Mineralogical Characterization and Firing Temperature Delineation on Minoan Pottery, Focusing on the Application of Micro-Raman Spectroscopy
Abstract
1. Introduction
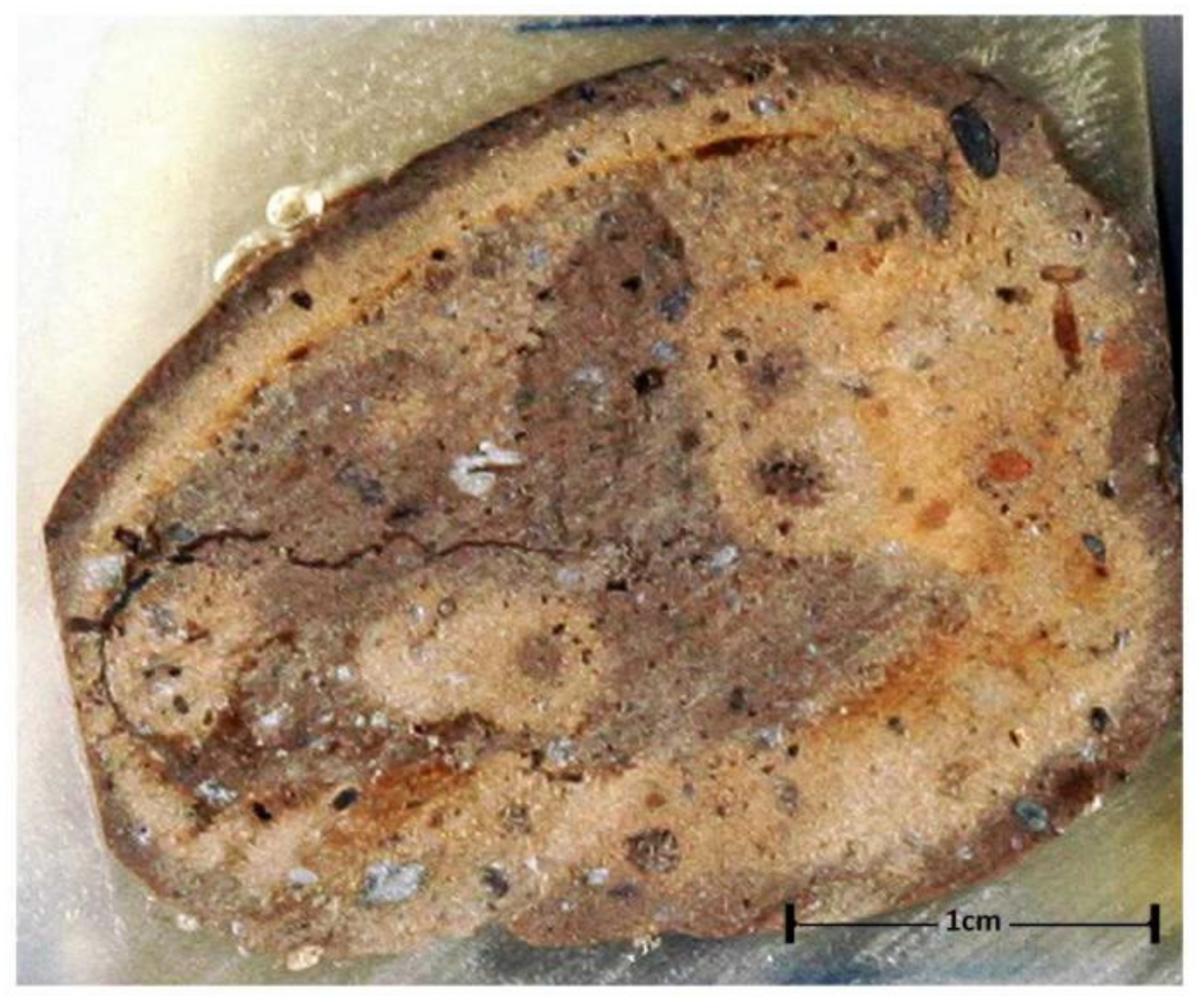
2. Materials and Methods
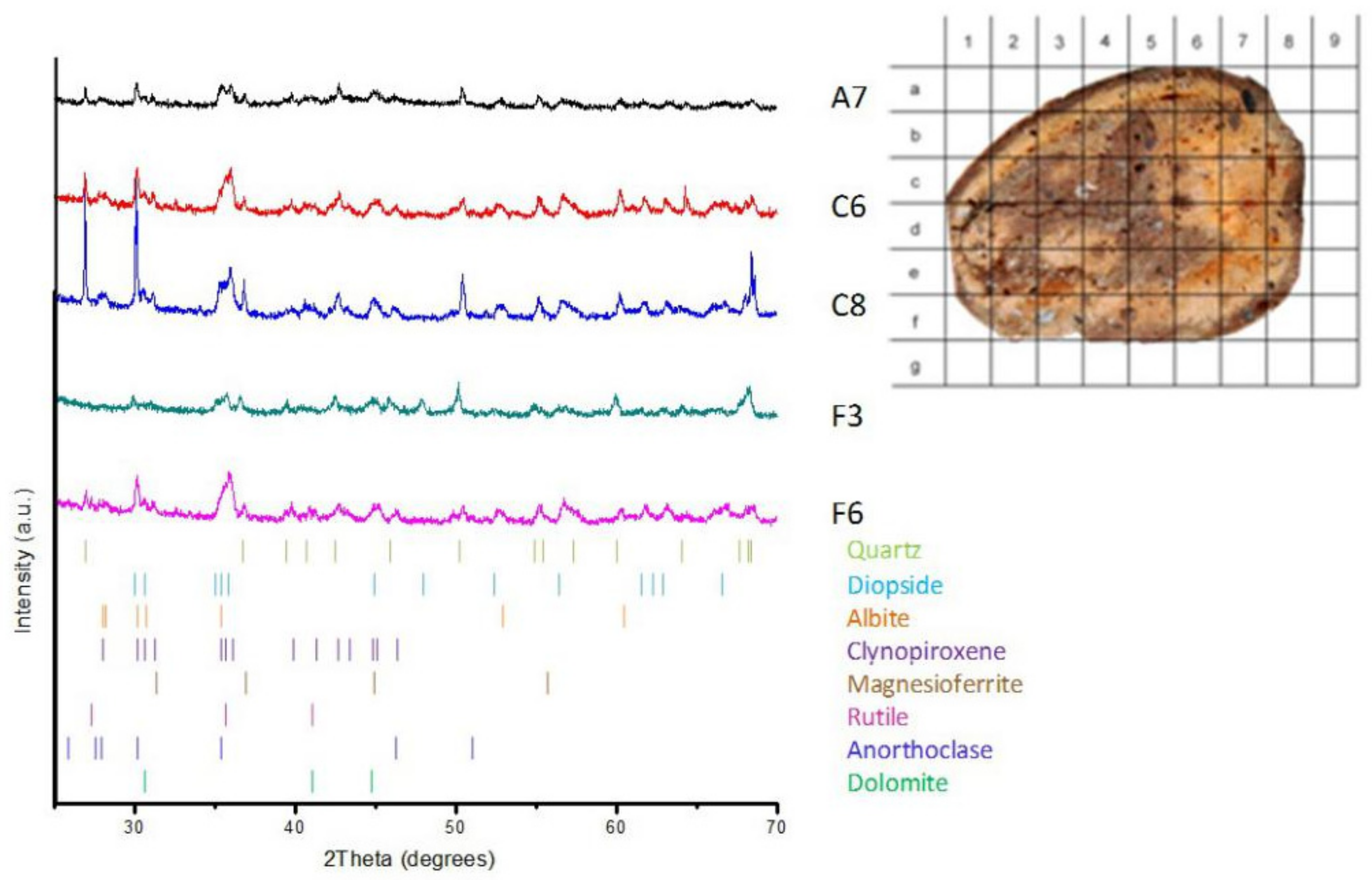
3. Results
3.1. Mineral Phase Identification
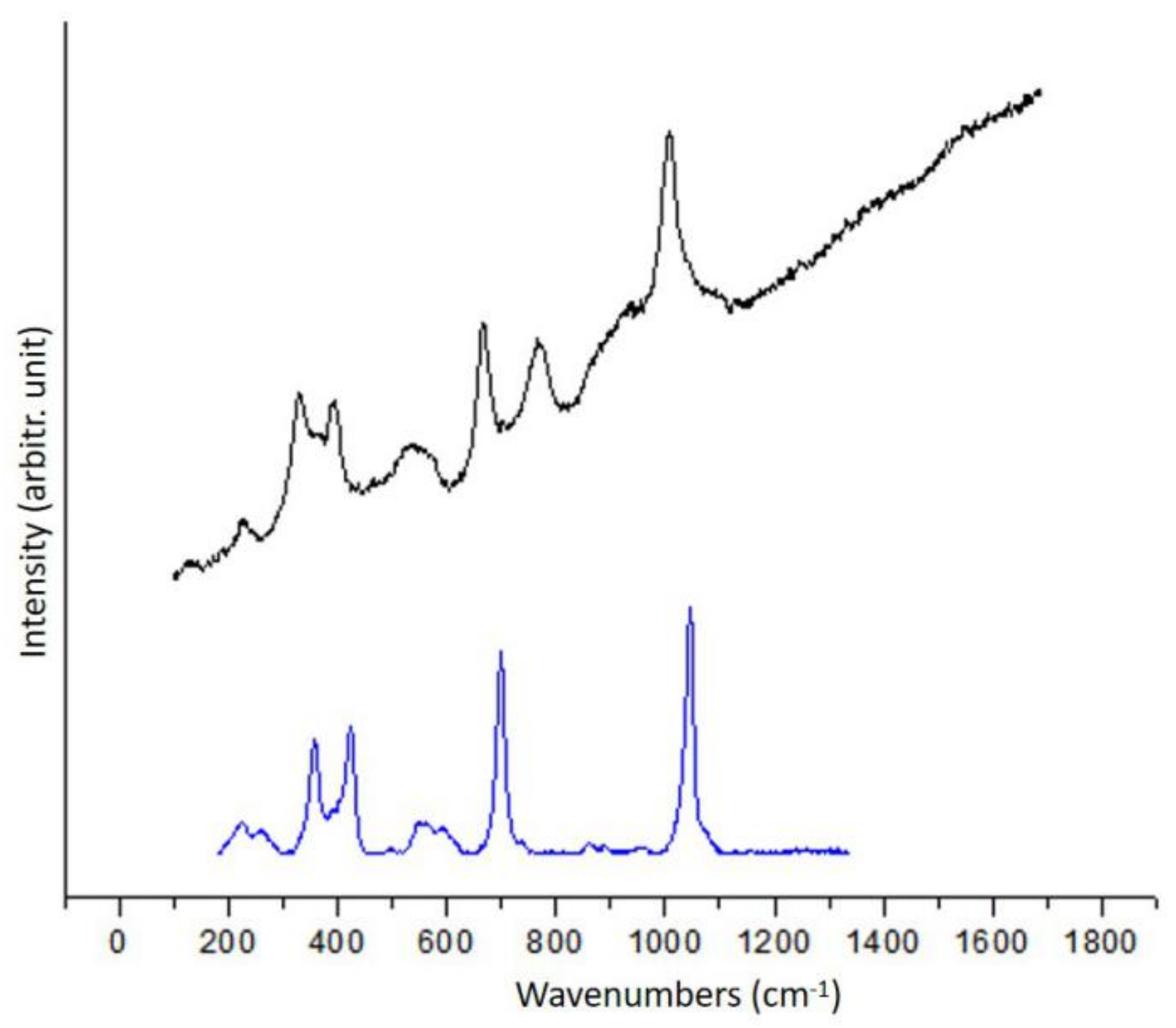
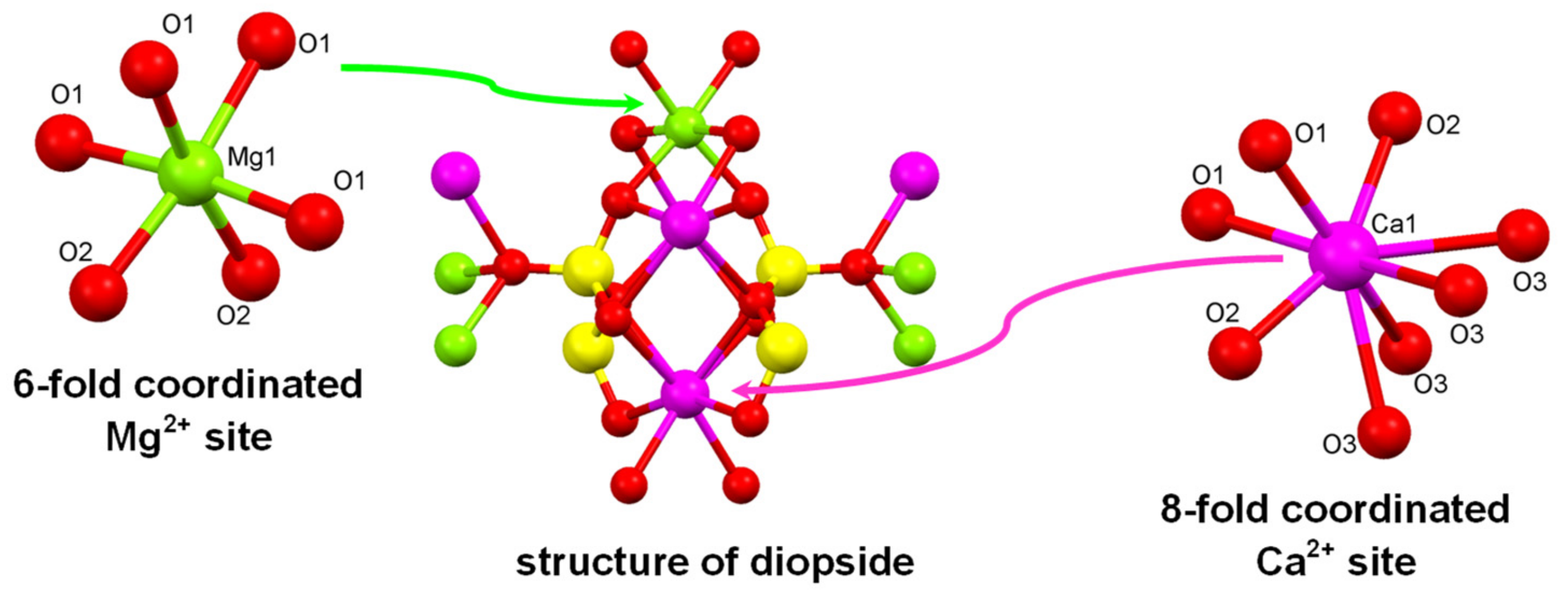
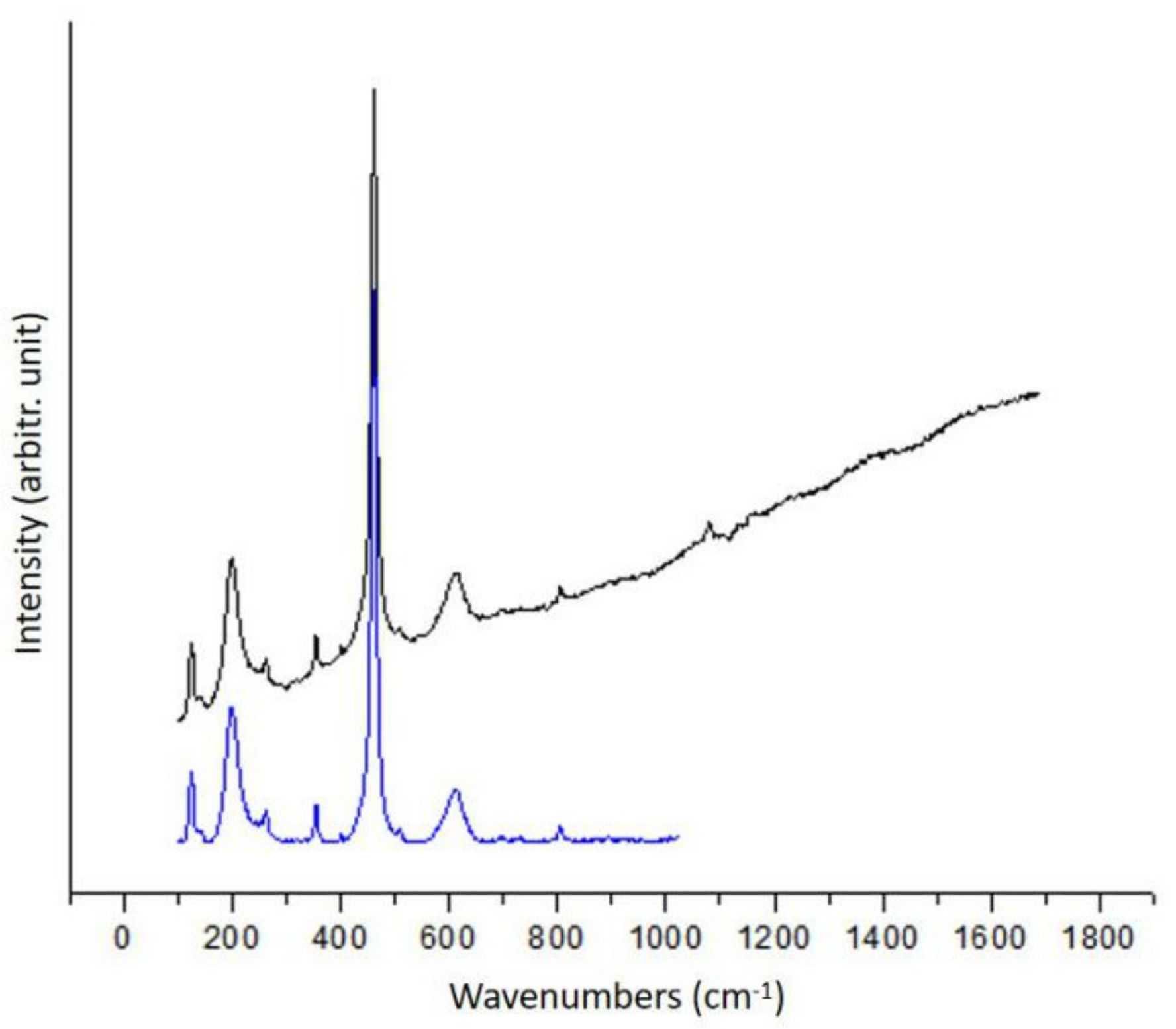
3.1.1. Diopside
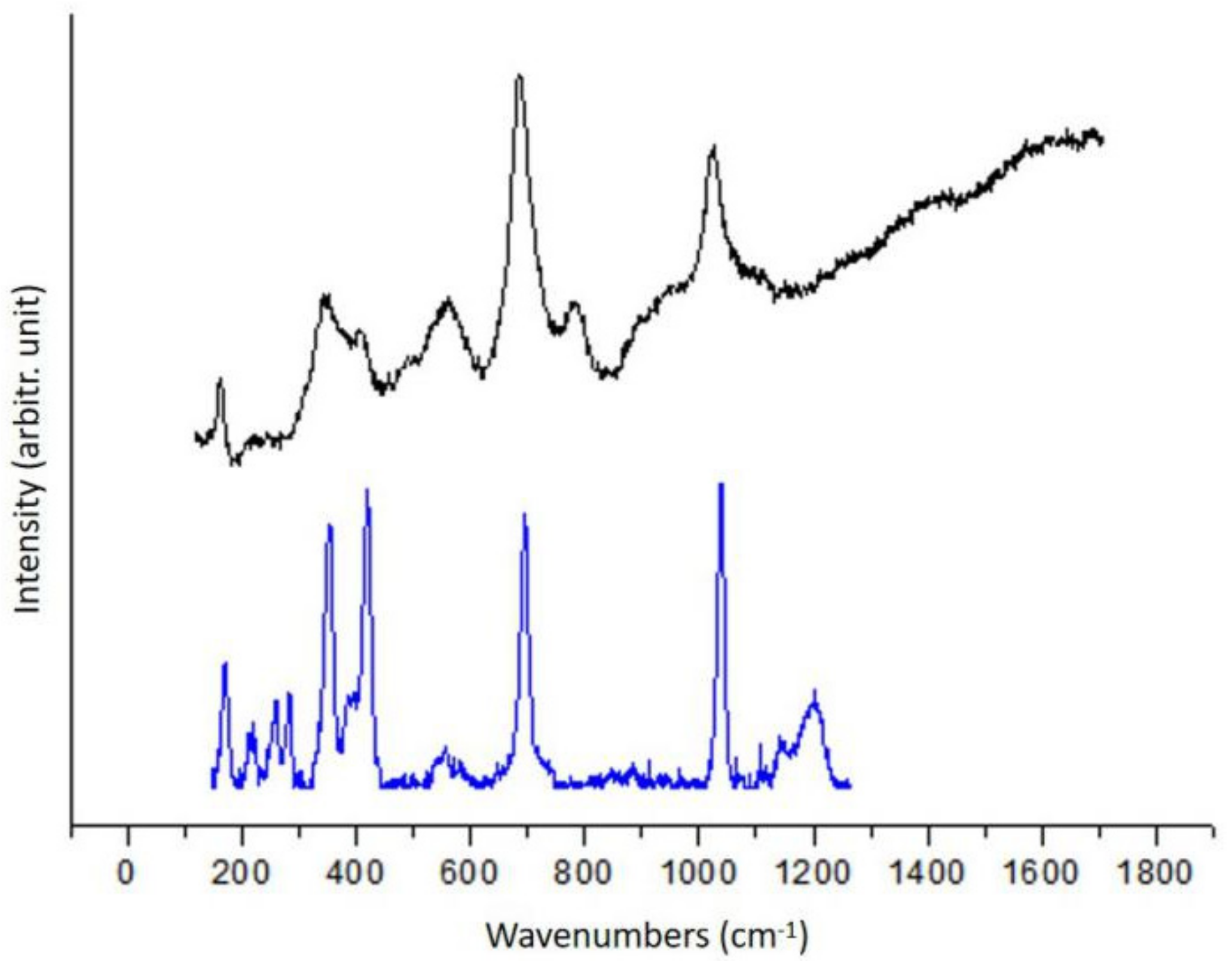
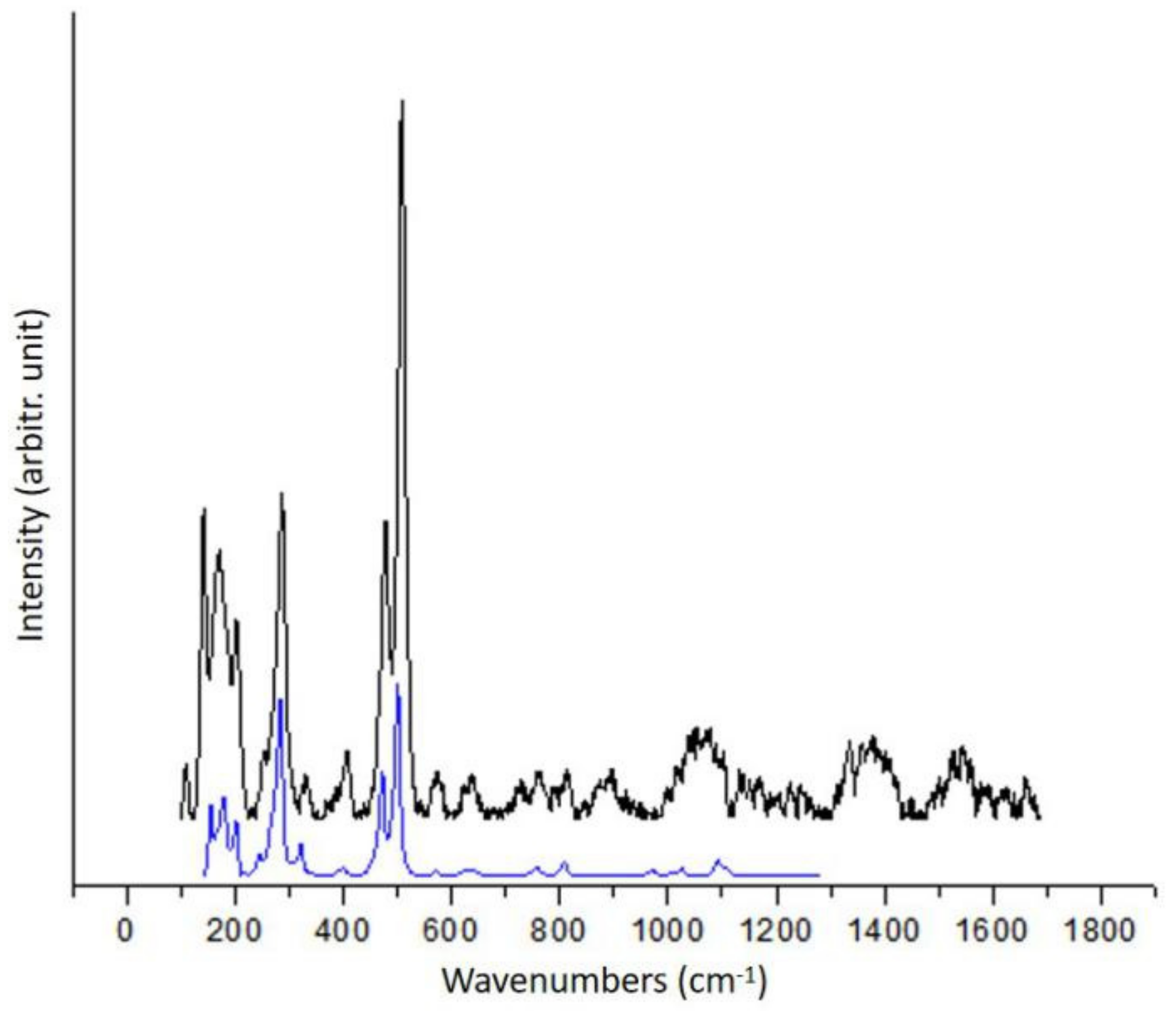
3.1.2. Quartz
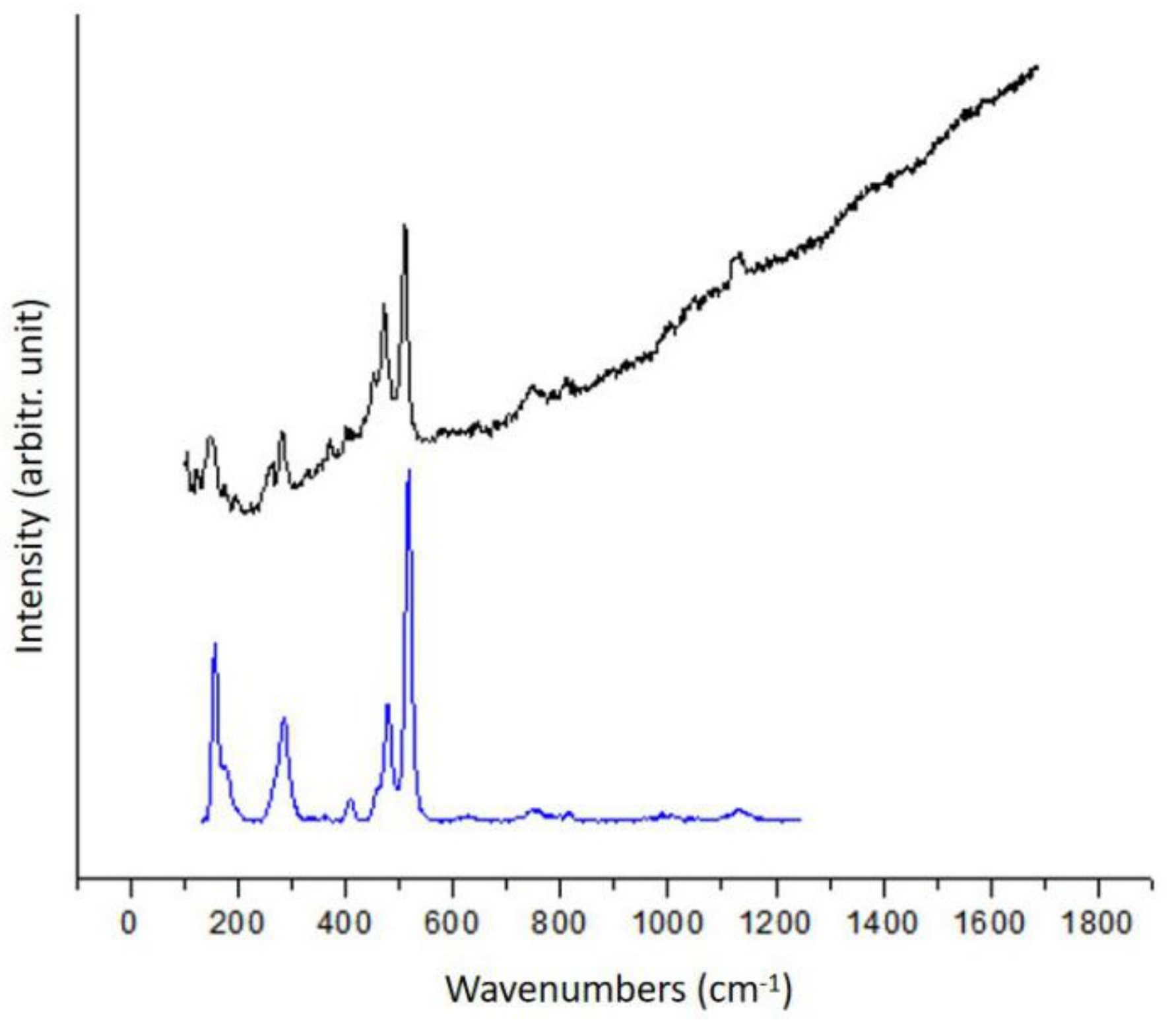
3.1.3. Feldspar Minerals
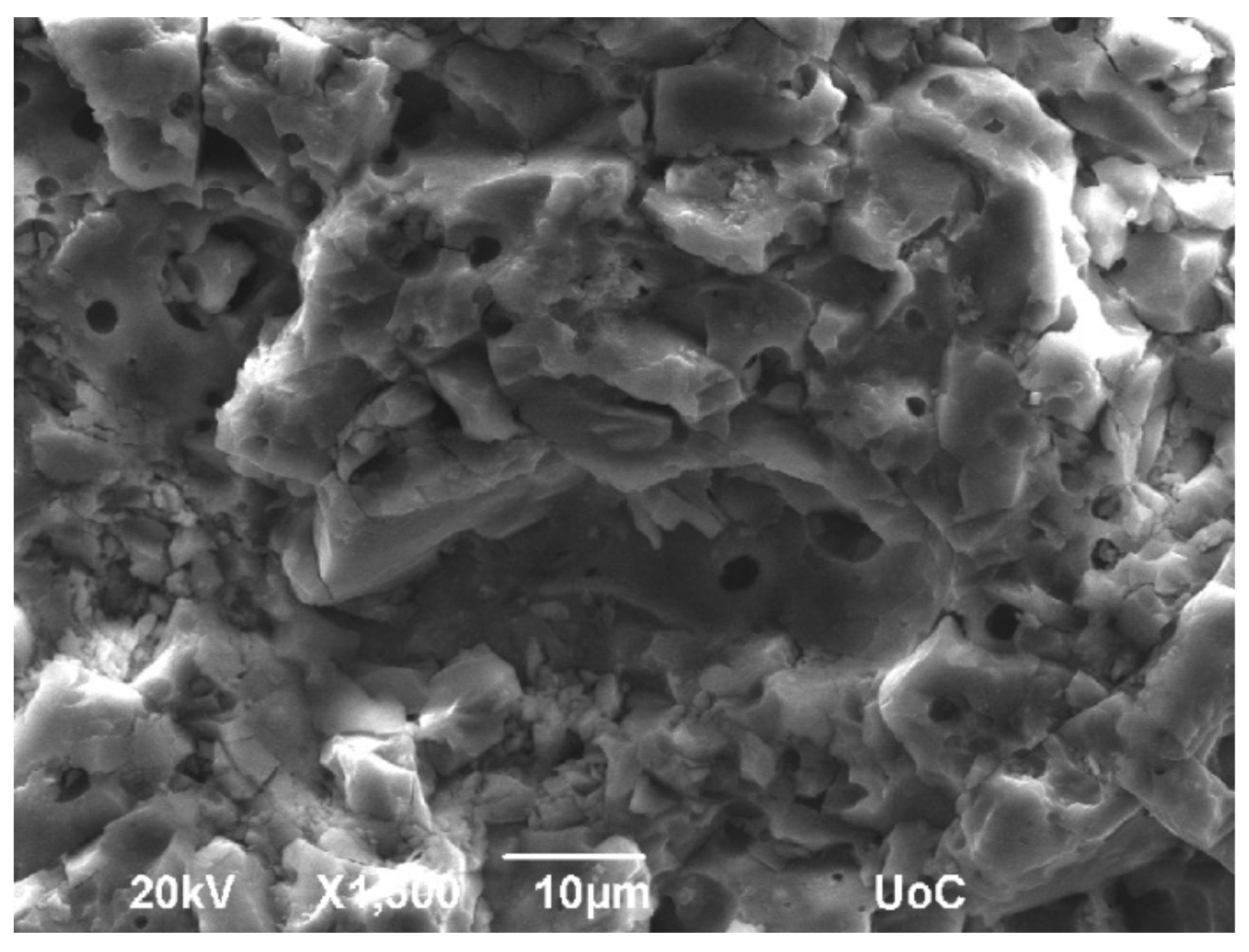
3.2. SEM-EDS
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Calas, G.; Hawthorne, F.C. Introduction to spectroscopic methods. Rev. Mineral. 1988, 18, 1–9. [Google Scholar]
- McMillan, P.F. Raman spectroscopy in mineralogy and geochemistry. Annu. Rev. Earth Planet. Sci. 1989, 17, 255–283. [Google Scholar] [CrossRef]
- Hutchinson, I.B.; Ingley, R.; Edwards, H.G.M.; Harris, L.; McHugh, M.; Malherbe, C.; Parnell, L. Raman spectroscopy on Mars: Identification of geological and bio-geological signatures in Martian analogues using miniaturized Raman spectrometers. Philos. Trans. R. Soc. 2014, 372, 20140204. [Google Scholar] [CrossRef] [PubMed]
- Perardi, A.; Zoppi, A.; Ruatta, L.; Davit, P. Modern trends in scientific studies on ancient ceramics. In Papers Presented at the 5th European Meeting on Ancient Ceramics, Athens, 1999; Kilikoglou, V., Hein, A., Maniatis, Y., Eds.; BAR International Series 1011; Archeopress: Oxford, UK, 2002; p. 371. [Google Scholar]
