Abstract
Human kin recognition activates substrates of the extended facial processing network, notably the right-hemisphere structures involved in self-face recognition and posterior medial cortical substrates. To understand the mechanisms underlying prosociality toward kin faces in comparison to other familiar faces, we investigated the neural correlates of implicit trustworthiness ratings to faces of actual kin and personal friends, controlling for activation to distracter faces. When controlling for activation associated with unknown faces, trustworthiness ratings of faces of kin, compared to friends, were associated with increased activation in the dorsal anterior cingulate cortex, posterior cingulate, and precuneous. On the other hand, trustworthiness ratings of friend faces, relative to kin faces, were associated with the lateral occipital gyrus and insular cortex. Trustworthiness ratings for unknown faces were only associated with activation in the fusiform gyrus. These findings suggest that we should employ medial cortical substrates known to be part of the self-other network when making implicit social judgements about kin, but not other classes of facial stimuli.
1. Introduction
Kin variably evoke a myriad of emotions such as love, irritation, trust, frustration, and altruism, among others. Kin selection theory [1] posits that organisms should differentially be altruistic toward and trust kin relative to non-kin based on the notion that any investment provides returns in the form of genetic representation in subsequent generations, or helping those genes that are shared among kin. Recent studies utilized modern techniques to further understand kin selection theory [2,3,4,5,6,7,8]. Because incorrect assignment/perception of kinship can have dire consequences (inbreeding depression, cuckoldry, deception), proximate (neural) mechanisms designed to detect and respond preferentially toward kin are predicted to have evolved [1,2,8]. Kin discrimination mechanisms allow individuals to modify their behavior with respect to genetic relatedness [9,10] and have been shown to impact a variety of behaviors [9,10,11,12,13,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29] in a variety of organisms, including humans.
Impression formation on the basis of facial processing occurs very quickly [30], and implicit trustworthiness judgements have been associated with activation in the limbic system, particularly the amygdala [31,32,33]. These important social judgements appear to occur in the absence of deep processing [34,35,36]. Several studies have demonstrated negative associations between trustworthiness ratings and activation in medial temporal lobe structures (including the amygdala, uncus, fusiform gyrus, and parahippocampal gyrus) [32,33]. The amygdala has been hypothesized to be involved in automatic face coding with respect to impression management and is predicted to drive social responses, appetitive or aversive, toward individuals. Similarly, several studies have demonstrated that attractive faces activate the neural substrates involved in reward processing [37,38,39,40] and that the amygdala encodes and/or processes the social valence and salience of a face based on important social information such as attractiveness and self-resemblance [41,42,43].
The proximate neural mechanisms associated with viewing faces of kin, relative to friends and strangers, has recently been documented [21,22,41,44,45]. Platek and Kemp [22] showed that activation to kin faces shared similar activation patterns when compared to participants viewing their own face (right parietal lobe), as well as activation in the posterior cingulate and precuneous. Another study showed that self-resembling faces, a putative proxy for kin faces, were associated with activation in ventral frontal reward substrates when regressed with trustworthiness ratings [46]. To date, no study has investigated the relationship between trustworthiness ratings and faces of actual kin. Here we regress ratings of trustworthiness on activation to faces of kin, friends, and unknown individuals in order to identify the proximate neural substrates that putatively drive implicit social judgements about kin vis-à-vis friends and unknown faces.
2. Materials and Methods
2.1. Participants/Stimuli
Ten volunteers (M age = 26.2; 5 female) were recruited from a university in northwest England. All subjects provided written informed consent. The local committee on research ethics approved the study.
