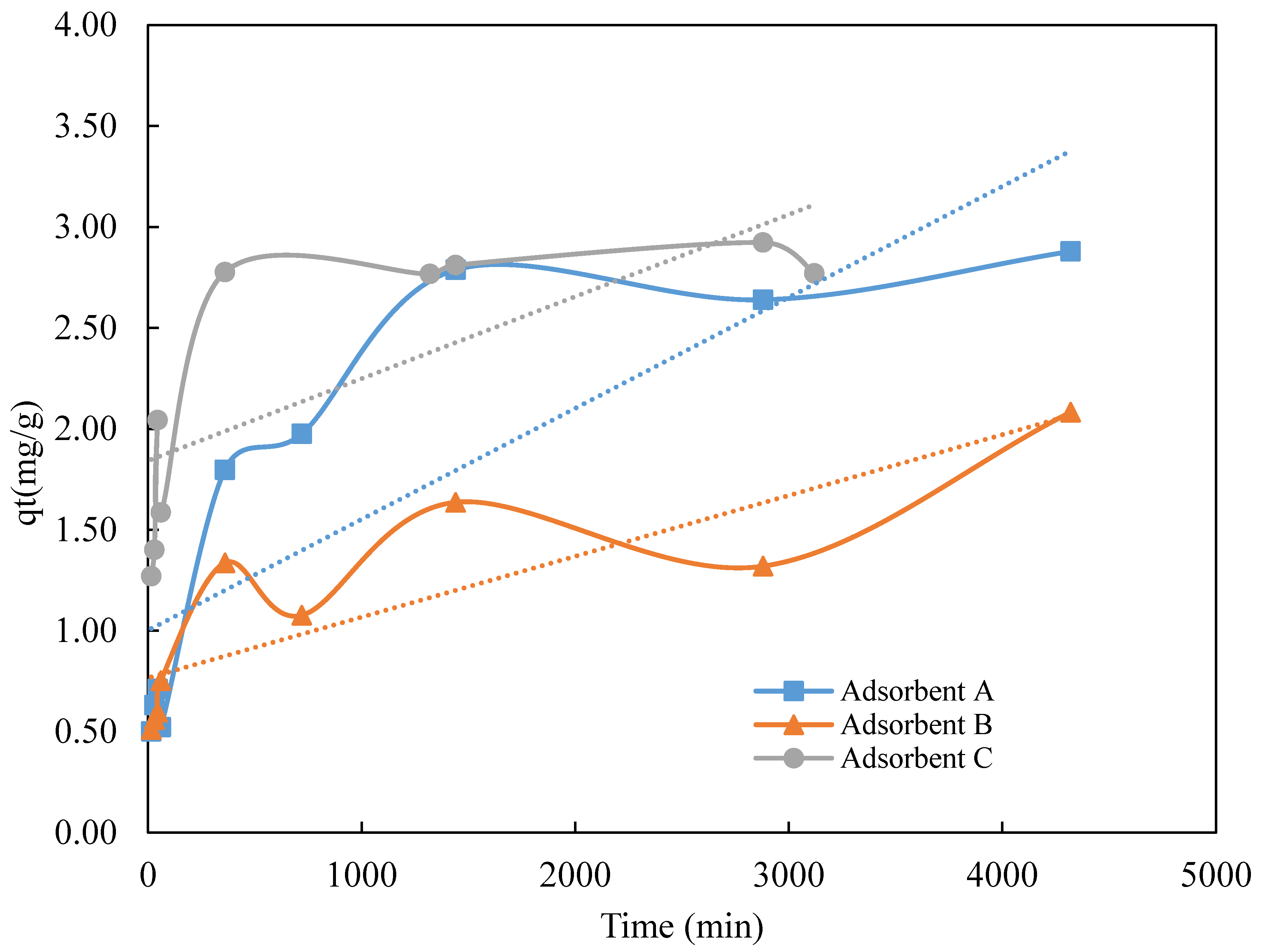
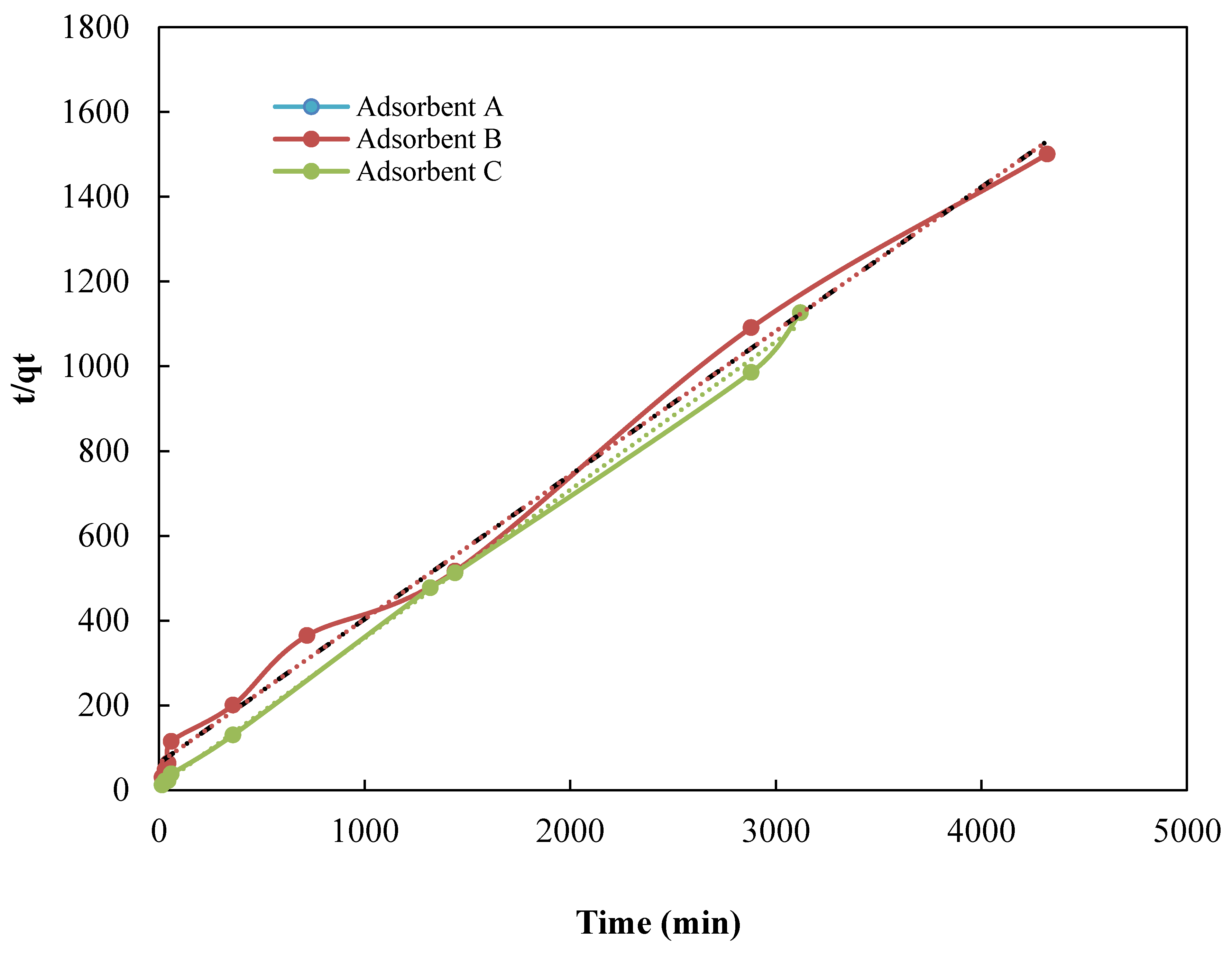
3.2. Kinetic Studies
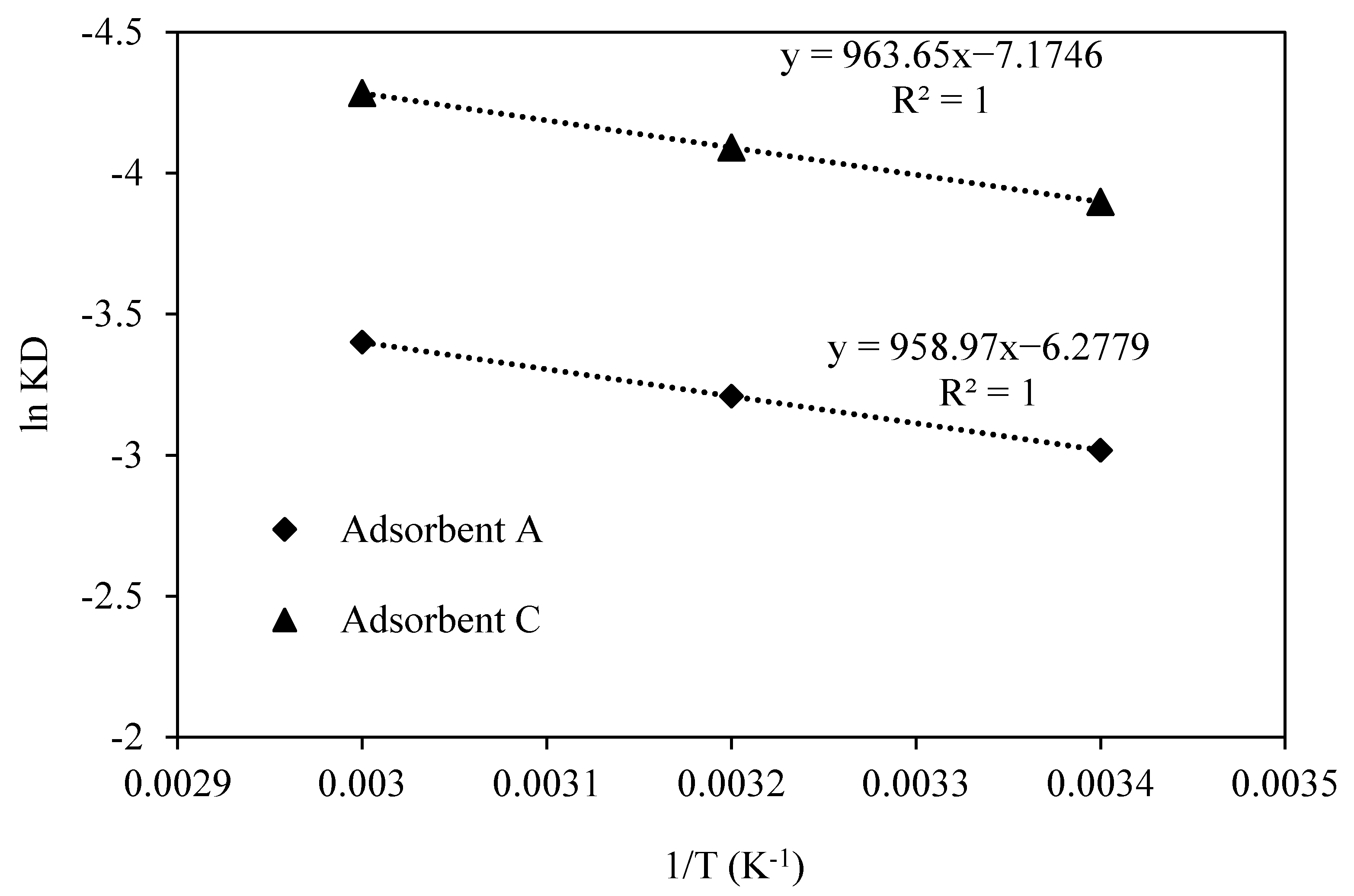
3.3. Adsorption Thermodynamic
4. Regeneration and Reusability
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Srivastava, V.C. An evaluation of desulfurization technologies for sulfur removal from liquid fuels. RSC Adv. 2012, 2, 759–783. [Google Scholar] [CrossRef]
- Choi, K.-H.; Kunisada, N.; Korai, Y.; Mochida, I.; Nakano, K. Facile ultra-deep desulfurization of gas oil through two-stage or -layer catalyst bed. Catal. Today 2003, 86, 277–286. [Google Scholar] [CrossRef]
- Da Costa, P.; Manoli, J.-M.; Potvin, C.; Djéga-Mariadassou, G. Deep HDS on doped molybdenum carbides: From probe molecules to real feedstocks. Catal. Today 2005, 107, 520–530. [Google Scholar] [CrossRef]
- Duan, F.; Chen, C.; Wang, G.; Yang, Y.; Liu, X.; Qin, Y. Efficient adsorptive removal of dibenzothiophene by graphene oxide-based surface molecularly imprinted polymer. RSC Adv. 2014, 4, 1469–1475. [Google Scholar] [CrossRef]
- Jafarinejad, S. Control and treatment of sulfur compounds specially sulfur oxides (SOx) emissions from the petroleum industry: A review. Chem. Int. 2016, 2, 242–253. [Google Scholar]
- Song, C. An overview of new approaches to deep desulfurization for ultra-clean gasoline, diesel fuel and jet fuel. Catal. Today 2003, 86, 211–263. [Google Scholar] [CrossRef]
- Ania, C.O.; Bandosz, T.J. Importance of Structural and Chemical Heterogeneity of Activated Carbon Surfaces for Adsorption of Dibenzothiophene. Langmuir 2005, 21, 7752–7759. [Google Scholar] [CrossRef]
