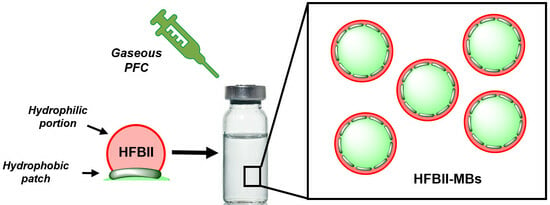
Hydrophobin-Coated Perfluorocarbon Microbubbles with Strong Non-Linear Acoustic Response
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of HFBII Aqueous Solutions
2.1.1. Preparation of MBs via the Vialmix™ Method
2.1.2. Preparation of MBs via the Polytron Method
2.1.3. Decantation of MBs
2.2. Size Distribution and Concentration
2.3. Optical Microscopy Analysis
2.4. Pressure Resistance Measurements
2.5. Spectral Attenuation Measurements
2.6. Harmonic Response
3. Results and Discussion
3.1. Influence of Different Core Gases and HFBII Dissolution Procedures
3.2. Influence of HFBII Initial Concentration
3.3. Comparison between the Vialmix™ and Polytron Preparation Methods
3.4. Echogenicity and Acoustic Characterization of HFBII-Shelled Microbubbles
3.5. Harmonic Response of HFBII-MBs
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Upadhyay, A.; Dalvi, V. Microbubble formulations: Synthesis, stability, modeling and biomedical applications. Ultrasound Med. Biol. 2019, 45, 301–343. [Google Scholar] [CrossRef] [PubMed]
- Navarro-Becerra, J.A.; Borden, M.A. Targeted Microbubbles for Drug, Gene, and Cell Delivery in Therapy and Immunotherapy. Pharmaceutics 2023, 15, 1625. [Google Scholar] [CrossRef] [PubMed]
- Roovers, S.; Segers, T.; Lajoinie, G.; Deprez, J.; Versluis, M.; De Smedt, S.C.; Lentacker, I. The role of ultrasound-driven microbubble dynamics in drug delivery: From microbubble fundamentals to clinical translation. Langmuir 2019, 35, 10173–101917. [Google Scholar] [CrossRef]
- Yusefi, H.; Helfield, B. Ultrasound Contrast Imaging: Fundamentals and Emerging Technology. Front. Phys. 2022, 10, 791145. [Google Scholar] [CrossRef]
- Kuriakose, M.; Borden, M.A. Microbubbles and nanodrops for photoacoustic tomography. Curr. Opin. Colloid Interface Sci. 2021, 55, 101464. [Google Scholar] [CrossRef]
- McMahon, D.; O’Reilly, M.A.; Hynynen, K. Therapeutic Agent Delivery Across the Blood–Brain Barrier Using Focused Ultrasound. Annu. Rev. Biomed. Eng. 2021, 23, 89–113. [Google Scholar] [CrossRef]
- Krafft, M.P.; Riess, J.G. Therapeutic oxygen delivery by perfluorocarbon-based colloids. Adv. Colloid Interface Sci. 2021, 294, 102407. [Google Scholar] [CrossRef]
- Duan, L.; Yang, L.; Jin, J.; Yang, F.; Liu, D.; Hu, K.; Wang, Q.; Yue, Y.; Gu, N. Micro/nano-bubble-assisted ultrasound to enhance the EPR effect and potential theranostic applications. Theranostics 2020, 10, 462–483. [Google Scholar] [CrossRef]
- Guo, R.; Xu, N.; Liu, Y.; Ling, G.; Yu, J.; Zhang, P. Functional ultrasound-triggered phase-shift perfluorocarbon nanodroplets for cancer therapy. Ultrasound Med. Biol. 2021, 47, 2064–2079. [Google Scholar] [CrossRef]
- Endo-Takahashi, Y.; Negishi, Y. Microbubbles and nanobubbles with ultrasound for systemic gene delivery. Pharmaceutics 2020, 12, 964. [Google Scholar] [CrossRef]
- Peruzzi, G.; Sinibaldi, G.; Silvani, G.; Ruocco, G.; Casciola, C.M. Perspectives on cavitation enhanced endothelial layer permeability. Colloids Surf. B Biointerfaces 2018, 168, 83–93. [Google Scholar] [CrossRef] [PubMed]
- Escoffre, J.-M.; Bouakaz, A. Minireview: Biophysical mechanisms of cell membrane sonopermeabilization. Knowns and unknowns. Langmuir 2019, 35, 10151–10165. [Google Scholar] [CrossRef]