- Zoppi, A.; Lofrumento, C.; Castellucci, E.M.; Migliorini, M.G. Micro-Raman technique for phase analysis on archaeological ceramics. Spectrosc. Eur. 2002, 14, 16–21. [Google Scholar]
- Legodi, M.A.; De Waal, D. Raman analysis of red-brown and gray shards from 16th and 17th century Portuguese shipwrecks. Cryst. Eng. 2003, 6, 287–299. [Google Scholar] [CrossRef]
- Akyuz, S.; Akyuz, T.; Basaran, S.; Bolcal, C.; Gulec, A. FT-IR and Micro-Raman spectroscopic study of decorated potteries from VI and VII century BC, excavated in ancient Ainos-Turkey. J. Mol. Struct. 2007, 834, 150–153. [Google Scholar] [CrossRef]
- Cavalheri, A.S.; Balan, A.M.; Künzli, R.; Constantino, C.J. Vibrational spectroscopy applied to the study of archeological ceramic artifacts from Guarani culture in Brazil. Vib. Spectrosc. 2010, 54, 164–168. [Google Scholar] [CrossRef]
- Bersani, D.; Lottici, P.P.; Virgenti, S.; Sodo, A.; Malvestuto, G.; Botti, A.; Catarsi, M. Multi-technique investigation of archaeological pottery from Parma (Italy). J. Raman Spectrosc. 2010, 41, 1556–1561. [Google Scholar] [CrossRef]
- Ballirano, P.; De Vito, C.; Medeghini, L.; Mignardi, S.; Ferrini, V.; Matthiae, P.; Lottici, P.P. A combined use of optical microscopy, X-ray powder diffraction and Micro-Raman spectroscopy for the characterization of ancient ceramic from Ebla (Syria). Ceram. Int. 2014, 40, 16409–16419. [Google Scholar] [CrossRef]