- Misra, P.; Badoga, S.; Dalai, A.K.; Adjaye, J. Enhancement of sulfur and nitrogen removal from heavy gas oil by using polymeric adsorbent followed by hydrotreatment. Fuel 2018, 226, 127–136. [Google Scholar] [CrossRef]
- Santos, A.L.; Reis, R.A.; Rossa, V.; Reis, M.M.; Costa, A.L.H.; Veloso, C.D.O.; Henriques, C.A.; Zotin, F.M.; Paredes, M.L.; Silveira, E.B.; et al. Silica–alumina impregnated with cerium, nickel, and molybdenum oxides for adsorption of sulfur and nitrogen compounds from diesel. Mater. Lett. 2012, 83, 158–160. [Google Scholar] [CrossRef]
- Velu, S.; Song, C.; Fuels, C.; Program, C.; Engineering, G. Zeolite-based adsorbents for Desulfurization of Jet fuel by Selective Adsorption. Fuel Chem. 2002, 47, 447–448. [Google Scholar]
- Yitzhaki, D.; Landau, M.; Berger, D.; Herskowitz, M. Deep desulfurization of heavy atmospheric gas oil with CoMoAl catalysts effect of sulfur adsorption. Appl. Catal. A Gen. 1995, 122, 99–110. [Google Scholar] [CrossRef]
- Lee, S.-W.; Ryu, J.W.; Min, W. SK Hydrodesulfurization (HDS) Pretreatment Technology for Ultralow Sulfur Diesel (ULSD) Production. Catal. Surv. Asia 2003, 7, 271–279. [Google Scholar] [CrossRef]
- Hendricks, D.W. Water Treatment Unit Processes: Physical and Chemical; CRC Press: Boca Raton, FL, USA, 2006. [Google Scholar]
- De La Cruz, A.B.; Boahene, P.; Vedachalam, S.; Dalai, A.; Adjaye, J. Adsorptive desulfurization through charge-transfer complex using mesoporous adsorbents. Fuel 2020, 269, 117379. [Google Scholar] [CrossRef]
- Laidler, K.J. The development of the Arrhenius equation. J. Chem. Educ. 1984, 61, 494–498. [Google Scholar] [CrossRef]
