Antioxidant Activity of Selected Medicinal Plants Used by Traditional Herbal Practitioners to Treat Cancer in Malawi
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Plant Materials
2.3. Sample Preparation
2.4. Moisture Content
2.5. Extraction of Phytochemicals
2.6. Preparation of 10 mg/mL and 1 mg/mL Stock and Working Plant Extracts, Respectively
2.7. Determination of FRAP and DPPH Antioxidant Activities
2.8. Total Phenolic, Flavonoid, and Alkaloid Contents
2.9. Statistical Analysis
3. Results
3.1. Moisture Content (%)
3.2. Yield (%) of the Crude Plant Extracts
3.3. FRAP and DPPH Antioxidant Activities of the Plants
3.4. Total Phenolic, Flavonoid, and Alkaloid Contents
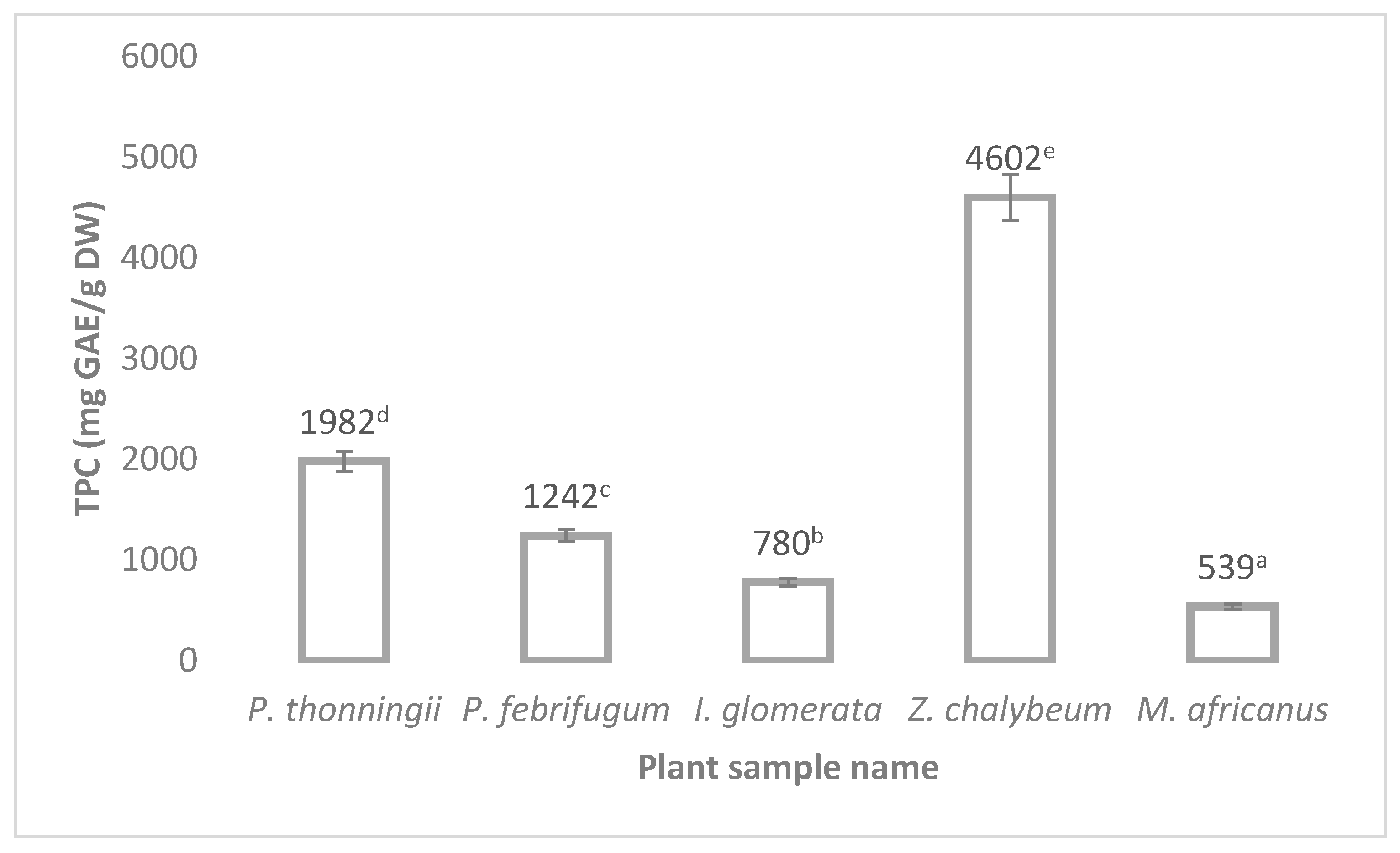
3.4.1. Total Phenolic Content (TPC)
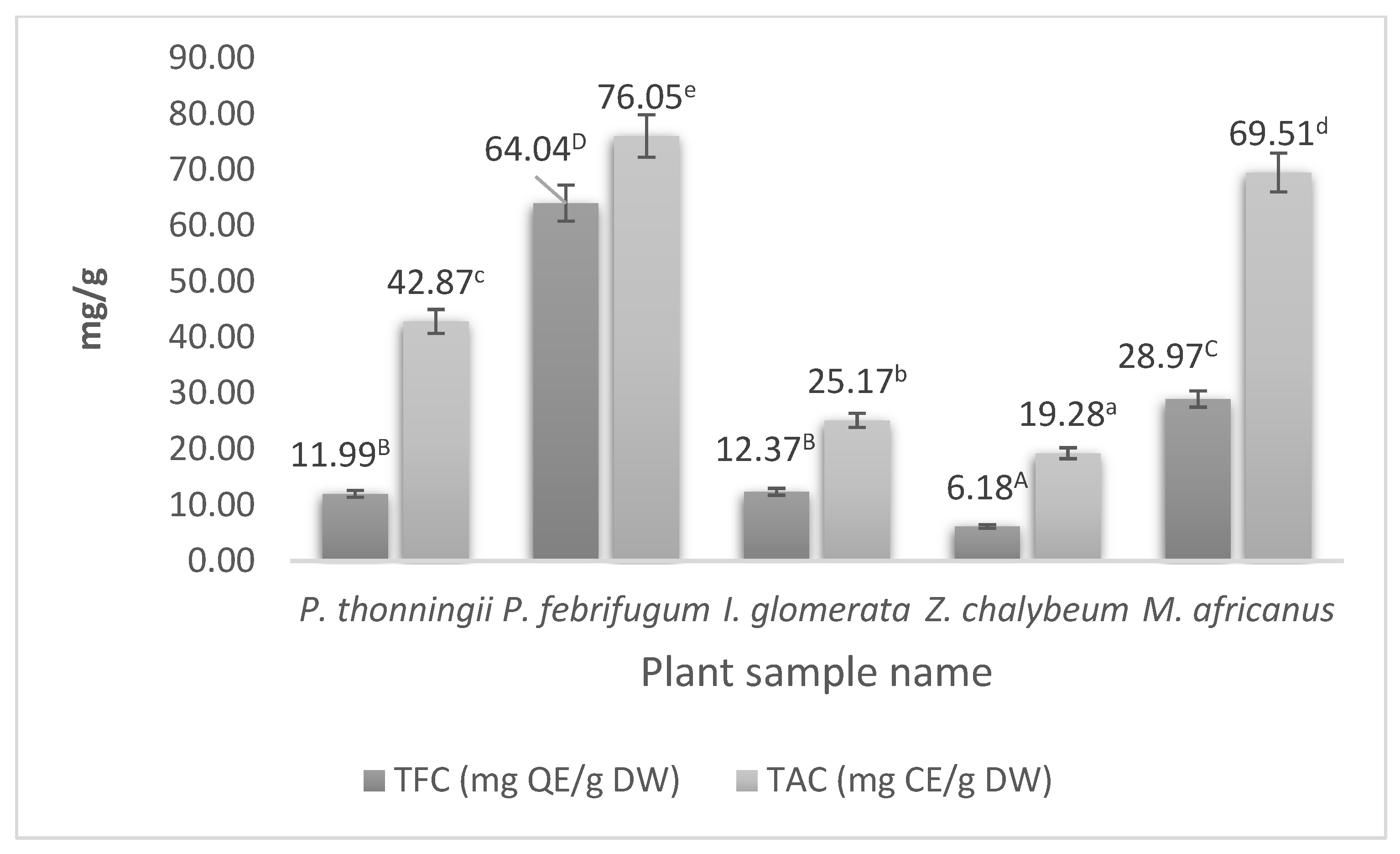
3.4.2. Total Flavonoid Content (TFC)
3.4.3. Total Alkaloid Content (TAC)
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lu, C.-H.; Chen, W.-T.; Hsieh, C.-H.; Kuo, Y.-Y.; Chao, C.-Y. Thermal Cycling-Hyperthermia in Combination with Polyphenols, Epigallocatechin Gallate and Chlorogenic Acid, Exerts Synergistic Anticancer Effect against Human Pancreatic Cancer PANC-1 Cells. PLoS ONE 2019, 14, e0217676. [Google Scholar] [CrossRef]
- Sato, H.; Shibata, M.; Shimizu, T.; Shibata, S.; Toriumi, H.; Ebine, T.; Kuroi, T.; Iwashita, T.; Funakubo, M.; Kayama, Y.; et al. Differential Cellular Localization of Antioxidant Enzymes in the Trigeminal Ganglion. Neuroscience 2013, 248, 345–358. [Google Scholar] [CrossRef]
- Glasauer, A.; Chandel, N.S. Targeting Antioxidants for Cancer Therapy. Biochem. Pharm. 2014, 92, 90–101. [Google Scholar] [CrossRef]