- Bersani, D.; Lottici, P.P. Raman spectroscopy of minerals and mineral pigments in archaeometry. J. Raman Spectrosc. 2016, 47, 499–530. [Google Scholar] [CrossRef]
- Medeghini, L.; Mignardi, S.; De Vito, C.; Bersani, D.; Lottici, P.P.; Turetta, M.; Costantini, J.; Bacchini, E.; Sala, M.; Nigro, L. The key role of Micro-Raman spectroscopy in the study of ancient pottery: The case of pre-classical Jordanian ceramics from the archaeological site of Khirbet al-Batrawy. Eur. J. Mineral. 2013, 25, 881–893. [Google Scholar] [CrossRef]
- Medeghini, L.; Lottici, P.P.; De Vito, C.; Mignardi, S.; Bersani, D. Micro-Raman spectroscopy and ancient ceramics: Applications and problems. J. Raman Spectrosc. 2014, 45, 1244–1250. [Google Scholar] [CrossRef]
- Frost, R.; Kristof, J.; Schmidt, J.; Kloprogge, T. Raman spectroscopy of potassium acetate-intercalated kaolinites at liquid nitrogen temperature. Spectrochim. Acta 2001, 57, 603–609. [Google Scholar] [CrossRef]
- Lofrumento, C.; Zoppi, A.; Castellucci, E.M. Micro-Raman spectroscopy of ancient ceramics: A study of French sigillata wares. J. Raman Spectrosc. 2004, 35, 650–655. [Google Scholar] [CrossRef]
- Trindade, M.J.; Dias, M.I.; Coroado, J.; Rocha, F. Mineralogical transformations of calcareous rich clays with firing: A comparative study between calcite and dolomite rich clays from Algarve, Portugal. Appl. Clay Sci. 2009, 42, 345–355. [Google Scholar] [CrossRef]