Upon agreeing to participate, each subject provided a digital photograph of a family member (kin face) and a close personal friend (friend face) via one of two methods: subjects either came to the lab with their friend and family member to have the pictures taken by the researchers, or subjects submitted a photograph of their friend or family member to the researchers. For those who opted for the latter method, participants were provided with explicit instructions on how to take and deliver (e-mail) the photographs. All photographs that were provided by participants were inspected for quality and lighting and discarded if they did not meet the strict requirements for lighting consistency, brightness, and size; the participant was then asked to send an additional photograph(s) to replace any inadequate ones. All participants were able to comply with the instructions and the final photographs used met the standards of the researcher and another inspector. All images were standardized for orientation (face tilt) and interpupillary distance. Additionally, two independent raters were asked to identify qualitative differences in the pictures and were unable to identify reliable differences in the photographic quality of the images. This procedure entailed sorting through all of the images and selecting images that they thought did not meet the study requirements or that were glaringly inconsistent when compared to other images in the group. If an independent rater identified a picture that was qualitatively different, they were asked to indicate what aspect of the face appeared unusual. Any photographs that were determined to be unusual resulted in the experimenter asking participants to provide another, different photo. This procedure was repeated until the raters no longer identified that image as being unusual. This happened for two sets of family photos. Participants did not report any differences in emotional valence to the photos, nor did the independent raters. All participants reported submission of photos of people that they liked, or were fond of. Length of acquaintance was not measured, but would be a good area for future research. Photos compiled from a freely available face database were used as unknown faces [47]. At the completion of participation, participants were asked if they recognized any of the unknown faces. No participant reported having recognized any of the unknown faces.
2.2. fMRI Acquisition
During scanning, participants were asked to make perceptual discriminations about whether the face was familiar or unfamiliar. No explicit instructions for how to scan the face were given. For each participant, one run/session of 205 volumes consisting of 42 interleaved slices (TR = 2.5, TE = 30(ms), FoV = 19.2 cm, flip angle = 85) were collected at 3 Tesla on a Siemens (Cambridge, UK) Trio scanner. The time of acquisition varied as stimuli were presented in a randomized, jittered fashion with variable null interstimulus intervals that ranged from 20% to 40% null events. Each image was presented 20 times. A high-resolution structural MP-RAGE scan was also collected and was used to coregister functional images.
Prior to analysis, all functional echo planar images (EPIs) were preprocessed using FSL-FEAT’s preprocessing tools. In short, the images were realigned and corrected for head movement and motion, normalized for intensity, and smoothed with a Gaussian kernel FWHM 6 mm3. Functional images were then statistically processed using FEAT-FLAME (part of the FSL package distribution [48,49,50,51]. Initially, we computed first-level analysis for the contrasts of each face class stimulus versus the baseline, which results in four contrast images per experiment: self vs. null, family vs. null, friend vs. null, and unknown vs. null [22].
2.3. Behavioral Procedures
Approximately four weeks after scanning, participants rated each face they saw during scanning for trustworthiness. Trustworthiness ratings for the difference between trust rating for kin face versus friend face, and vice versa, controlling for ratings of unknown/distractor faces (D), were then calculated using the equation:
where K is the trustworthiness rating for kin faces, Fr is the trustworthiness rating for friend faces, and D is the trustworthiness rating for distractor faces. In order to show the effect for friend faces, the signs were reversed. We call this variable the calculation for relative trustworthiness for kin (CTfa) and friend (CTfr), respectively. In order to create a similar score for trustworthiness ratings to unknown faces, we modified the equation as follows:
which represents the mean trustworthiness ratings to unknown faces when controlling for each of kin and friend faces (CTun).
2.4. fMRI Trustworthiness Analysis
Each CT score was parametrically regressed on first-level analyses using FEAT [48,50,51]. Mixed-effects modeling was employed using FSL-FLAME methods and the statistical parametric threshold was set to uncorrected p < 0.005.
3. Results
Ratings of trustworthiness to kin faces (CTfa) were associated with increased activation in the right superior parietal lobe (38, −36, 52; z = 4.47), precuneous (12, −48, 46; z = 3.67), posterior cingulate gyrus (6, −42, 28; z = 2.61), dorsal anterior cingulate gyrus (2, −8, 44; z = 3.54), and right superior frontal gyrus (22, 16, 44; z = 3.71) (see Figure 1).
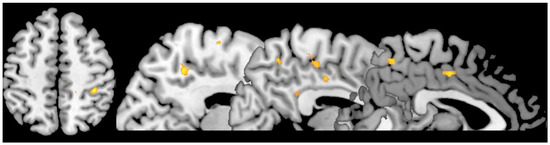
Figure 1.