- Field, J.A.; Sierra-Alvarez, R. Biodegradability of chlorinated solvents and related chlorinated aliphatic compounds. Rev. Environ. Sci. Bio Technol. 2004, 3, 185–254. [Google Scholar] [CrossRef]
- Crowson, R.; Hargrove, D.; Pout, C.R. Chlorine regeneration of platinum group metal zeolite catalysts. U.S. Patent 3,986,982, 19 October 1976. [Google Scholar]
- Okawa, K.; Suzuki, K.; Takeshita, T.; Nakano, K. Regeneration of granular activated carbon with adsorbed trichloroethylene using wet peroxide oxidation. Water Res. 2007, 41, 1045–1051. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, Y.; Luo, W.; Elzatahry, A.A.; Cheng, X.; Alghamdi, A.; Abdullah, A.M.; Deng, Y.; Zhao, D. Synthesis of Ordered Mesoporous Silica with Tunable Morphologies and Pore Sizes via a Nonpolar Solvent-Assisted Stöber Method. Chem. Mater. 2016, 28, 2356–2362. [Google Scholar] [CrossRef]
- Almarri, M.; Ma, X.; Song, C. Selective Adsorption for Removal of Nitrogen Compounds from Liquid Hydrocarbon Streams over Carbon- and Alumina-Based Adsorbents. Ind. Eng. Chem. Res. 2009, 48, 951–960. [Google Scholar] [CrossRef]
- Anirudhan, T.; Radhakrishnan, P. Thermodynamics and kinetics of adsorption of Cu(II) from aqueous solutions onto a new cation exchanger derived from tamarind fruit shell. J. Chem. Thermodyn. 2008, 40, 702–709. [Google Scholar] [CrossRef]
- Saadi, R.; Saadi, Z.; Fazaeli, R.; Fard, N.E. Monolayer and multilayer adsorption isotherm models for sorption from aqueous media. Korean J. Chem. Eng. 2015, 32, 787–799. [Google Scholar] [CrossRef]