- Maggetti, M. Phase analysis and its significance for technology and origin. In Archaeological Ceramics; Olin, J.S., Ed.; Smithsonian Institution Press: Boston, MA, USA, 1982; pp. 121–133. [Google Scholar]
- Peters, T.; Iberg, R. Mineralogical changes during firing of calcium-rich brick clays. Ceram. Bull. 1978, 57, 503–509. [Google Scholar]
- Duminuco, P.; Messiga, B.; Riccardi, M.P. Firing process of natural clays. Some microtextures and related phase compositions. Thermochim. Acta 1998, 321, 185–190. [Google Scholar] [CrossRef]
- Riccardi, M.P.; Messiga, B.; Duminuco, P. An approach to the dynamics of clay firing. Appl. Clay Sci. 1999, 15, 393–409. [Google Scholar] [CrossRef]
- Cultrone, G.; Rodriguez-Navarro, C.; Sebastian, E.; Cazalla, O.; De La Torre, M.J. Carbonate and silicate phase reactions during ceramic firing. Eur. J. Mineral. 2001, 13, 621–634. [Google Scholar] [CrossRef]
- Jordán, M.M.; Sanfeliu, T.; de la Fuente, C. Firing transformations of Tertiary clays used in the manufacturing of ceramic tile bodies. Appl. Clay Sci. 2001, 20, 87–95. [Google Scholar] [CrossRef]
- Traoré, K.; Kabré, T.S.; Blanchart, P. Gehlenite and anorthite crystallization from kaolinite and calcite mix. Ceram. Int. 2003, 9, 377–383. [Google Scholar] [CrossRef]
- Maggetti, M. Composition of Roman pottery from Lausanne (Switzerland). Br. Mus. Occas. Pap. 1981, 19, 33–49. [Google Scholar]
- De Benedetto, G.; Laviano, R.; Sabbatini, L.; Zambonin, P. Infrared spectroscopy in the mineralogical characterization of ancient pottery. J. Cult. Herit. 2002, 3, 177–186. [Google Scholar] [CrossRef]
