Effects of Intensity-Specific Acute Exercise on Paired-Associative Memory and Memory Interference
Abstract
1. Introduction
2. Methods
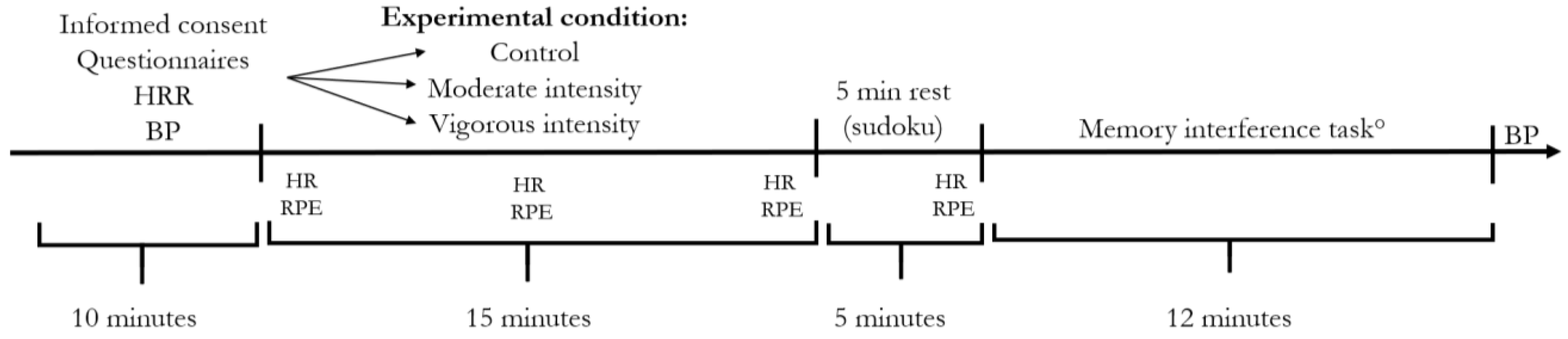
2.1. Study Design
2.2. Participants
2.3. Experimental Conditions
2.4. Memory
- Participants studied set 1, which included eight noun-pairs, half containing interference pairs (AB). Following this study period, they completed a 20-s arithmetic distractor task.
- Participants then completed Cued Recall 1, which involved the cued recall of Study Set 1. Participants were exposed to the stem word (and a blank) and were required to recall its pair. Following this cued recall, they completed a 20-s arithmetic distractor task.
- Participants then studied Set 2, which included eight noun-pairs, half of them containing interference pairs (AC) from Set 1, with the other half being new noun-pairs (FG). Following this study period, they completed a 20-s arithmetic distractor task.
- Participants then completed Cued Recall 2, which involved the cued recall of Study Set 2. Participants were exposed to the stem word (and a blank) and were required to recall its pair. Following this cued recall, they completed a 20-s arithmetic distractor task.
- Participants then completed a modified–modified free recall (MMFR), which involved the cued recall of Set 1 and Set 2. Since some of the words (A words) were paired with two different words (B and C words), participants were instructed to recall both words, if they could recall both. From the MMFR cued recall test, Proactive Interference (PI) was calculated by subtracting the percentage of FG pairs recalled from the percentage of AC pairs recalled. From the MMFR cued recall test, Retroactive Interference (RI) was calculated by subtracting the percentage of DE pairs recalled from the percentage of AB pairs recalled. Higher PI and RI scores are indicators of proactive and retroactive facilitation, and thus, correspond with decreased memory interference. That is, a lower PI and RI score indicate a greater (worse, unfavorable) memory interference effect.
3. Statistical Analysis
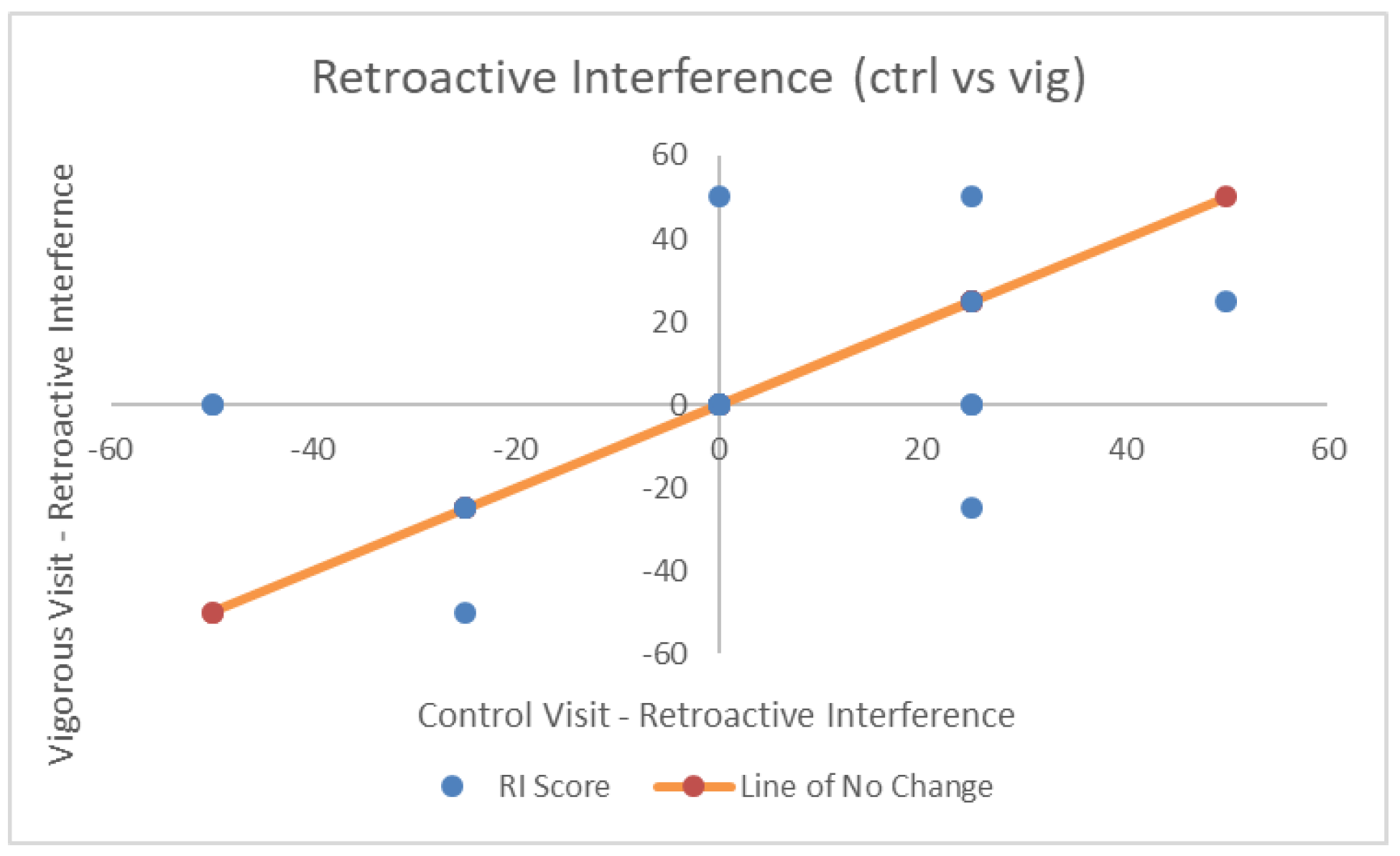
4. Results
5. Discussion
Author Contributions
Funding
Conflicts of Interest
References
- Frith, E.; Addoh, O.; Mann, J.R.; Windham, B.G.; Loprinzi, P.D. Individual and Combined Associations of Cognitive and Mobility Limitations on Mortality Risk in Older Adults. Mayo. Clin. proc. 2017, 92, 1494–1501. [Google Scholar] [CrossRef]
- Bird, C.M.; Burgess, N. The hippocampus and memory: Insights from spatial processing. Nat. rev. Neurosci. 2008, 9, 182–194. [Google Scholar] [CrossRef]
- Weilbacher, R.A.; Gluth, S. The Interplay of Hippocampus and Ventromedial Prefrontal Cortex in Memory-Based Decision Making. Brain sci. 2016, 7, 4. [Google Scholar] [CrossRef] [PubMed]
- Tulving, E. Elements of Episodic Memory; Oxford University Press: Oxford, UK, 1983. [Google Scholar]
- Baddeley, A. Working memory and language: An overview. J. Commun. Disord. 2003, 36, 189–208. [Google Scholar] [CrossRef]
- Warburton, D.E.R.; Bredin, S.S.D. Health benefits of physical activity: A systematic review of current systematic reviews. Curr. Opin. Cardiol. 2017, 32, 541–556. [Google Scholar] [CrossRef]
- Loprinzi, P.D.; Herod, S.M.; Cardinal, B.J.; Noakes, T.D. Physical activity and the brain: A review of this dynamic, bi-directional relationship. Brain Res. 2013, 1539, 95–104. [Google Scholar] [CrossRef] [PubMed]
- Ponce, P.; Loprinzi, P.D. A bi-directional model of exercise and episodic memory function. Med. Hypotheses 2018, 117, 3–6. [Google Scholar] [CrossRef]
- Sng, E.; Frith, E.; Loprinzi, P.D. Temporal Effects of Acute Walking Exercise on Learning and Memory Function. Am. J. Health Promot. 2017. [Google Scholar] [CrossRef] [PubMed]