- An, S.; Ranaweera, R.; Luo, I. Harnessing bubble behaviors for developing new analytical strategies. Analyst 2020, 145, 7782–7795. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Hossack, J.A.; Klibanov, A.L. From anatomy to functional and molecular biomarker imaging and therapy: Ultrasound is safe, ultrafast, portable, and inexpensive. Investig. Radiol. 2020, 55, 559–572. [Google Scholar] [CrossRef] [PubMed]
- Park, B.; Yoon, S.; Choi, Y.; Jang, J.; Park, S.; Choi, J. Stability of engineered micro or nanobubbles for biomedical applications. Pharmaceutics 2020, 12, 1089. [Google Scholar] [CrossRef]
- Versluis, M.; Stride, E.; Lajoinie, G.; Dollet, B.; Segers, T. Ultrasound contrast agent modeling: A review. Ultrasound Med. Biol. 2020, 46, 2117–2144. [Google Scholar] [CrossRef]
- Lee, L.; Cavalieri, F.; Ashokkumar, M. Exploring new applications of lysozyme-shelled microbubbles. Langmuir 2019, 35, 9997–10006. [Google Scholar] [CrossRef] [PubMed]
- Shi, D.; Wallyn, J.; Nguyen, D.-V.; Perton, F.; Felder-Flesch, D.; Bégin-Colin, S.; Maaloum, M.; Krafft, M.P. Microbubbles decorated with dendronized magnetic nanoparticles for biomedical imaging: Effective stabilization via fluorous interactions. Beilstein J. Nanotechnol. 2019, 10, 2103–2115. [Google Scholar] [CrossRef] [PubMed]
- Helfield, B. A review of phospholipid encapsulated ultrasound contrast agent microbubble physics. Ultrasound Med. Biol. 2019, 45, 282–300. [Google Scholar] [CrossRef]
- Langeveld, S.A.G.; Schwieger, C.; Beekers, I.; Blaffert, J.; van Rooij, T.; Blume, A.; Kooiman, K. Ligand distribution and lipid phase behavior in phospholipid-coated microbubbles and monolayers. Langmuir 2020, 36, 3221–3233. [Google Scholar] [CrossRef]
- Frinking, P.; Segers, T.; Luan, Y.; Tranquart, F. Three decades of ultrasound contrast agents: A review of the past, present and future improvements. Ultrasound Med. Biol. 2020, 46, 892–908. [Google Scholar] [CrossRef]
- Linder, M.B. Hydrophobins: Proteins that self-assemble at interfaces. Curr. Opin. Colloid Interface Sci. 2009, 14, 356–363. [Google Scholar] [CrossRef]
- Berger, B.W.; Sallada, N.D.J. Hydrophobins: Multifunctional biosurfactants for interface engineering. Biol. Eng. 2019, 13, 10. [Google Scholar] [CrossRef] [PubMed]
- Szilvay, G.R.; Paananen, A.; Laurikainen, K.; Vuorimaa, E.; Lemmetyinen, H.; Peltonen, J.; Linder, M.B. Self-assembled hydrophobin protein films at the air-water interface: Structural analysis and molecular engineering. Biochemistry 2007, 46, 2345–2354. [Google Scholar] [CrossRef] [PubMed]
- Lo, V.C.; Ren, Q.; Pham, C.L.L.; Morris, V.K.; Kwan, A.H.; Sunde, M. Fungal hydrophobin proteins produce self-assembling protein films with diverse structure and chemical stability. Nanomaterials 2014, 4, 827–843. [Google Scholar] [CrossRef] [PubMed]
- Cox, A.R.; Aldred, D.L.; Russell, A.B. Exceptional stability of food foams using class II hydrophobin HFBII. Food Hydrocoll. 2009, 23, 366–376. [Google Scholar] [CrossRef]
- Dokouhaki, M.; Hung, A.; Kasapis, S.; Gras, S.L. Hydrophobins and chaplins: Novel bio-surfactants for food dispersions. Trends Food Sci. Technol. 2021, 111, 378–387. [Google Scholar] [CrossRef]
- Akanbi, M.H.J.; Post, E.; Meter-Arkema, A.; Rink, R.; Robillard, G.T.; Wang, X.; Wösten, H.A.B.; Scholtmeijer, K. Use of hydrophobins in formulation of water insoluble drugs for oral administration. Colloids Surf. B Biointerfaces 2010, 75, 526–531. [Google Scholar] [CrossRef]