- Lafuente, B.; Downs, R.T.; Yang, H.; Stone, N. The power of databases: The RRUFF project. In Highlights in Mineralogical Crystallography; Armbruster, T., Danisi, R.M., Eds.; W. De Gruyter: Berlin, Germany, 2015; pp. 1–303. [Google Scholar]
- Moropoulou, A.; Bakolas, A.; Bisbikou, K. Thermal analysis as a method of characterizing ancient ceramic technologies. Thermochim. Acta 1995, 2570, 743–753. [Google Scholar] [CrossRef]
- Maritan, L.; Nodari, L.; Mazzoli, C.; Milano, A.; Russo, U. Influence of firing conditions on ceramic products: Experimental study on clay rich in organic matter. Appl. Clay Sci. 2006, 31, 1–15. [Google Scholar] [CrossRef]
- Buzatu, A.; Buzgar, N. The Raman study of single-chain silicates. Analele Ştiinţifice ale Universităţii, A.I.; Cuza Iaşi 2010, LVI, 108–125. [Google Scholar]
- Prencipe, M.; Mantovani, L.; Tribaudino, M.; Bersani, D.; Lottici, P.P. The Raman spectrum of diopside: A comparison between ab initio calculated and experimentally measured frequencies. Eur. J. Mineral. 2012, 24, 457–464. [Google Scholar] [CrossRef]
- Flemming, R.L. Micro X-ray diffraction (μXRD): A versatile technique for characterization of Earth and planetary materials. Can. J. Earth Sci. 2007, 44, 1333–1346. [Google Scholar] [CrossRef]
- Sendova, M.; Zhelyaskov, V.; Scalera, M.; Ramsey, M. Micro-Raman spectroscopic study of pottery fragments from the Lapatsa tomb, Cyprus, ca 2500 BC. J. Raman Spectrosc. 2005, 36, 829–833. [Google Scholar] [CrossRef]