Statistical parametric maps showing activation to the CTfa condition (uncorrected, p < 0.005, see text for coordinates and details).
Ratings of trustworthiness of friend faces (CTfr) were associated with increased activation in the left insular cortex (−32, 18, −12; z = 3.10), left parahippocampal gyrus (−16, −28, −14; z = 2.77), and left lateral occipital cortex (−42, −70, 36; z = 3.23), but not the posterior cingulate or dorsal anterior cingulate (see Figure 2a). Ratings of trustworthiness of unknown faces (CTun) were only associated with increased activation in the left inferior lateral occipital/fusiform gyrus (−0, −62, −4; z = 4.22) (Figure 2b).
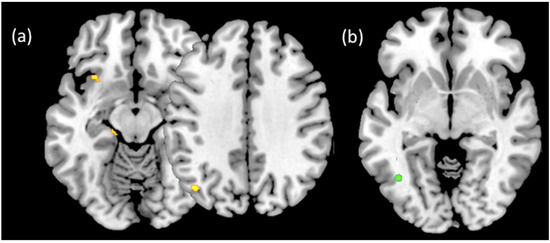
Figure 2.
Statistical parametric maps showing activation associated with increased trustworthiness ratings in the (a) CTfr and (b) CTun conditions (uncorrected, p < 0.005; see text for coordinates and details).
4. Discussion
Several studies have demonstrated negative associations between trustworthiness ratings and activation in the medial temporal lobe (e.g., amygdala, uncus, parahippocampal gyrus [32,33]) to unknown or unfamiliar faces. The amygdala appears to be involved in automatic face coding with respect to impression management [30], and is predicted to drive social responses toward individuals [32]. Similarly, several studies have demonstrated that attractive faces activate neural substrates implicated in reward processing [38,39,40,52], as do self-resembling faces [22], a putative proxy for kin faces. Here we found that implicit judgments of trustworthiness toward actual kin faces relative to friend faces activated medial cortical substrates including the precuneous, and anterior and posterior cingulate gyri. Medial cortical substrates have been implicated in self-information processing [21,22,25,46,53,54,55,56].
Self-face resemblance has been shown to increase ratings of attractiveness and trust [13,57,58]. The current findings extend these behavioral studies by showing that the affectively regulated prosocial behavior toward self-resembling faces is mediated by proximate neural substrates located in the medial portions of the cortex that respond parametrically to implicit social judgements about actual faces of kin. Thus, the self-processing network that resides in the medial portions of the cortex—including, but not limited to the precuneous, anterior and posterior cingulate gyri, and medial prefrontal gyrus—appears to be a critical neural network involved in differentiating how allocation of trust is expressed to varying classes of faces/individuals. It appears that as kin faces tap and activate neural substrates that are also commonly activated in response to self-information processing (e.g., self-face recognition), self-referential phenotypic matching may occur. If such self-referential phenotypic matching takes the form of matching kin to a neurocognitively stored template [15], then these findings suggest that the more a face activates self-referent substrates, the more trust ought to be allocated to such individuals.
In fact, two studies support that claim. Krill and Platek [59] asked participants to play the social exclusion game CyberBall with three classes of faces: self-resembling, same race, and other race. They showed that as self-reference decreased, so too did the level of activation and self-reported ‘upset’ during the game. In particular, while the self-resembling and same-race face activated regions of the anterior cingulate gyrus, a putative neural alarm center, in response to social exclusion, the other-race face (i.e., least resembling and least likely to match a stored self-referent template) did not. In other words, participants felt the greatest sense of betrayal by being excluded by faces that possibly tapped their neurocognitively stored self-referent phenotypic match.