- Sarparanta, M.P.; Bimbo, L.M.; Mäkilä, E.M.; Salonen, J.J.; Laaksonen, P.H.; Helariutta, A.M.K.; Linder, M.B.; Hirvonen, J.T.; Laaksonen, T.J.; Santos, H.A.; et al. The mucoadhesive and gastroretentive properties of hydrophobin-coated porous silicon nanoparticle oral drug delivery systems. Biomaterials 2012, 11, 3353–3362. [Google Scholar] [CrossRef]
- Maiolo, D.; Pigliacelli, C.; Sánchez Moreno, P.; Violatto, M.B.; Talamini, L.; Tirotta, I.; Piccirillo, R.; Zucchetti, M.; Morosi, L.; Frapolli, R.; et al. Bioreducible Hydrophobin-Stabilized Supraparticles for Selective Intracellular Release. ACS Nano 2017, 11, 9413–9423. [Google Scholar] [CrossRef]
- Niu, B.; Li, M.; Jia, J.; Zhang, C.; Fan, Y.-Y.; Li, W. Hydrophobin-enhanced stability, dispersions and release of curcumin nanoparticles in water. J. Biomater. Sci. Polym. Ed. 2020, 31, 1793–1805. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Han, Z.; Song, B.; Yu, L.; Ma, Z.; Xu, H.; Qiao, M. Effective drug delivery system based on hydrophobin and halloysite clay nanotubes for sustained release of doxorubicin. Colloids Surf. A Physicochem. Eng. Asp. 2021, 628, 127351. [Google Scholar] [CrossRef]
- Pigliacelli, C.; D’Elicio, A.; Milani, R.; Terraneo, G.; Resnati, G.; Baldelli Bombelli, F.; Metrangolo, P. Hydrophobin-stabilized dispersions of PVDF nanoparticles in water. J. Fluor. Chem. 2015, 177, 62–69. [Google Scholar] [CrossRef]
- Milani, R.; Monogioudi, E.; Baldrighi, M.; Cavallo, G.; Arima, V.; Marra, L.; Zizzari, A.; Rinaldi, R.; Linder, M.; Resnati, G.; et al. Hydrophobin: Fluorosurfactant-like properties without fluorine. Soft Matter 2013, 9, 6505–6514. [Google Scholar] [CrossRef]
- Dichiarante, V.; Milani, R.; Metrangolo, P. Natural surfactants towards a more sustainable fluorine chemistry. Green. Chem. 2018, 20, 13–27. [Google Scholar] [CrossRef]
- Ayaz, N.; Dichiarante, V.; Pigliacelli, C.; Repossi, J.; Gazzera, L.; Borreggio, M.; Maiolo, D.; Chirizzi, C.; Bergamaschi, G.; Chaabane, L.; et al. Hydrophobin-coated solid fluorinated nanoparticles for 19F-MRI. Adv. Mater. Interfaces 2021, 9, 2101677. [Google Scholar] [CrossRef]
- Kordts, M.; Kampe, M.; Kerth, A.; Hinderberger, D. Structure Formation in Class I and Class II Hydrophobins at the Air-Water Interface under Multiple Compression/Expansion Cycles. ChemistryOpen 2018, 7, 1005–1013. [Google Scholar] [CrossRef]
- Gazzera, L.; Milani, R.; Pirrie, L.; Schmutz, M.; Blanck, C.; Resnati, G.; Metrangolo, P.; Krafft, M.P. Design of highly stable echogenic microbubbles through controlled assembly of their hydrophobin shell. Angew. Chem. Int. Ed. 2016, 55, 10263–10267. [Google Scholar] [CrossRef]
- de Jong, N.; Hoff, L.; Skotland, T.; Bom, N. Absorption and scatter of encapsulated gas filled microspheres: Theoretical considerations and some measurements. Ultrasonics 1992, 30, 95–103. [Google Scholar] [CrossRef]
- Riccobelli, D.; Al-Terke, H.H.; Laaksonen, P.; Metrangolo, P.; Paananen, A.; Ras, R.H.A.; Ciarletta, P.; Vella, D. Flattened and wrinkled encapsulated droplets: Shape-morphing induced by gravity and evaporation. Phys. Rev. Lett. 2023, 130, 218202. [Google Scholar] [CrossRef]