- Hossain, M.A.; Shah, M.D. A Study on the Total Phenols Content and Antioxidant Activity of Essential Oil and Different Solvent Extracts of Endemic Plant Merremia Borneensis. Arab. J. Chem. 2015, 8, 66–71. [Google Scholar] [CrossRef]
- Moriasi, G.; Ireri, A.; Ngugi, M.P. In Vitro Antioxidant Activities of the Aqueous and Methanolic Stem Bark Extracts of Piliostigma thonningii (Schum.). J. Evid. Based Complement. Altern. Med. 2020, 25, 2515690X20937988. [Google Scholar] [CrossRef]
- Huang, W.-J.; Zhang, X.; Chen, W.-W. Role of Oxidative Stress in Alzheimer’s Disease. Biomed. Rep. 2016, 4, 519–522. [Google Scholar] [CrossRef]
- Liguori, I.; Russo, G.; Curcio, F.; Bulli, G.; Aran, L.; Della-Morte, D.; Gargiulo, G.; Testa, G.; Cacciatore, F.; Bonaduce, D.; et al. Oxidative Stress, Aging, and Diseases. CIA 2018, 13, 757–772. [Google Scholar] [CrossRef] [PubMed]
- Ojo, M.C.; Osunsanmi, F.O.; Cele, N.D.; Zharare, G.E.; Mosa, R.A.; Opoku, A.R. In Vitro and in Vivo Antioxidant Potentials of the Methanolic Crude Extract from Inula Glomerata Oliv. & Hiern (Asteraceae) and Salacia Kraussii (Harv.) Harv (Celastraceae). BLACPMA 2021, 20, 416–426. [Google Scholar] [CrossRef]
- Pizzino, G.; Irrera, N.; Cucinotta, M.; Pallio, G.; Mannino, F.; Arcoraci, V.; Squadrito, F.; Altavilla, D.; Bitto, A. Oxidative Stress: Harms and Benefits for Human Health. Oxidative Med. Cell. Longev. 2017, 2017, 8416763. [Google Scholar] [CrossRef] [PubMed]
- Halliwell, B.; Gutteridge, J. Free Radicals in Biology and Medicine, 5th ed.; Oxford University Press: Oxford, UK, 2015. [Google Scholar]