Platek and Krill [42] showed a possible perceptual effect that mediates such a neurocognitively held self-referent phenotypic matching. They investigated activation in the amygdala, specifically, to faces that shared varying degrees of self-resemblance. They showed people a same-race self-resembling face, a same-race non-self-resembling face, an other-race self-resembling face (using a facial transformation technique), and an other-race non-self-resembling face. Activation in the bilateral amygdala revealed a nonlinear pattern; i.e., the amygdala was most activated by same-race self-resembling faces and other-race non-self-resembling faces. Platek and Krill argue that same-race self-resembling faces and other-race non-self-resembling faces anchor the end points of a theoretical valence-based distribution for self-referent phenotypic matching. In others words, people who resemble you and share your race are highly likely to be your kin, while people who do not look like you and do not share your race are least likely to be your kin. Interestingly, they showed that same-race non-self-resembling and other-race self-resembling faces showed similar and lower levels of activation in the amygdala, suggesting a neural trade-off between cues to group membership that include race and self-resemblance perceptions.
Because the amygdala and medial cortical substrates are highly connected, it could be the case that increased arousal to self-resembling and kin faces starts a cascade of activations aimed at showing increased levels of altruism, trust, etc. On the other hand, faces that do not activate arousal substrates in this way do not start this cascade and thus are not subject to the same degree of appetitive behavioral interaction. The mechanism of this hypothesis may be increases in affiliative hormones such as oxytocin [60,61] that might be mediated by a variety of ontogenetic experiences [62]. If our hypothesis is correct, it would suggest that variability in response to kin and non-kin, but not familiar faces, could be modulated by emotional expression, among other things. This, of course, is a matter of conjecture and requires further research.
This research is not without its limitations. For example, asking participants to provide images of their family and friends presents the possibility that the images of individuals that occupied a particularly positive place in participants’ evaluative process were more likely to be included. Additionally, it would have been ideal to ask participants to provide a number of images of different family members and friends so that a random selection of the faces could have been used as stimuli. This might have alleviated some bias toward selecting stimuli that were associated a priori with participants’ positive attributions (it is also possible that an array of images that were all positively biased might have been provided by the participants). Ideally, images of family members and friends would have been taken without the participant’s knowledge, thus randomly controlling for valence about the individual. Unfortunately, that was not possible. Participants were not instructed to bring a photo of someone they liked/disliked, but simply to provide an image of someone who fit the requisite criteria. However, no participant self-reported having a negative feeling about either the family member or friend whose photograph they provided. An additional limitation is the lack of images that varied with respect to genealogical distribution. Ideally, a set of images that represented several points of genetic relatedness would have been preferred to allow for modeling of the coefficient of relatedness. This could be a ripe area for future research. Participants were not asked how long they knew the family member or familiar face. Length of acquaintance could impact levels of trust and variably activate the proposed social arousal network we are proposing. To our knowledge, no one has tested the effects of length of acquaintance on the neural correlates of familiarity of faces. This could be a very exciting area of future research on familiar face processing. Participants were also not given any instructions on how to scan the face—i.e., what part to look at first, second, and so forth. This might be particularly interesting when measuring this response if participants are known to scan faces differently, such as those with autism. Typically, developing children and adults tend to begin the scan of a face by looking at the eyes. In contrast, children and adults with autism tend to scan a face by first looking at the mouth. How, or if, this would have an effect on trustworthiness ratings is unknown. Lastly, the sample size was small, making this a 1) brief and 2) preliminary report on this finding. Here we investigated the link between kinship, friendship, and social judgement. Future investigations should involve larger sample sizes that vary in terms of genealogical and ethnic/cultural makeup.
Recent findings suggest cultural variance in self‒other representation with respect to trait adjective judgments [45]. While this is an area that requires further research in light of the current findings, our sample was entirely Western. The characterization of a similar response in non-Western cultures would be fascinating, but is beyond the scope of this paper.
5. Conclusions
In conclusion, it appears that implicit prosocial judgments about kin, compared to friends and unknown individuals, is associated with activation in medial substrates that are known to be associated with self-related processing. A recent imaging study [63] demonstrated that activation to self-resembling faces interacts with structural facial characteristics that predict ratings of (un)trustworthiness. Interestingly, they showed that faces that resembled subjects by approximately 50% activated medial cortical substrates [21]. This is consistent with a series of investigations showing that medial cortical substrates are implicated in responding to self-resemblance and the hypothesis that these areas may be associated with making important kin-based and sociality-based judgments about how to react to such individuals. The current findings add to this growing area of research by showing that implicit ratings of trustworthiness of faces that approximate kin, compared to nonkin, are evaluated at a socially important level.