- Schneider, M.; Arditi, M.; Barrau, M.-B.; Brochot, J.; Broillet, A.; Ventrone, R.; Yan, F. BR1: A new ultrasonographic contrast agent based on sulfur hexafluoride-filled microbubbles. Investig. Radiol. 1995, 30, 451–457. [Google Scholar] [CrossRef] [PubMed]
- Fournier, L.; de La Taille, T.; Chauvierre, C. Microbubbles for human diagnosis and therapy. Biomaterials 2023, 294, 122025. [Google Scholar] [CrossRef] [PubMed]
- Segers, T.; de Jong, N.; Versluis, M. Uniform scattering and attenuation of acoustically sorted ultrasound contrast agents: Modeling and experiments. J. Acoust. Soc. Am. 2016, 140, 2506–2517. [Google Scholar] [CrossRef] [PubMed]
- Mielke, S.; Liu, X.; Krafft, M.P.; Tanaka, M. Influence of semifluorinated alkane surface domains on phase behavior and linear and nonlinear viscoelasticity of phospholipid monolayers. Langmuir 2020, 36, 781–788. [Google Scholar] [CrossRef]




| Headspace Gas | Core Gas | Time after Activation (min) | DV (μm) | DN (μm) | Total MBs/mL (×106) | <2–8 μm> MBs/mL (×106) | Pc50 (mmHg) |
|---|---|---|---|---|---|---|---|
| N2 | N2 | 10 | 12 | 1.4 | 40.0 | 2.2 | 50 |
| 180 | n.d. | n.d. | n.d. | n.d. | n.d. | ||
| SF6 | 10 | 12 | 2 | 70.0 | 9.4 | 56 | |
| 180 | 12 | 2.4 | 62.0 | 9.6 | 84 | ||
| C4F10 | 10 | 14 | 1.5 | 220.0 | 11.5 | 76 | |
| 180 | 14 | 1.6 | 224.0 | 13.0 | 72 | ||
| C6F14- saturated N2 | 10 | 14 | 5 | 19.0 | 2.4 | 81 | |
| 180 | 13 | 5 | 21.0 | 3.7 | 115 | ||
| C6F14 | N2 | 10 | 11 | 1.6 | 135.3 | 22.1 | n.d. |
| 180 | 10 | 2 | 94.4 | 21.4 | 113 | ||
| SF6 | 10 | 12 | 1.8 | 174.0 | 22.3 | n.d. | |
| 180 | 12 | 2 | 106.3 | 23.3 | 110 | ||
| C4F10 | 10 | 14 | 1.4 | 214.3 | 14.2 | n.d. | |
| 180 | 14 | 1.5 | 217.1 | 15.8 | 118 | ||
| C6F14- saturated N2 | 10 | 12 | 1 | 18.1 | 0.4 | n.d. | |
| 180 | 12 | 1 | 21.6 | 0.2 | n.d. |
| Core Gas | Time after Activation (min) | DV (μm) | DN (μm) | Total MBs/mL (×106) | <2–8 μm> MBs/mL (×106) | Pc50 (mmHg) |
|---|---|---|---|---|---|---|
| SF6 | 10 | 12 | 2 | 573.0 | 64.9 | 86 |
| 180 | 12 | 2 | 604.0 | 55.0 | 81 | |
| C4F10 | 10 | 12 | 1 | 2541.0 | 85.6 | 64 |
| 180 | 12 | 1 | 2324.0 | 72.8 | 73 | |
| C6F14-saturated N2 | 10 | 14 | 2 | 50.0 | 3.5 | 85 |
| 180 | 14 | 2 | 69.0 | 3.5 | 79 |
| Core Gas | [HFBII] (mg/mL) | DV (μm) | DN (μm) | Total MBs/mL (×106) | <2–8 μm> MBs/mL (×106) |
|---|---|---|---|---|---|
| SF6 | 0.2 | 12 | 1 | 138.8 | 7.3 |
| 1 | 13 | 1 | 247.7 | 11.2 | |
| C4F10 | 0.2 | 10 | 2 | 349.0 | 61.3 |
| 1 | 11 | 1 | 377.9 | 22.7 | |
| C6F14-saturated N2 | 0.2 | 8 | 1 | 70.6 | 2.9 |
| 1 | 14 | 2 | 89.5 | 7.0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dichiarante, V.; Salzano, G.; Bussat, P.; Gaud, E.; Cherkaoui, S.; Metrangolo, P. Hydrophobin-Coated Perfluorocarbon Microbubbles with Strong Non-Linear Acoustic Response. Chemistry 2024, 6, 299-311. https://doi.org/10.3390/chemistry6020016
Dichiarante V, Salzano G, Bussat P, Gaud E, Cherkaoui S, Metrangolo P. Hydrophobin-Coated Perfluorocarbon Microbubbles with Strong Non-Linear Acoustic Response. Chemistry. 2024; 6(2):299-311. https://doi.org/10.3390/chemistry6020016
Chicago/Turabian StyleDichiarante, Valentina, Giuseppina Salzano, Philippe Bussat, Emmanuel Gaud, Samir Cherkaoui, and Pierangelo Metrangolo. 2024. "Hydrophobin-Coated Perfluorocarbon Microbubbles with Strong Non-Linear Acoustic Response" Chemistry 6, no. 2: 299-311. https://doi.org/10.3390/chemistry6020016