- Bouayed, J.; Bohn, T. Exogenous Antioxidants—Double-Edged Swords in Cellular Redox State: Health Beneficial Effects at Physiologic Doses versus Deleterious Effects at High Doses. Oxidative Med. Cell. Longev. 2010, 3, 228–237. [Google Scholar] [CrossRef]
- Aguilar, T.A.F.; Navarro, B.C.H.; Pérez, J.A.M. Endogenous Antioxidants: A Review of Their Role in Oxidative Stress. In A Master Regulator of Oxidative Stress—The Transcription Factor Nrf2; Morales-Gonzalez, J.A., Morales-Gonzalez, A., Madrigal-Santillan, E.O., Eds.; InTech: Brisbane, Australia, 2016. [Google Scholar] [CrossRef]
- Arulselvan, P.; Fard, M.T.; Tan, W.S.; Gothai, S.; Fakurazi, S.; Norhaizan, M.E.; Kumar, S.S. Role of Antioxidants and Natural Products in Inflammation. Oxidative Med. Cell. Longev. 2016, 2016, 5276130. [Google Scholar] [CrossRef] [PubMed]
- Afolayan, M.; Srivedavyasasri, R.; Asekun, O.T.; Familoni, O.B.; Orishadipe, A.; Bourgaud, F.; Ibrahim, M.A.; Ross, S.A. Phytochemical Study of Piliostigma Thonningii, a Medicinal Plant Grown in Nigeria. Med. Chem. Res. 2018, 27, 2325–2330. [Google Scholar] [CrossRef] [PubMed]
- Kurmukov, A.G. Phytochemistry of Medicinal Plants. In Medicinal Plants of Central Asia: Uzbekistan and Kyrgyzstan; Eisenman, S.W., Zaurov, D.E., Struwe, L., Eds.; Springer: New York, NY, USA, 2013. [Google Scholar] [CrossRef]
- Zhang, Y.-J.; Gan, R.-Y.; Li, S.; Zhou, Y.; Li, A.-N.; Xu, D.-P.; Li, H.-B. Antioxidant Phytochemicals for the Prevention and Treatment of Chronic Diseases. Molecules 2015, 20, 21138–21156. [Google Scholar] [CrossRef]