- Legodi, M.A.; De Waal, D. The preparation of magnetite, goethite, hematite and maghemite of pigment quality from mill scale iron waste. Dyes Pigments 2007, 74, 161–168. [Google Scholar] [CrossRef]
- Williams, Q. A Handbook of Physical Constants: Mineral Physics and Crystallography; Ahrens, T.J., Ed.; American Geophysical Union: Washington, DC, USA, 1995; Volume 2, p. 291. [Google Scholar]
- Deer, W.A.; Howie, R.A.; Zussman, J. An Introduction to Rock-Forming Minerals, 2nd ed.; Pearson, Prentice Hall: New York, NY, USA, 1991. [Google Scholar]
- Sharma, S.; Simons, B.; Yoder, H.S., Jr. Raman study of anorthite, calcium Tschermak’s pyroxene, and gehlenite in crystalline and glassy states. Am. Mineral. 1983, 68, 1113–1125. [Google Scholar]
- Matson, D.W.; Sharma, S.K.; Philpotts, J.A. Raman spectra of some tectosilicates and of glasses along the orthoclase–anorthite and nepheline–anorthite joins. Am. Mineral. 1986, 71, 694–704. [Google Scholar]
- Freeman, J.; Wang, A.; Kuebler, K.; Jolliff, B.; Haskin, L. Characterization of natural feldspars by Raman spectroscopy for future planetary exploration. Can. Mineral. 2008, 46, 1477–1500. [Google Scholar] [CrossRef]
- McKeown, D. Raman spectroscopy and vibrational analyses of albite: From 25 °C through the melting temperature. Am. Mineral. 2005, 90, 1506–1517. [Google Scholar] [CrossRef]
- Ribbe, P.H.; Hofmeister, A.M. Feldspar Mineralogy, 2nd ed.; The Mineralogical Society of American: Chantilly, VA, USA, 1983. [Google Scholar]
- Smith, J.V. Feldspar Minerals. Crystal structure and physical properties I. In Feldspar Minerals; Springer: Berlin/Heidelberg, Germany, 1974. [Google Scholar]