Author Contributions
Conceptualization, S.M.P.; methodology, S.M.P. Formal analysis, S.M.P. and J.C.H. writing—original draft preparation, writing—review and editing, S.M.P. and J.C.H. funding acquisition, S.M.P.
Funding
This research was funded by The Pioneer Fund, Inc. via a small investigative research grant to SMP.
Acknowledgments
The authors would like to thank Georgia Gwinnett College for support during this research. Also, the authors thank the MARIARC center MRI technicians for their assistance with data collection.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Hamilton, W. The genetical evolution of social behaviour. II. J. Theor. Biol. 1964, 7, 17–52. [Google Scholar] [CrossRef]
- Bourke, A.F.G. Hamilton’s rule and the causes of social evolution. Philos. Trans. R. Soc. B Biol. Sci. 2014, 369, 20130362. [Google Scholar] [CrossRef] [PubMed]
- Gardner, A.; West, S.; Wild, G. The genetical theory of kin selection. J. Evol. Biol. 2011, 24, 1020–1043. [Google Scholar] [CrossRef] [PubMed]
- Gorrell, J.C.; McAdam, A.G.; Coltman, D.W.; Humphries, M.M.; Boutin, S. Adopting kin enhances inclusive fitness in asocial red squirrels. Nat. Commun. 2010, 1, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Leadbeater, E.; Carruthers, J.M.; Green, J.P.; Rosser, N.S.; Field, J. Nest Inheritance Is the Missing Source of Direct Fitness in a Primitively Eusocial Insect. Science 2011, 333, 874–876. [Google Scholar] [CrossRef] [PubMed]
- Linksvayer, T.; Wade, M. Theoretical predictions for sociogenomic data: The effects of kin selection and sex-limited expression on the evolution of social insect genomes. Front. Ecol. Evol. 2016, 4, 65. [Google Scholar] [CrossRef]
- Warner, M.; Mikheyev, A.; Linksvayer, T. Genomic signature of kin selection in an ant with obligately sterile workers. Mol. Biol. Evol. 2017, 34, 1780–1787. [Google Scholar] [CrossRef]
- West, S.A.; Gardner, A. Adaptation and Inclusive Fitness. Curr. Biol. 2013, 23, R577–R584. [Google Scholar] [CrossRef]
- Charpentier, M.; Crawford, J.; Boulet, M.; Drea, C. Message ‘scent’: Lemurs detect the genetic relatedness and quality of conspecifics via olfactory cues. Anim. Behav. 2010, 80, 101–108. [Google Scholar] [CrossRef]
- Lehmann, L.; Perrin, N. Altruism, Dispersal, and Phenotype-Matching Kin Recognition. Am. Nat. 2002, 159, 451–468. [Google Scholar] [CrossRef]
- Daly, M.; Wilson, M.I. Whom are newborn babies said to resemble? Evol. Hum. Behav. 1982, 3, 69–78. [Google Scholar] [CrossRef]
- Daly, M.; Wilson, M.I. The Truth about Cinderella: A Darwinian View of Parental Love; Yale University Press: New Haven, CT, USA, 1998. [Google Scholar]
- DeBruine, L.M. Facial resemblance enhances trust. Proc. R. Soc. Lond. Ser. B Biol. Sci. 2002, 269, 1307–1312. [Google Scholar] [CrossRef] [PubMed]
- Debruine, L.; Jones, B.; Perrett, D. Women’s attractiveness judgments of self-resembling faces change across the menstrual cycle. Horm. Behav. 2005, 47, 379–383. [Google Scholar] [CrossRef] [PubMed]
- Hauber, M.E.; Sherman, P.W. Self-referent phenotype matching: theoretical considerations and empirical evidence. Trends Neurosci. 2001, 24, 609–616. [Google Scholar] [CrossRef]