- Loprinzi, P.D.; Edwards, M.K.; Frith, E. Potential avenues for exercise to activate episodic memory-related pathways: A narrative review. Eur. J. Neurosci. 2017, 46, 2067–2077. [Google Scholar] [CrossRef] [PubMed]
- Siddiqui, A.; Loprinzi, P.D. Experimental Investigation of the Time Course Effects of Acute Exercise on False Episodic Memory. J. Clin. Med. 2018, 7, 157. [Google Scholar] [CrossRef]
- Haynes Iv, J.T.; Frith, E.; Sng, E.; Loprinzi, P.D. Experimental Effects of Acute Exercise on Episodic Memory Function: Considerations for the Timing of Exercise. Psychol. Rep. 2018. [Google Scholar] [CrossRef] [PubMed]
- Frith, E.; Sng, E.; Loprinzi, P. Randomized Controlled Trial Considering Varied Exercises for Reducing Proactive Memory Interference. J. Clin. Med. 2018, 7, 147. [Google Scholar] [CrossRef] [PubMed]
- Haynes, J.; Loprinzi, P. Acute cardiovascular exercise on proactive memory interference. J. Cogn. Enhanc. 2018, 3, 139–143. [Google Scholar] [CrossRef]
- Chang, Y.K.; Labban, J.D.; Gapin, J.I.; Etnier, J.L. The effects of acute exercise on cognitive performance: A meta-analysis. Brain Res. 2012, 1453, 87–101. [Google Scholar] [CrossRef] [PubMed]
- Lambourne, K.; Tomporowski, P. The effect of exercise-induced arousal on cognitive task performance: A meta-regression analysis. Brain Res. 2010, 1341, 12–24. [Google Scholar] [CrossRef]
- McMorris, T.; Hale, B.J. Differential effects of differing intensities of acute exercise on speed and accuracy of cognition: a meta-analytical investigation. Brain Cogn. 2012, 80, 338–351. [Google Scholar] [CrossRef]
- Roig, M.; Nordbrandt, S.; Geertsen, S.S.; Nielsen, J.B. The effects of cardiovascular exercise on human memory: A review with meta-analysis. Neurosci. Biobehav. Rev. 2013, 37, 1645–1666. [Google Scholar] [CrossRef] [PubMed]
- McMorris, T.; Sproule, J.; Turner, A.; Hale, B.J. Acute, intermediate intensity exercise, and speed and accuracy in working memory tasks: A meta-analytical comparison of effects. Physiol. Behav. 2011, 102, 421–428. [Google Scholar] [CrossRef]
- Loprinzi, P.D. Intensity-specific effects of acute exercise on human memory function: Considerations for the timing of exercise and the type of memory. Health Promot. Perspect. 2018, 8, 255–262. [Google Scholar] [CrossRef]
- Loprinzi, P.D.; Ponce, P.; Frith, E. Hypothesized mechanisms through which acute exercise influences episodic memory. Physiol. Int. 2018, 105, 285–297. [Google Scholar] [CrossRef]
- Poo, M.M.; Pignatelli, M.; Ryan, T.J.; Tonegawa, S.; Bonhoeffer, T.; Martin, K.C.; Rudenko, A.; Tsai, L.H.; Tsien, R.W.; Fishell, G.; et al. What is memory? The present state of the engram. BMC Biol. 2016, 14, 40. [Google Scholar] [CrossRef]
- Audiffren, M. Acute exercise and psychological functions: A cognitive-energetic approach. Exerc. Cogn. Funct. 2009, 3–39. [Google Scholar] [CrossRef]
- Noble, E.E.; Mavanji, V.; Little, M.R.; Billington, C.J.; Kotz, C.M.; Wang, C. Exercise reduces diet-induced cognitive decline and increases hippocampal brain-derived neurotrophic factor in CA3 neurons. Neurobiol. Learn. Mem. 2014, 114, 40–50. [Google Scholar] [CrossRef] [PubMed]
- Davis, R.L.; Zhong, Y. The Biology of Forgetting-A Perspective. Neuron 2017, 95, 490–503. [Google Scholar] [CrossRef] [PubMed]
- Osgood, C.E. An investigation into the causes of retroactive interference. J. Exp. Psychol. 1948, 38, 132–154. [Google Scholar] [CrossRef] [PubMed]
- Shimamura, A.P.; Jurica, P.J.; Mangels, J.A.; Gershberg, F.B.; Knight, R.T. Susceptibility to Memory Interference Effects following Frontal Lobe Damage: Findings from Tests of Paired-Associate Learning. J. Cogn. Neurosci. 1995, 7, 144–152. [Google Scholar] [CrossRef]
- Bridge, D.J. Memory & Cognition: What Difference Does Gender Make? Available online: https://surface.syr.edu/honors_capstone/655/ (accessed on 25 May 2019).