- Winter, J.K.; Okamura, F.P.; Ghose, S. A high-temperature structural study of high albite, monalbite, and the analbite to monalbite phase transition. Am. Mineral. 1979, 64, 409–423. [Google Scholar]
- Faust, G.T. The fusion relations of iron-orthoclase. Am. Miner. 1936, 21, 735–763. [Google Scholar]
- Maniatis, Y.; Tite, M.S. Technological examination of Neolithic-Bronze Age pottery from central and southeast Europe and from the Near East. J. Archaeol. Sci. 1981, 8, 59–76. [Google Scholar] [CrossRef]
- Born, M.; Huang, K. Dynamical Theory of Crystal Lattices; Clarendon Press: Oxford, UK, 1954; p. 199. [Google Scholar]
- Colomban, P. On-site Raman study of artwork: Procedure and illustrative examples. J. Raman Spectrosc. 2017, 49, 921–934. [Google Scholar] [CrossRef]
- Caggiani, M.; Colomban, P. Raman microspectroscopy for Cultural Heritage. Phys. Sci. Rev. 2018. [Google Scholar] [CrossRef]

| Element | Diopside | Quartz | Albite | Orthoclase | ||||
|---|---|---|---|---|---|---|---|---|
| Weight % | Atomic % | Weight % | Atomic % | Weight % | Atomic % | Weight % | Atomic % | |
| O K | 50.51 | 65.84 | 60.33 | 72.9 | 53.39 | 66.54 | 47.36 | 63.71 |
| Na K | 0.69 | 0.63 | - | - | 5.02 | 4.36 | 0.37 | 0.35 |
| Mg K | 8.91 | 7.64 | 0.29 | 0.23 | - | - | 1.17 | 1.03 |
| Al K | 2.98 | 2.3 | 1.22 | 0.87 | 10.14 | 7.5 | 14.86 | 11.86 |
| Si K | 23.92 | 17.76 | 37.26 | 25.64 | 27.91 | 19.82 | 22.33 | 17.12 |