- Lacy, R.C.; Sherman, P.W. Kin Recognition by Phenotype Matching. Am. Nat. 1983, 121, 489–512. [Google Scholar] [CrossRef]
- Lieberman, D.; Tooby, J.; Cosmides, L. The architecture of human kin detection. Nature 2007, 445, 727–731. [Google Scholar] [CrossRef]
- Neff, B.D.; Sherman, P.W. Decision making and recognition mechanisms. Proc. R. Soc. B Biol. Sci. 2002, 269, 1435–1441. [Google Scholar] [CrossRef] [PubMed]
- Platek, S.M. Unconcious reactions to children’s faces: the effects of resemblance. Evol. Cogn. 2002, 8, 207–214. [Google Scholar]
- Platek, S.M.; Raines, D.M.; Gallup, J.G.G.; Mohamed, F.B.; Thomson, J.W.; Myers, T.E.; Panyavin, I.S.; Levin, S.L.; Davis, J.A.; Fonteyn, L.C.M.; et al. Reactions to children’s faces: Males are more affected by resemblance than females are, and so are their brains. Evol. Hum. Behav. 2004, 25, 394–405. [Google Scholar] [CrossRef]
- Platek, S.M.; Krill, A.L.; Kemp, S.M. The neural basis of facial resemblance. Neurosci. Lett. 2008, 437, 76–81. [Google Scholar] [CrossRef]
- Platek, S.M.; Kemp, S.M. Is family special to the brain? An event-related fMRI study of familiar, familial, and self-face recognition. Neuropsychologia 2009, 47, 849–858. [Google Scholar] [CrossRef] [PubMed]
- Platek, S.M.; Burch, R.L.; Panyavin, I.S.; Wassserman, B.H., Jr.; Gallup, G.G. Reactions towards children’s faces: Resemblance matters more for females than males. Evol. Hum. Behav. 2002, 23, 159–166. [Google Scholar] [CrossRef]
- Platek, S.M.; Critton, R.L.; Burch, R.L.; Frederick, D.A.; Myers, T.E., Jr.; Gallup, G.G. How much paternal resemblance is enough? Sex differences in the reaction to resemblance but not in ability to detect resemblance. Evol. Hum. Behav. 2003, 24, 81–87. [Google Scholar] [CrossRef]
- Platek, S.M.; Keenan, J.P.; Mohamed, F.B. Sex differences in the neural correlates of child facial resemblance: An event-related fMRI study. NeuroImage 2005, 25, 1336–1344. [Google Scholar] [CrossRef] [PubMed]
- Rushton, J.P.; Russell, R.J.H.; Wells, P.A. Genetic similarity theory: Beyond kin selection. Behav. Genet. 1984, 14, 179–193. [Google Scholar] [CrossRef] [PubMed]
- Strassmann, J.; Owen, G.; Queller, D. Kin discrimination and cooperation in microbes. Annu. Rev. Microbiol. 2011, 65, 349–367. [Google Scholar] [CrossRef] [PubMed]
- Volk, A.; Quinsey, V.L. The influence of infant facial cues on adoption preferences. Hum. Nat. 2002, 13, 437–455. [Google Scholar] [CrossRef]
- Volk, A.A.; Quinsey, V.L. Parental Investment and Resemblance: Replications, Refinements, and Revisions. Evol. Psychol. 2007, 5, 1–14. [Google Scholar] [CrossRef]
- Willis, J.; Todorov, A. First impressions: Making up your mind after a 100-ms exposure to a face. Psychol. Sci. 2006, 17, 592–598. [Google Scholar] [CrossRef]
- Bzdok, D.; Langner, R.; Hoffstaedter, F.; Turetsky, B.; Zilles, K.; Eickhoff, S. The modular neuroarchitecture of social judgements on faces. Cereb. Cortex 2011, 22, 951–961. [Google Scholar] [CrossRef]
- Engell, A.D.; Haxby, J.V.; Todorov, A. Implicit Trustworthiness Decisions: Automatic Coding of Face Properties in the Human Amygdala. J. Cogn. Neurosci. 2007, 19, 1508–1519. [Google Scholar] [CrossRef] [PubMed]