- Erickson, K.I.; Leckie, R.L.; Weinstein, A.M. Physical activity, fitness, and gray matter volume. Neurobiol. Aging 2014, 35. [Google Scholar] [CrossRef]
- Samrani, G.; Bäckman, L.; Persson, J. Age-differences in the temporal properties of proactive interference in working memory. Psychol. Aging 2017, 32, 722–731. [Google Scholar] [CrossRef] [PubMed]
- Bennett, R. Proactive interference in short-term memory: Fundamental forgetting processes. J. Verbal Learn. Verbal Behav. 1975, 123–144. [Google Scholar] [CrossRef]
- Hung, C.L.; Tseng, J.W.; Chao, H.H.; Hung, T.M.; Wang, H.S. Effect of Acute Exercise Mode on Serum Brain-Derived Neurotrophic Factor (BDNF) and Task Switching Performance. J. Clin. Med. 2018, 7, 301. [Google Scholar] [CrossRef]
- Siddiqui, S.V.; Chatterjee, U.; Kumar, D.; Siddiqui, A.; Goyal, N. Neuropsychology of prefrontal cortex. Indian J. Psychiatry 2008, 50, 202–208. [Google Scholar]
- Guise, K.G.; Shapiro, M.L. Medial Prefrontal Cortex Reduces Memory Interference by Modifying Hippocampal Encoding. Neuron 2017, 94, 183–192. [Google Scholar] [CrossRef]
- Ahmed, O.J.; Mehta, M.R. Running speed alters the frequency of hippocampal gamma oscillations. J. Neurosci. 2012, 32, 7373–7383. [Google Scholar] [CrossRef]
- Zheng, J.; Stevenson, R.F.; Mander, B.A.; Mnatsakanyan, L.; Hsu, F.P.K.; Vadera, S.; Knight, R.T.; Yassa, M.A.; Lin, J.J. Multiplexing of Theta and Alpha Rhythms in the Amygdala-Hippocampal Circuit Supports Pattern Separation of Emotional Information. Neuron 2019, 102, 887–898. [Google Scholar] [CrossRef] [PubMed]
- Thomas, A.G.; Dennis, A.; Bandettini, P.A.; Johansen-Berg, H. The effects of aerobic activity on brain structure. Front. Psychol. 2012. [Google Scholar] [CrossRef] [PubMed]
- Loprinzi, P.D.; Frith, E.; Edwards, M.K.; Sng, E.; Ashpole, N. The Effects of Exercise on Memory Function Among Young to Middle-Aged Adults: Systematic Review and Recommendations for Future Research. Am. J. Health Promot. 2018, 32, 691–704. [Google Scholar] [CrossRef] [PubMed]
- Sudo, M.; Komiyama, T.; Aoyagi, R.; Nagamatsu, T.; Higaki, Y.; Ando, S. Executive function after exhaustive exercise. Eur. J. Appl. Physiol. 2017, 117, 2029–2038. [Google Scholar] [CrossRef] [PubMed]
- Endo, K.; Matsukawa, K.; Liang, N.; Nakatsuka, C.; Tsuchimochi, H.; Okamura, H.; Hamaoka, T. Dynamic exercise improves cognitive function in association with increased prefrontal oxygenation. J. Physiol. Sci. 2013, 63, 287–298. [Google Scholar] [CrossRef]
- McNerney, M.W.; Radvansky, G.A. Mind racing: The influence of exercise on long-term memory consolidation. Memory 2015, 23, 1140–1151. [Google Scholar] [CrossRef]
- Delancey, D.; Frith, E.; Sng, E.; Loprinzi, P.D. Randomized controlled trial examining the long-term memory effects of acute exercise during the memory consolidation stage of memory. J. Cogn. Enhance. 2018, 1–6. [Google Scholar] [CrossRef]
- Frith, E.; Sng, E.; Loprinzi, P.D. Randomized controlled trial evaluating the temporal effects of high-intensity exercise on learning, short-term and long-term memory, and prospective memory. Eur. J. Neurosci. 2017, 46, 2557–2564. [Google Scholar] [CrossRef]
- Sng, E.; Frith, E.; Loprinzi, P.D. Temporal Effects of Acute Walking Exercise on Learning and Memory Function. Am. J. Health Promot. 2018, 32, 1518–1525. [Google Scholar] [CrossRef] [PubMed]
- Jubelt, L.E.; Barr, R.S.; Goff, D.C.; Logvinenko, T.; Weiss, A.P.; Evins, A.E. Effects of transdermal nicotine on episodic memory in non-smokers with and without schizophrenia. Psychopharmacology (Berl) 2008, 199, 89–98. [Google Scholar] [CrossRef] [PubMed]
- Klaming, R.; Annese, J.; Veltman, D.J.; Comijs, H.C. Episodic memory function is affected by lifestyle factors: A 14-year follow-up study in an elderly population. Neuropsychol. Dev. Cogn. Sect. B Aging Neuropsychol. Cogn. 2017, 24, 528–542. [Google Scholar] [CrossRef]
- Henry, J.D.; Rendell, P.G. A review of the impact of pregnancy on memory function. J. Clin. Exp. Neuropsychol. 2007, 29, 793–803. [Google Scholar] [CrossRef]
- Labban, J.D.; Etnier, J.L. Effects of acute exercise on long-term memory. Res. Q. Exerc. Sport 2011, 82, 712–721. [Google Scholar] [CrossRef]
- Sherman, S.M.; Buckley, T.P.; Baena, E.; Ryan, L. Caffeine Enhances Memory Performance in Young Adults during Their Non-optimal Time of Day. Front. Psychol. 2016, 7, 1764. [Google Scholar] [CrossRef] [PubMed]
- Wammes, J.D.; Good, T.J.; Fernandes, M.A. Autobiographical and episodic memory deficits in mild traumatic brain injury. Brain Cogn. 2017, 111, 112–126. [Google Scholar] [CrossRef]
- Ilieva, I.P.; Hook, C.J.; Farah, M.J. Prescription Stimulants’ Effects on Healthy Inhibitory Control, Working Memory, and Episodic Memory: A Meta-analysis. J. Cogn. Neurosci. 2015, 27, 1069–1089. [Google Scholar] [CrossRef] [PubMed]
- Hindocha, C.; Freeman, T.P.; Xia, J.X.; Shaban, N.D.C.; Curran, H.V. Acute memory and psychotomimetic effects of cannabis and tobacco both ‘joint’ and individually: A placebo-controlled trial. Psychol. Med. 2017, 47, 2708–2719. [Google Scholar] [CrossRef]
- Le Berre, A.P.; Fama, R.; Sullivan, E.V. Executive Functions, Memory, and Social Cognitive Deficits and Recovery in Chronic Alcoholism: A Critical Review to Inform Future Research. Alcohol. Clin. Exp. Res. 2017, 41, 1432–1443. [Google Scholar] [CrossRef] [PubMed]
- Loprinzi, P.D.; Edwards, M.K.; Frith, E. Exercise and Prospective Memory. J. Lifestyle Med. 2018, 8, 51–59. [Google Scholar] [CrossRef]
- Wade, B.; Loprinzi, P.D. The Experimental Effects of Acute Exercise on Long-Term Emotional Memory. J. Clin. Med. 2018, 7, 486. [Google Scholar] [CrossRef]
- Wingate, S.; Crawford, L.; Frith, E.; Loprinzi, P.D. Experimental investigation of the effects of acute exercise on memory interference. Health Promot. Perspect. 2018, 8, 208–214. [Google Scholar] [CrossRef] [PubMed]
- Clinic, M. Exercise Intensity: How to Measure It. Available online: https://www.mayoclinic.org/healthy-lifestyle/fitness/in-depth/exercise-intensity/art-20046887 (accessed on 15 February 2019).