- Fei, L.; Rui, J.; Wang, R.; Lu, Y.; Yang, X. Equilibrium and kinetic studies on the adsorption of thiophene and benzothiophene onto NiCeY zeolites. RSC Adv. 2017, 7, 23011–23020. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Yu, F.; Cheng, W.; Wang, J.; Ma, J. Adsorption Equilibrium and Kinetics of the Removal of Ammoniacal Nitrogen by Zeolite X/Activated Carbon Composite Synthesized from Elutrilithe. J. Chem. 2017, 2017, 1–9. [Google Scholar] [CrossRef]
- Foo, K.; Hameed, B. Insights into the modeling of adsorption isotherm systems. Chem. Eng. J. 2010, 156, 2–10. [Google Scholar] [CrossRef]
- Whalen, J.W. Physical chemistry of surfaces, fourth edition (Adamson, Arthur W.). J. Chem. Educ. 1983, 60, A322. [Google Scholar] [CrossRef] [Green Version]
| Variables | Symbol | Range | |
|---|---|---|---|
| Temperature (°C) | A1 | 22 | 60 |
| Time (min) | B1 | 15 | 60 |
| Adsorbent loading (g) | C1 | 0.25 | 0.75 |
| Run Number | Adsorbent Loading (g) | Temperature (°C) | Time (min) |
|---|---|---|---|
| 1 | 0.75 | 22 | 60 |
| 2 | 0.75 | 27 | |
| 3 | 0.25 | 60 | |
| 4 | 0.25 | 27 | |
| 5 | 0.75 | 60 | 60 |
| 6 | 0.75 | 27 | |
| 7 | 0.25 | 60 | |
| 8 | 0.25 | 27 | |
| 9 | 0.5 | 41 | 43 |
| 10 | 0.5 | 43 | |
| 11 | 0.5 | 71 | |
| 12 | 0.5 | 43 | |
| 13 | 0.5 | 43 | |
| 14 | 0.5 | 43 | |
| 15 | 0.5 | 15 | |
| 16 | 0.5 | 43 | |
| 17 | 0.92 | 43 | |
| 18 | 0.08 | 43 | |
| 19 | 0.5 | 73 | 43 |
| Adsorbent | Linear Equation |
|---|---|
| A | Total S removal = −5.37+0.17X + 111.04Y + 68.99Z − 0.60XY − 0.44XZ − 75.31YZ |
| B | Total S removal = 50.73 + 0.34X – 55.31Y – 19.13Z + 0.30XY – 0.17XZ + 48.46 YZ |
| C | Total S removal = 103.46 – 0.01X – 1.63Y – 104.90Z – 0.54XY + 0.27XZ + 116.77YZ |