- Winston, J.; Strange, B.; O’Doherty, J.; Dolan, R.; Strange, B. Automatic and intentional brain responses during evaluation of trustworthiness of faces. Nat. Neurosci. 2002, 5, 277–283. [Google Scholar] [CrossRef] [PubMed]
- Bargh, J.; Schwader, K.; Hailey, S.; Dyer, R.; Boothby, E. Automaticity in social-cognitive processes. Trends Cogn. Sci. 2012, 16, 593–605. [Google Scholar] [CrossRef] [PubMed]
- Bargh, J.A.; Chartrand, T.L. The unbearable automaticity of being. Am. Psychol. 1999, 54, 462–479. [Google Scholar] [CrossRef]
- Hassin, R.; Uleman, J.; Bargh, J. The new unconscious; Oxford University Press: New York, NY, USA, 2005. [Google Scholar]
- O’Doherty, J.; Kringelbach, M.L.; Rolls, E.T.; Hornak, J.; Andrews, C.; Kringelbach, M.; Rolls, E. Abstract reward and punishment representations in the human orbitofrontal cortex. Nat. Neurosci. 2001, 4, 95–102. [Google Scholar] [CrossRef]
- O’Doherty, J.; Winston, J.; Critchley, H.; Perrett, D.; Burt, D.; Dolan, R.; Burt, M. Beauty in a smile: The role of medial orbitofrontal cortex in facial attractiveness. Neuropsychologia 2003, 41, 147–155. [Google Scholar] [CrossRef]
- Tsukiura, T.; Cabeza, R. Remembering beauty: Roles of orbitofrontal and hippocampal regions in successful memory encoding of attractive faces. Neuroimage 2011, 54, 653–660. [Google Scholar] [CrossRef]
- Winston, J.S.; O’Doherty, J.; Kilner, J.M.; Perrett, D.I.; Dolan, R.J. Brain systems for assessing facial attractiveness. Neuropsychologia 2007, 45, 195–206. [Google Scholar] [CrossRef]
- Bartels, A.; Zeki, S. The neural correlates of maternal and romantic love. Neuroimage 2004, 21, 1155–1166. [Google Scholar] [CrossRef]
- Platek, S.M.; Krill, A.L. Self-face resemblance attenuates other-race face effect in the amygdala. Brain Res. 2009, 1284, 156–160. [Google Scholar] [CrossRef]
- Santos, A.; Mier, D.; Kirsch, P.; Meyer-Lindenberg, A. Evidence for a general face salience signal in human amygdala. Neuroimage 2011, 54, 3111–3116. [Google Scholar] [CrossRef] [PubMed]
- Bornstein, M.; Aterberry, M.; Mash, C. Differentiated brain activity in response to faces of “own” versus “unfamiliar” babies in primipara mothers: An electrophysiological study. Dev. Neuropsychol. 2013, 38, 365–385. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Q.; Song, Y.; Hu, S.; Li, X.; Tian, M.; Zhen, Z.; Dong, Q.; Kanwisher, N.; Liu, J. Heritability of the Specific Cognitive Ability of Face Perception. Curr. Biol. 2010, 20, 137–142. [Google Scholar] [CrossRef] [PubMed]
- Platek, S.M.; Krill, A.L.; Wilson, B. Implicit trustworthiness ratings of self-resembling faces activate brain centers involved in reward. Neuropsychologia 2009, 47, 289–293. [Google Scholar] [CrossRef] [PubMed]
- Minear, M.; Park, D.C. A lifespan database of adult facial stimuli. Behav. Res. Methods Instrum. Comput. 2004, 36, 630–633. [Google Scholar] [CrossRef] [PubMed]
- Beckmann, C.F.; Jenkinson, M.; Smith, S.M. General multilevel linear modeling for group analysis in FMRI. Neuroimage 2003, 20, 1052–1063. [Google Scholar] [CrossRef]
- Critchley, H.D.; Tang, J.; Glaser, D.; Butterworth, B.; Dolan, R.J. Anterior cingulate activity during error and autonomic response. Neuroimage 2005, 27, 885–895. [Google Scholar] [CrossRef] [PubMed]