- Garber, C.E.; Blissmer, B.; Deschenes, M.R.; Franklin, B.A.; Lamonte, M.J.; Lee, I.M.; Nieman, D.C.; Swain, D.P. Quantity and quality of exercise for developing and maintaining cardiorespiratory, musculoskeletal, and neuromotor fitness in apparently healthy adults: Guidance for prescribing exercise. Med. Sci. Sports Exerc. 2011, 43, 1334–1359. [Google Scholar] [CrossRef]
- Litt, R.A.; Wang, H.C.; Sailah, J.; Badcock, N.A.; Castles, A. Paired associate learning deficits in poor readers: The contribution of phonological input and output processes. Q. J. Exp. Psychol. (Hove) 2019, 72, 616–633. [Google Scholar] [CrossRef] [PubMed]
- Elvevåg, B.; Egan, M.F.; Goldberg, T.E. Paired-associate learning and memory interference in schizophrenia. Neuropsychologia 2000, 38, 1565–1575. [Google Scholar] [CrossRef]
- Iodice, R.; Meilan, J.J.G.; Ramos, J.C.; Small, J.A. Sentence Context and Word-Picture Cued-Recall Paired-Associate Learning Procedure Boosts Recall in Normal and Mild Alzheimer’s Disease Patients. Behav. Neurol. 2018, 2018, 7401465. [Google Scholar] [CrossRef]
- Ebert, P.L.; Anderson, N.D. Proactive and retroactive interference in young adults, healthy older adults, and older adults with amnestic mild cognitive impairment. J. Int. Neuropsychol. Soc. 2009, 15, 83–93. [Google Scholar] [CrossRef]