| Adsorbent | qe (exp) (mg·g−1) | Pseudo-First Order Model | Pseudo-Second Order Model | ||||
|---|---|---|---|---|---|---|---|
| qe(cal) (mg·g−1) | K1 (min−1) | R2 | qe (cal) (mg·g−1) | K2 (g·mg−1·min−1) | R2 | ||
| A | 2.79 | −0.00112 | 3.5 × 10−4 | 0.39 | 1.01 | 1.59 × 10−2 | 0.99 |
| B | 2.08 | −0.00021 | 3.4 × 10−5 | 0.18 | 1.20 | 1.22 × 10−2 | 0.92 |
| C | 2.78 | −0.00117 | −9.0 × 10−4 | 0.33 | 1.03 | 9.21 × 10−3 | 0.99 |
| Adsorbent | Activation Energy (Ea) (kJ/mol) | ΔH (kJ/mol) | ΔS (J/mol K) |
|---|---|---|---|
| A | 8.27 | 15.5 | −85.2 |
| C | 8.29 | 15.3 | −77.1 |
| Temperature (K) | ΔG (kJ/mol) | |
|---|---|---|
| Adsorbent A | Adsorbent C | |
| 295 | 9.62 | 7.45 |
| 318 | 10.08 | 7.94 |
| 333 | 10.56 | 8.37 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Botana-de la Cruz, A.; Boahene, P.E.; Vedachalam, S.; Dalai, A.K.; Adjaye, J. Mesoporous Adsorbents for Desulfurization of Model Diesel Fuel: Optimization, Kinetic, and Thermodynamic Studies. Fuels 2020, 1, 47-58. https://doi.org/10.3390/fuels1010005
Botana-de la Cruz A, Boahene PE, Vedachalam S, Dalai AK, Adjaye J. Mesoporous Adsorbents for Desulfurization of Model Diesel Fuel: Optimization, Kinetic, and Thermodynamic Studies. Fuels. 2020; 1(1):47-58. https://doi.org/10.3390/fuels1010005
Chicago/Turabian StyleBotana-de la Cruz, Anakaren, Philip E. Boahene, Sundaramurthy Vedachalam, Ajay K. Dalai, and John Adjaye. 2020. "Mesoporous Adsorbents for Desulfurization of Model Diesel Fuel: Optimization, Kinetic, and Thermodynamic Studies" Fuels 1, no. 1: 47-58. https://doi.org/10.3390/fuels1010005