- Woolrich, M.W.; Behrens, T.E.; Beckmann, C.F.; Jenkinson, M.; Smith, S.M. Multilevel linear modelling for FMRI group analysis using Bayesian inference. Neuroimage 2004, 21, 1732–1747. [Google Scholar] [CrossRef] [PubMed]
- Worsley, K.J.; Evans, A.C.; Marrett, S.; Neelin, P. A Three-Dimensional Statistical Analysis for CBF Activation Studies in Human Brain. Br. J. Pharmacol. 1992, 12, 900–918. [Google Scholar] [CrossRef] [PubMed]
- Aharon, I.; Etcoff, N.; Ariely, D.; Chabris, C.F.; O’Connor, E.; Breiter, H.C. Beautiful faces have variable reward value: fMRI and behavioral evidence. Neuron 2001, 32, 537–551. [Google Scholar]
- Gusnard, D.A.; Akbudak, E.; Shulman, G.L.; Raichle, M.E. Medial prefrontal cortex and self-referential mental activity: Relation to a default mode of brain function. Proc. Natl. Acad. Sci. USA 2001, 98, 4259–4264. [Google Scholar] [CrossRef] [PubMed]
- Jenkins, A.; Mitchell, J. Medial prefrontal cortex subserves diverse forms of self-reflection. Soc. Neurosci. 2010, 6, 211–218. [Google Scholar] [CrossRef] [PubMed]
- Lou, H.C.; Luber, B.; Crupain, M.; Keenan, J.P.; Nowak, M.; Kjaer, T.W.; Sackeim, H.A.; Lisanby, S.H. Parietal cortex and representation of the mental Self. Proc. Natl. Acad. Sci. USA 2004, 101, 6827–6832. [Google Scholar] [CrossRef] [PubMed]
- Vogeley, K.; Bussfeld, P.; Newen, A.; Herrmann, S.; Happe, F.; Falkai, P.; Maier, W.; Shah, N.J.; Fink, G.R.; Zilles, K. Mind Reading: Neural Mechanisms of Theory of Mind and Self-Perspective. Neuroimage 2001, 14, 170–181. [Google Scholar] [CrossRef] [PubMed]
- Kocsor, F.; Rezneki, R.; Juhasz, S.; Bereczkei, T. Preference for facial self-resemblance and attractiveness in human mate choice. Arch. Sex. Behav. 2011, 40, 1263–1270. [Google Scholar] [CrossRef] [PubMed]
- Platek, S.M.; Thomson, J.W. Facial Resemblance Exaggerates Sex-Specific Jealousy-Based Decisions. Evol. Psychol. 2007, 5, 223–231. [Google Scholar] [CrossRef]
- Krill, A.; Platek, S.M. In-Group and Out-Group Membership Mediates Anterior Cingulate Activation to Social Exclusion. Front. Evol. Neurosci. 2009, 1, 1. [Google Scholar] [CrossRef] [PubMed]
- Hrdy, S. Mothers and Others: The Evolutionary Origins of Mutual Understanding; Belknap Press of Harvard University Press: Cambridge, MA, USA, 2009. [Google Scholar]
- Riem, M.; Bakermans-Kranenburg, M.; Pieper, S.; Tops, M.; Boksem, M.; Vermeiren, R.; van Ijzendoorn, M.; Rombouts, S. Oxytocin modulates amygdala, insula, and inferior frontal gyrus responses to infant crying: A randomized controlled trial. Biol. Psychiatry 2011, 70, 291–297. [Google Scholar] [CrossRef] [PubMed]
- Cameron, N.M.; Shahrokh, D.; Del Corpo, A.; Dhir, S.K.; Szyf, M.; Champagne, F.A.; Meaney, M.J.; Cameron, N. Epigenetic Programming of Phenotypic Variations in Reproductive Strategies in the Rat Through Maternal Care. J. Neuroendocr. 2008, 20, 795–801. [Google Scholar] [CrossRef] [PubMed]
- Verosky, S.C.; Todorov, A. Differential neural responses to faces physically similar to the self as a function of their valence. NeuroImage 2010, 49, 1690–1698. [Google Scholar] [CrossRef] [PubMed]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).