- Curiel, R.E.; Crocco, E.; Acevedo, A.; Duara, R.; Agron, J.; Loewenstein, D.A. A New Scale for the Evaluation of Proactive and Retroactive Interference in Mild Cognitive Impairment and Early Alzheimer’s Disease. J. Aging Sci. 2013, 1. [Google Scholar] [CrossRef]
- Barnes, J.M.; Underwood, B.J. Fate of first-list associations in transfer theory. J. Exp. Psychol. 1959, 58, 97–105. [Google Scholar] [CrossRef] [PubMed]
- Burton, R.L.; Lek, I.; Caplan, J.B. Associative independence revisited: Competition between conflicting associations can be resolved or even reversed in one trial. Q. J. Exp. Psychol. (Hove) 2017, 70, 832–857. [Google Scholar] [CrossRef]
- Lee, S.Y.; Song, X.Y. Evaluation of the Bayesian and Maximum Likelihood Approaches in Analyzing Structural Equation Models with Small Sample Sizes. Multivar. Behav. Res. 2004, 39, 653–686. [Google Scholar] [CrossRef] [PubMed]
- Hox, J.; Van de Schoot, R.; Matthijsse, S. How few countries will do? Comparative survey analysis from a Bayesian perspective. Surv. Res. Methods 2012, 6, 87–93. [Google Scholar]
- Jarosz, A.F.; Wiley, J. What Are the Odds? A Practical Guide to Computing and Reporting Bayes Factors. J. Probl. Solving 2014, 7, 1932–6246. [Google Scholar] [CrossRef]
- Mandolesi, L.; Polverino, A.; Montuori, S.; Foti, F.; Ferraioli, G.; Sorrentino, P.; Sorrentino, G. Effects of Physical Exercise on Cognitive Functioning and Wellbeing: Biological and Psychological Benefits. Front. Psychol. 2018, 9, 509. [Google Scholar] [CrossRef]