| K K | 0.66 | 0.35 | 0.31 | 0.15 | 3.12 | 1.59 | 2.51 | 1.38 |
| Ca K | 5.94 | 3.09 | - | - | 0.41 | 0.2 | 0.7 | 0.38 |
| Fe K | 6.39 | 2.39 | 0.09 | 0.21 | 9.78 | 3.77 | ||
| Totals | 100.00 | 100.00 | 100.00 | 100.00 | ||||
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Grammatikakis, I.E.; Kyriakidis, E.; Demadis, K.D.; Cabeza Diaz, A.; Leon-Reina, L. Mineralogical Characterization and Firing Temperature Delineation on Minoan Pottery, Focusing on the Application of Micro-Raman Spectroscopy. Heritage 2019, 2, 2652-2664. https://doi.org/10.3390/heritage2030163
Grammatikakis IE, Kyriakidis E, Demadis KD, Cabeza Diaz A, Leon-Reina L. Mineralogical Characterization and Firing Temperature Delineation on Minoan Pottery, Focusing on the Application of Micro-Raman Spectroscopy. Heritage. 2019; 2(3):2652-2664. https://doi.org/10.3390/heritage2030163
Chicago/Turabian StyleGrammatikakis, Ioannis E., Evangelos Kyriakidis, Konstantinos D. Demadis, Aurelio Cabeza Diaz, and Laura Leon-Reina. 2019. "Mineralogical Characterization and Firing Temperature Delineation on Minoan Pottery, Focusing on the Application of Micro-Raman Spectroscopy" Heritage 2, no. 3: 2652-2664. https://doi.org/10.3390/heritage2030163
APA StyleGrammatikakis, I. E., Kyriakidis, E., Demadis, K. D., Cabeza Diaz, A., & Leon-Reina, L. (2019). Mineralogical Characterization and Firing Temperature Delineation on Minoan Pottery, Focusing on the Application of Micro-Raman Spectroscopy. Heritage, 2(3), 2652-2664. https://doi.org/10.3390/heritage2030163