- Ferguson, L.; Cantrelle, J.; Loprinzi, P.D. Experimental effects of exercise on forgetting. OBM Integr. Complement. Med. 2018, 3, 034. [Google Scholar] [CrossRef]
- Loprinzi, P.D.; Frith, E. A brief primer on the mediational role of BDNF in the exercise-memory link. Clin. Physiol. Funct. Imaging 2018. [Google Scholar] [CrossRef]
| Study Set 1: AB, DE | Cued Recall 1: A__, D__ | Study Set 2: AC, FG | Cued Recall 2: A__, F__ | MMFR: A__ __ D__ __ F__ __ |
|---|---|---|---|---|
| Blouse Nickel | Pepper ________ | Clothing House | Jacket _______ | Blouse ____ ____ |
| Paint Bread | Clothing ________ | Jacket Wheel | Clothing ______ | Chalk ____ ____ |
| Onion Bottle | Blouse ________ | Blouse Bridge | Mirror______ | Jacket ____ ____ |
| Pepper Cloud | Chalk ________ | Mirror Zipper | Paint _______ | Paint ____ ____ |
| Button Phone | Button ________ | Button Breakfast | Sister _______ | Mirror ____ ____ |
| Clothing Steak | Paint _______ | Sister Wallet | Liquid _______ | Onion ___ ___ |
| Chalk Pillow | Brain ________ | Liquid Plant | Blouse _______ | Button ____ ____ |
| Brain Orange | Onion ______ | Paint Dollar | Button ______ | Sister ____ _____ |
| Pepper ____ ____ | ||||
| Clothing ____ ____ | ||||
| Brain ____ ____ | ||||
| Liquid ____ ____ |
| Variable | Point Estimate | SD | |
|---|---|---|---|
| Gender, female (%) | 68.4 | - | |
| Age, mean years | 20.32 | 1.41 | |
| Race, Non-Hispanic White (%) | 78.9 | - | |
| Race, Non-Hispanic Black (%) | 15.8 | - | |
| Race, Mexican American (%) | 5.3 | - | |
| BMI, mean kg/m2 | 24.25 | 4.66 | |
| Average min/week of exercise | 231.37 | 25.96 |
| Control (n = 19) | Moderate, 50% HHR (n = 19) | Vigorous, 80% HHR (n = 19) | ||||
|---|---|---|---|---|---|---|
| Variable | Mean | SD | Mean | SD | Mean | SD |
| Heart rate at rest, bpm | 69.5 | 11.8 | 69.4 | 14.7 | 71.6 | 12.4 |
| Heart rate at 7.5 min, bpm | - | - | 134.6 | 8.0 | 172.9 | 7.2 |
| Heart rate at 15 min, bpm | - | - | 135.8 | 9.2 | 176.6 | 5.9 |
| Heart rate 5 min post, bpm | 72.3 | 12.4 | 84.0 | 14.2 | 91.9 | 16.8 |
| Control | Moderate | Vigorous | Results | |
|---|---|---|---|---|
| Variable | Mean (SD) | Mean (SD) | Mean (SD) | |
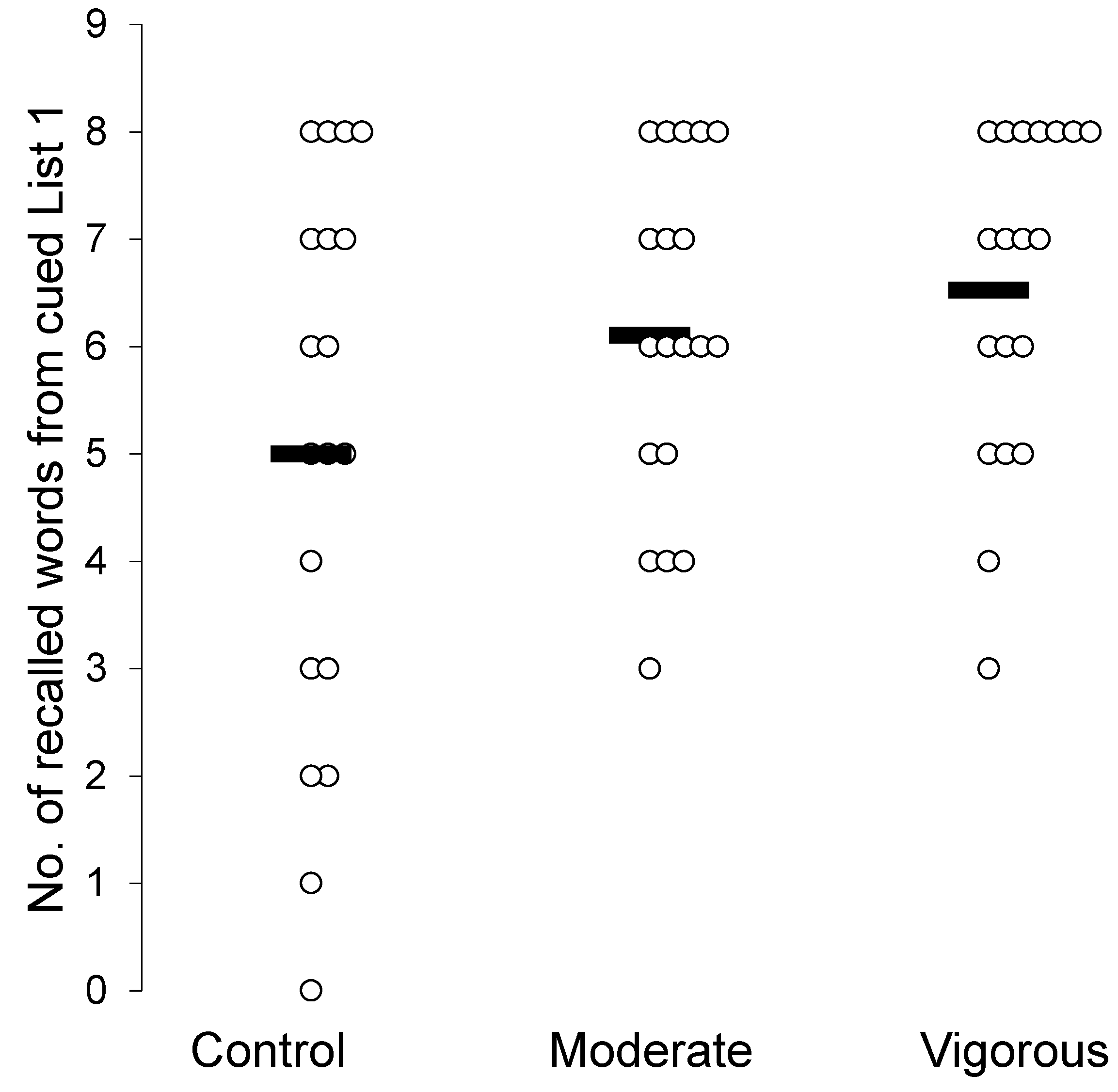
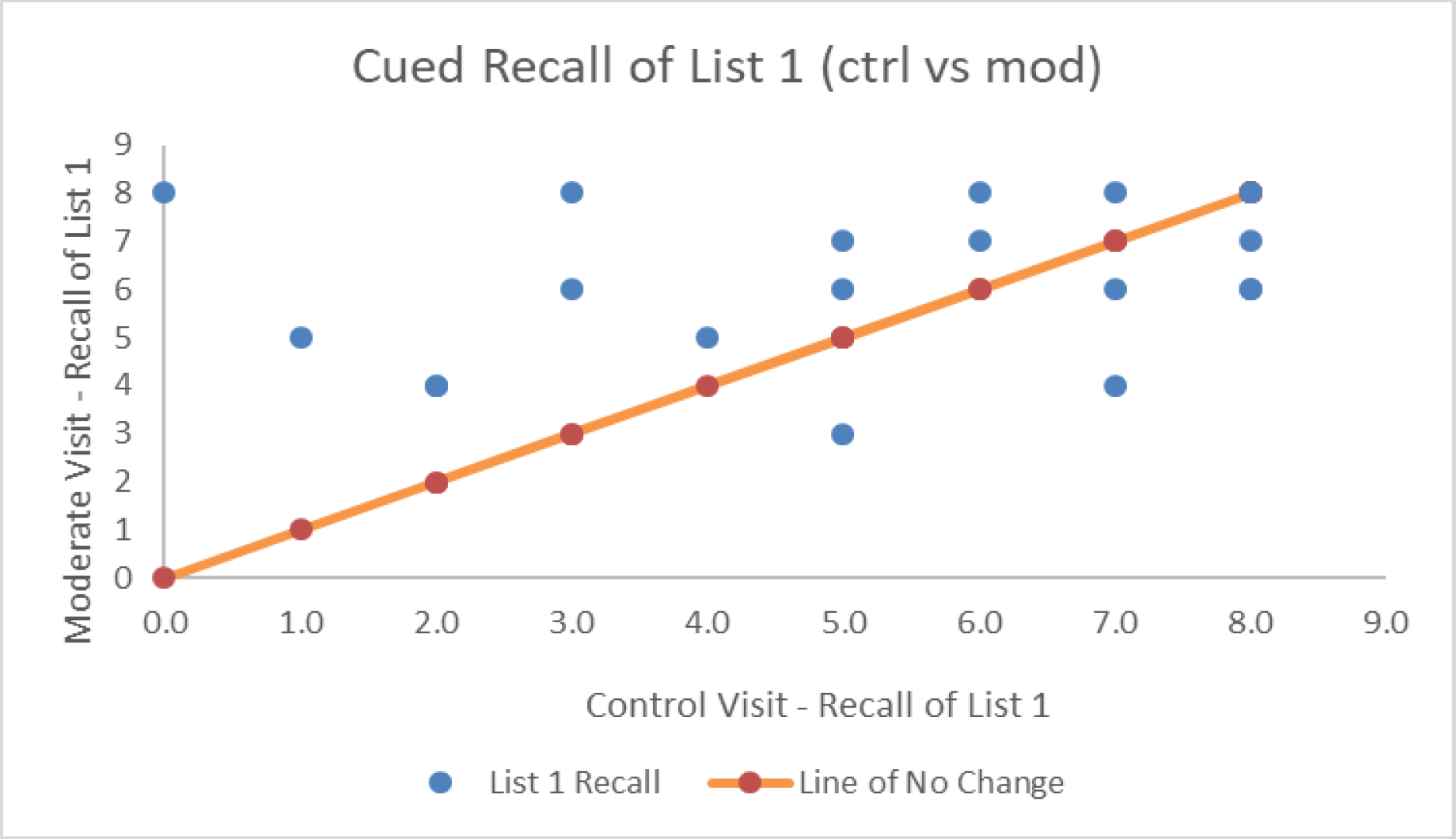
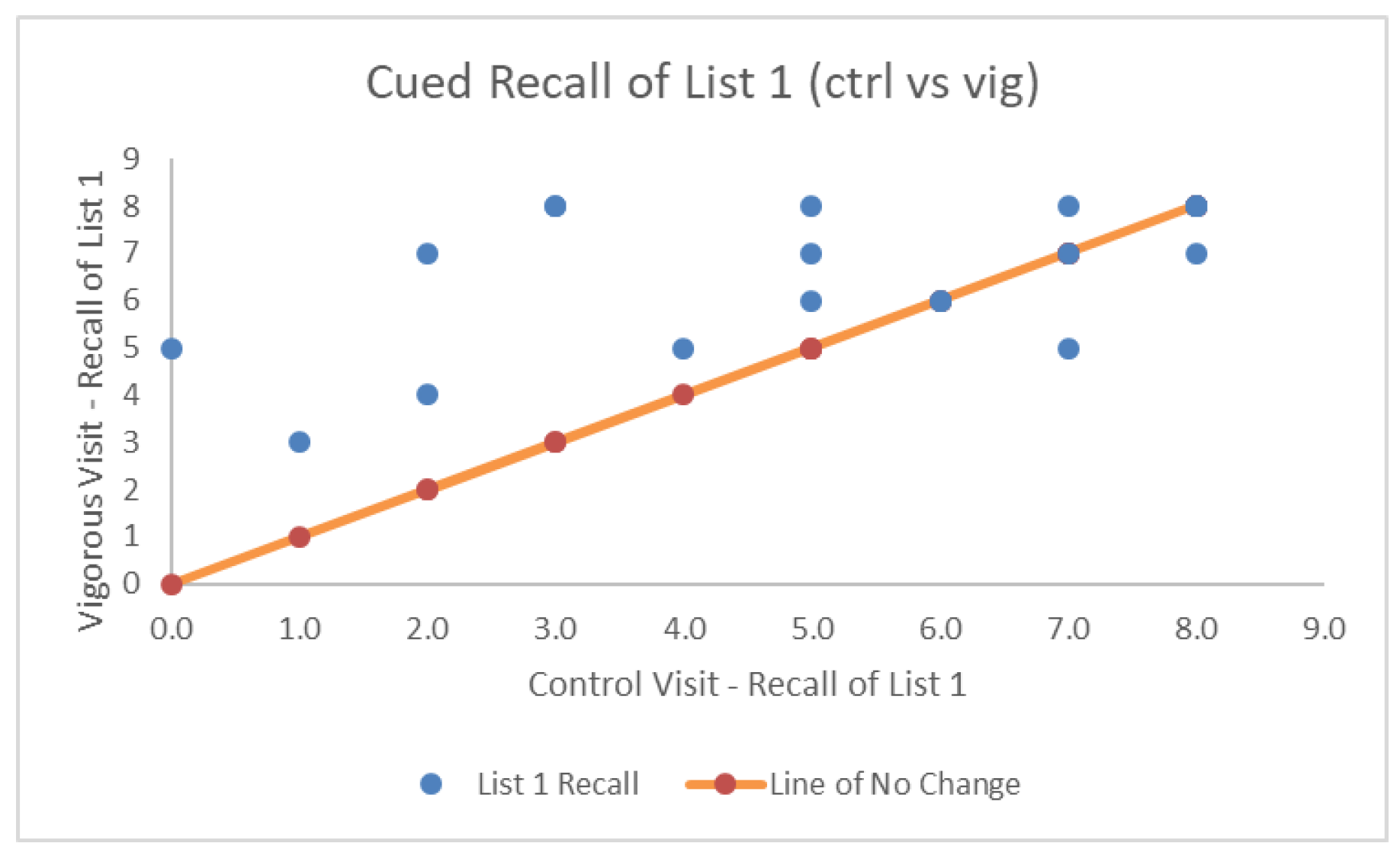
| # Correct Cued Recall List 1 | 5.00 (2.56) | 6.11 (1.59) | 6.53 (1.54) | F(2,36) = 4.63, p = 0.01*, η2p = 0.205, BF = 4.05 |
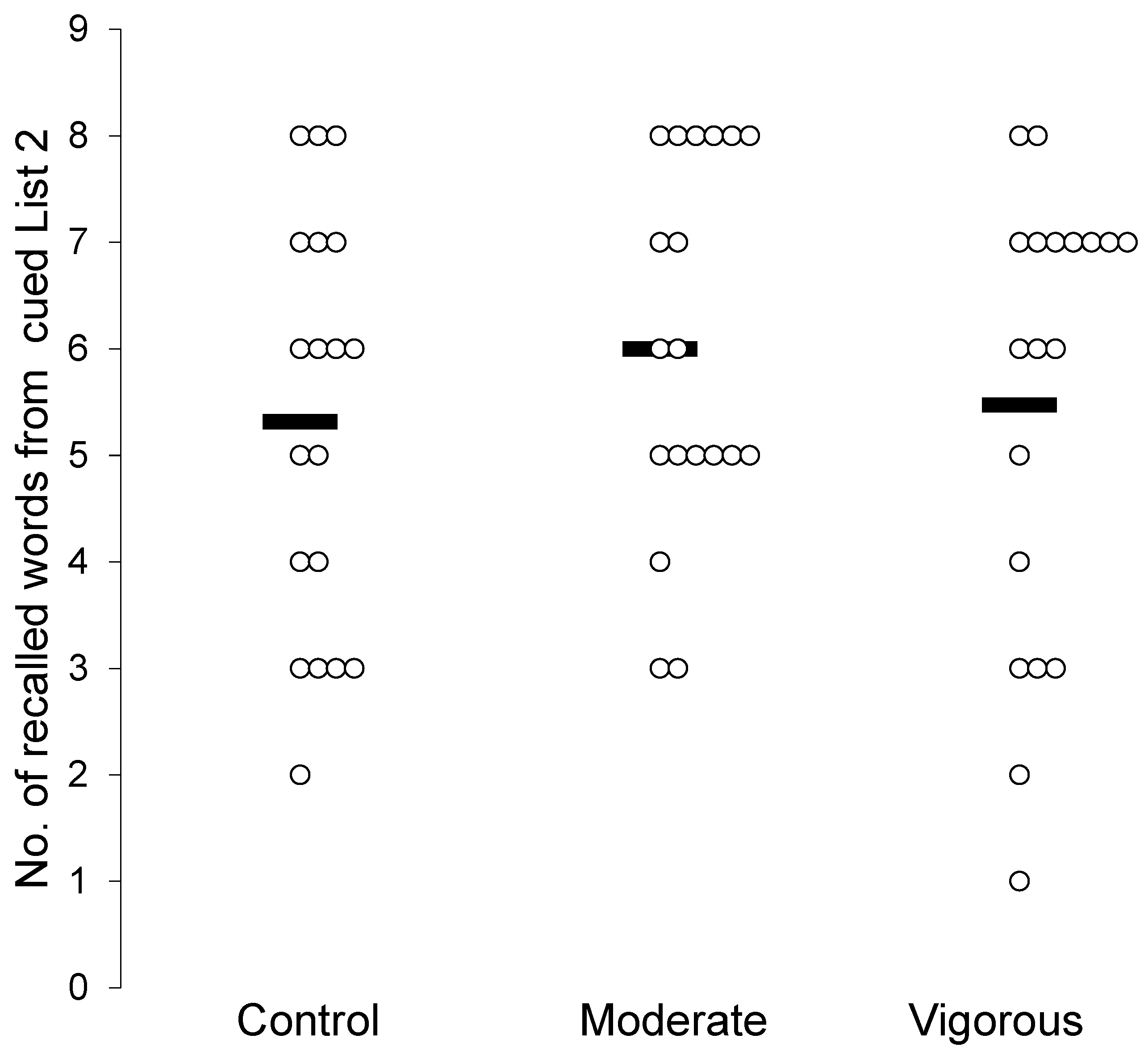
| # Correct Cued Recall List 2 | 5.31 (1.94) | 6.00 (1.73) | 5.47 (2.14) | F(2,36) = 1.71, p = 0.19, η2p = 0.087, BF = 0.47 |
| # Correct MMFR AB List | 2.10 (1.24) | 2.47 (1.21) | 3.05 (0.97) | F(2,36) = 4.97, p = 0.01*, η2p = 0.217, BF = 4.94 |
| # Correct MMFR DE List | 2.10 (1.44) | 2.36 (1.21) | 3.00 (1.15) | F(2,36) = 4.72, p = 0.01*, η2p = 0.208, BF = 3.76 |
| # Correct MMFR AC List | 2.21 (1.22) | 2.57 (1.21) | 2.57 (1.30) | F(2,36) = 0.795, p = 0.46, η2p = 0.042, BF = 0.25 |
| # Correct MMFR FG List | 2.63 (1.16) | 3.26 (0.87) | 2.84 (1.25) | F(2,36) = 3.20, p = 0.052, η2p = 0.151, BF = 0.51 |
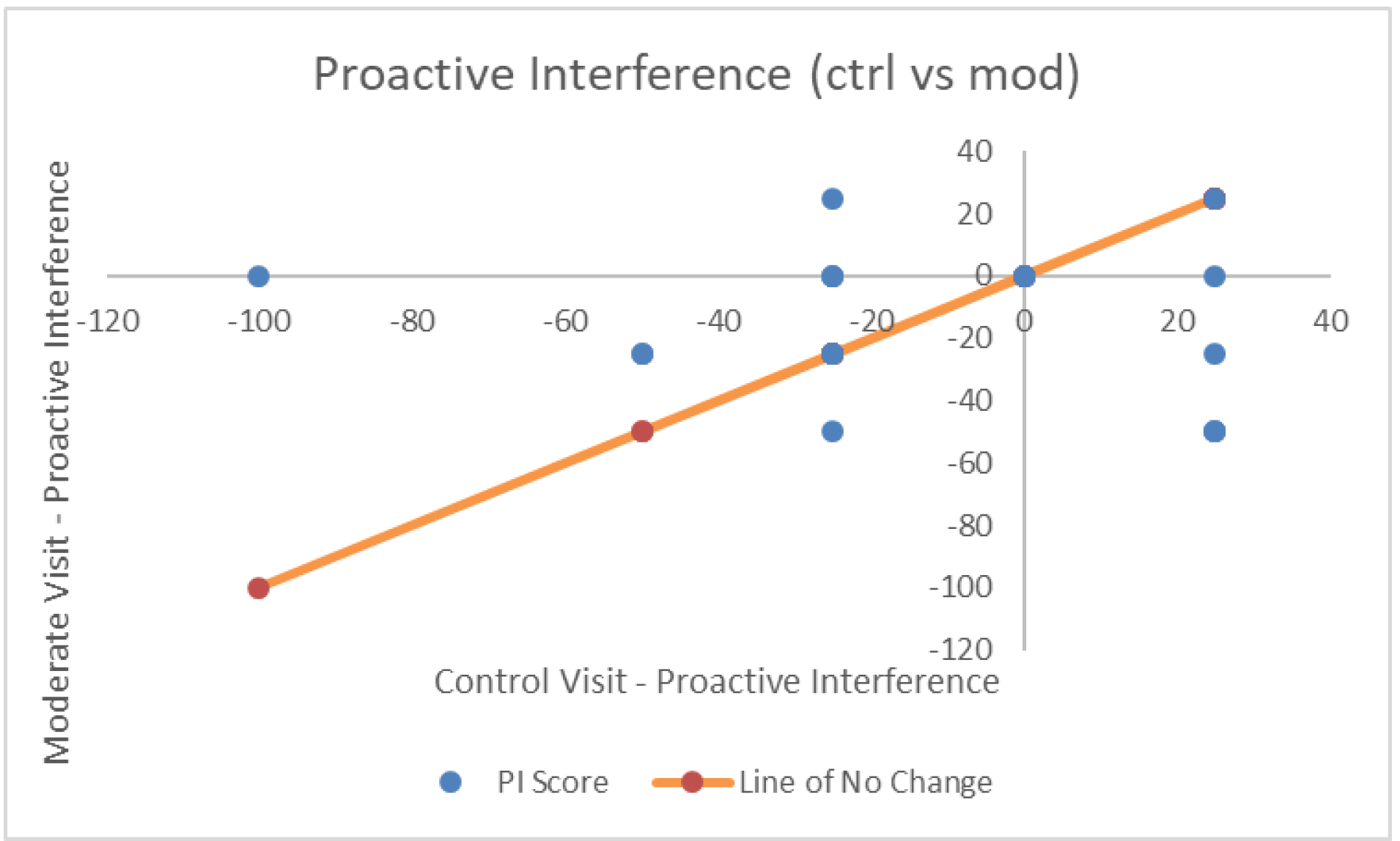
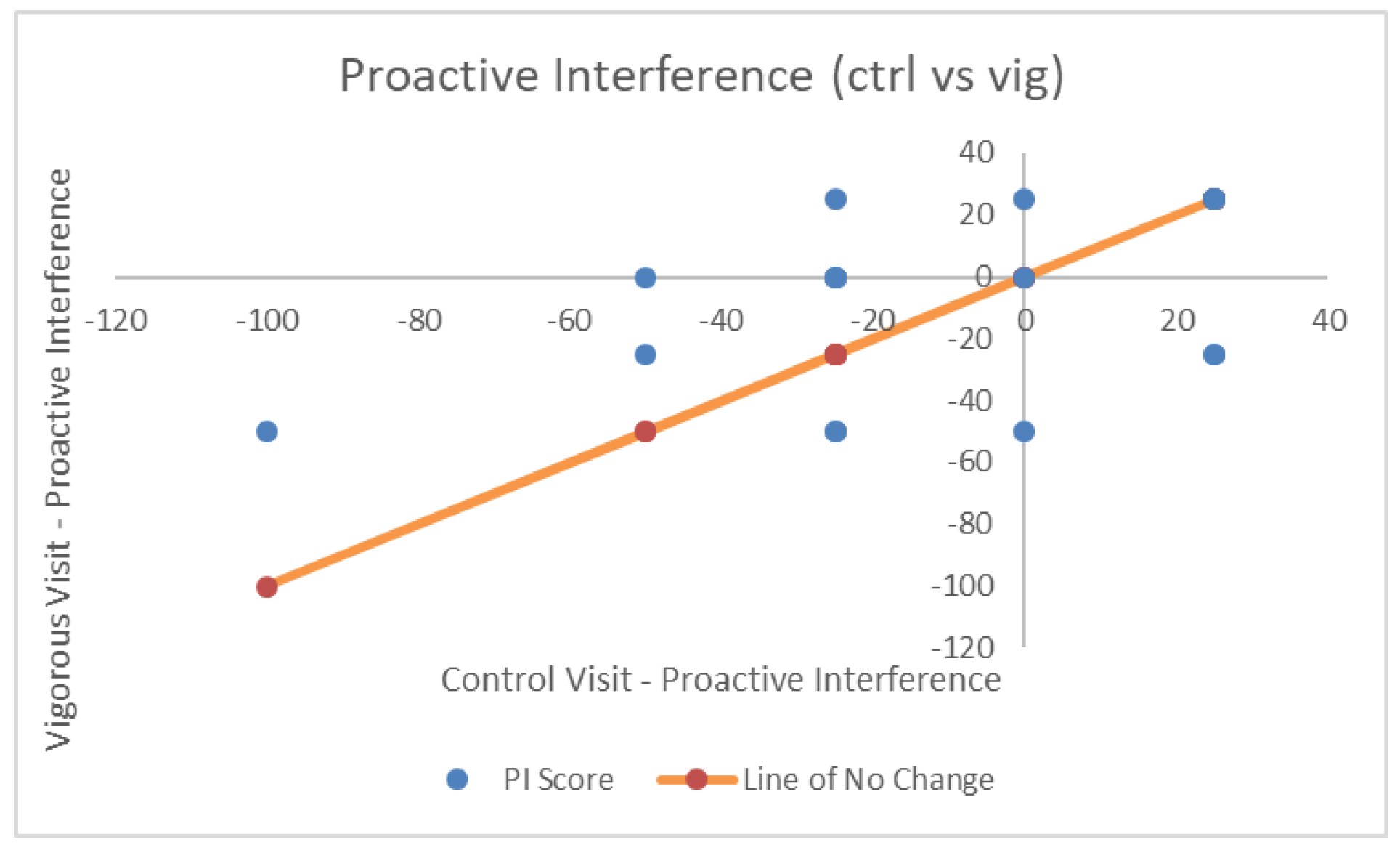
| Proactive Interference | −11.84 (33.71) | −14.47 (24.03) | −6.58 (28.67) | F(2,36) = 0.411, p = 0.66, η2p = 0.022, BF = 0.82 |
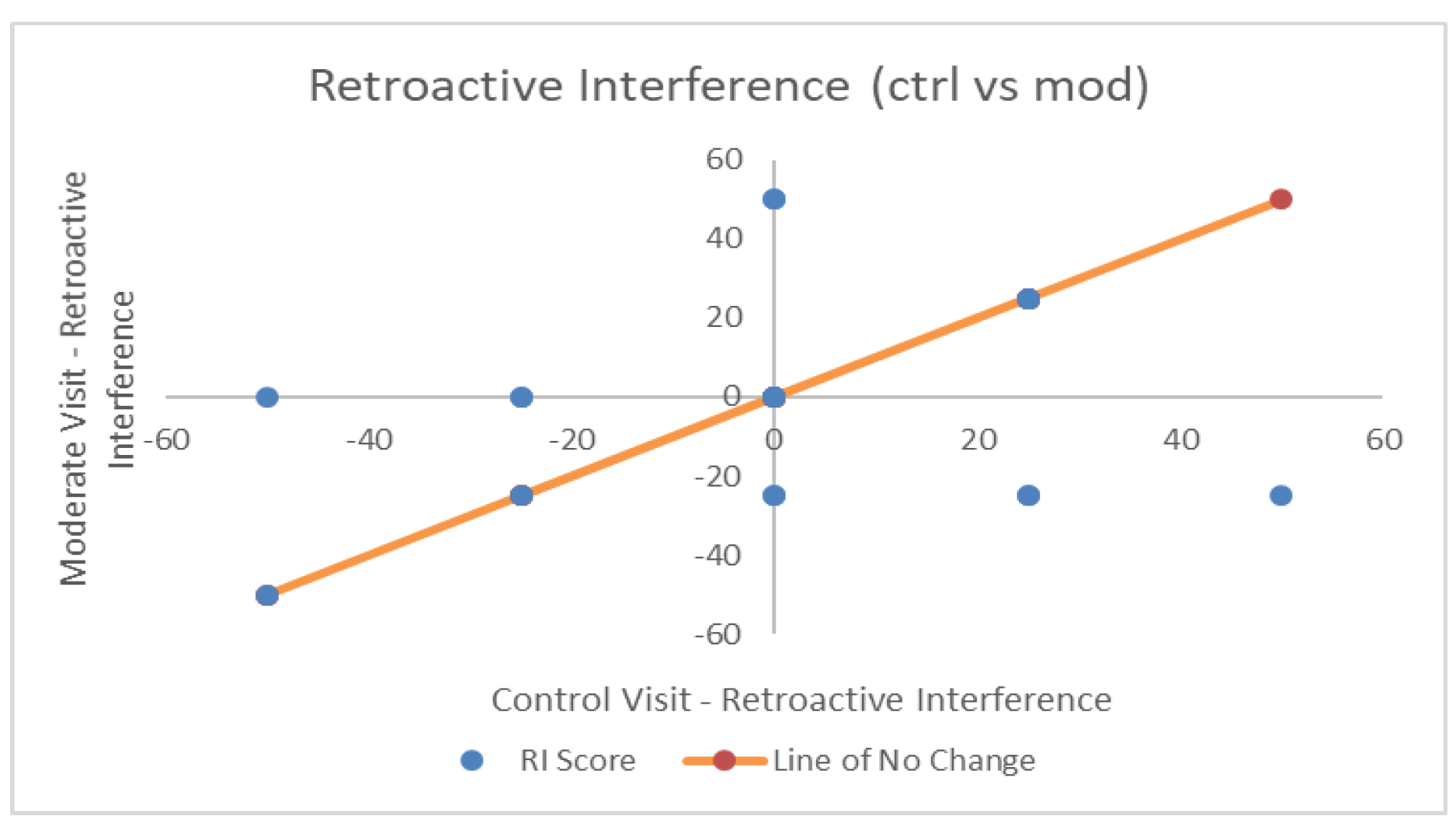
| Retroactive Interference | 0.00 (25.36) | −1.32 (26.96) | 1.32 (24.25) | F(2,36) = 0.062, p = 0.94, η2p = 0.003, BF = 0.14 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Crawford, L.; Loprinzi, P. Effects of Intensity-Specific Acute Exercise on Paired-Associative Memory and Memory Interference. Psych 2019, 1, 290-305. https://doi.org/10.3390/psych1010020
Crawford L, Loprinzi P. Effects of Intensity-Specific Acute Exercise on Paired-Associative Memory and Memory Interference. Psych. 2019; 1(1):290-305. https://doi.org/10.3390/psych1010020
Chicago/Turabian StyleCrawford, Lindsay, and Paul Loprinzi. 2019. "Effects of Intensity-Specific Acute Exercise on Paired-Associative Memory and Memory Interference" Psych 1, no. 1: 290-305. https://doi.org/10.3390/psych1010020
APA StyleCrawford, L., & Loprinzi, P. (2019). Effects of Intensity-Specific Acute Exercise on Paired-Associative Memory and Memory Interference. Psych, 1(1), 290-305. https://doi.org/10.3390/psych1010020