- Zhen, J.; Guo, Y.; Villani, T.; Carr, S.; Brendler, T.; Mumbengegwi, D.R.; Kong, A.-N.T.; Simon, J.E.; Wu, Q. Phytochemical Analysis and Anti-Inflammatory Activity of the Extracts of the African Medicinal Plant Ximenia Caffra. J. Anal. Methods Chem. 2015, 2015, 948262. [Google Scholar] [CrossRef] [PubMed]
- Lourenço, S.C.; Moldão-Martins, M.; Alves, V.D. Antioxidants of Natural Plant Origins: From Sources to Food Industry Applications. Molecules 2019, 24, 4132. [Google Scholar] [CrossRef]
- Liu, Z.; Ren, Z.; Zhang, J.; Chuang, C.-C.; Kandaswamy, E.; Zhou, T.; Zuo, L. Role of ROS and Nutritional Antioxidants in Human Diseases. Front. Physiol. 2018, 9, 477. [Google Scholar] [CrossRef]
- Alagbe, J.O. Proximate, Mineral and Phytochemical Analysis of Piliostigma Thonningii Stem Bark and Roots. Int. J. Biol. Phys. Chem. Stud. 2019, 1, 1–7. [Google Scholar]
- Hyun, T.K.; Kim, H.-C.; Ko, Y.-J.; Kim, J.-S. Antioxidant, α-Glucosidase Inhibitory and Anti-Inflammatory Effects of Aerial Parts Extract from Korean Crowberry (Empetrum nigrum Var. Japonicum). Saudi J. Biol. Sci. 2016, 23, 181–188. [Google Scholar] [CrossRef]
- Chikuni, A.C.; Maliwichi, C.P.; Mwanyambo, M.L. National Herbarium and Botanical Gardens of Malawi. An Overview of Traditional Medicine and Medicinal Plant Research in Malawi: Achievements and Priorities. Conserve Afr. Med. Plants 2013, 1–5. Available online: https://www.glinus.com/2013/12/an-overview-of-traditional-medicine-and.html (accessed on 10 April 2023).
- Mwamatope, B.; Tembo, D.; Chikowe, I.; Kampira, E.; Nyirenda, C. Total Phenolic Contents and Antioxidant Activity of Senna Singueana, Melia Azedarach, Moringa Oleifera and Lannea Discolor Herbal Plants. Sci. Afr. 2020, 9, 7. [Google Scholar] [CrossRef]
- Konan, Y.; Witabouna, K.M.; Bassirou, B.; Kagoyire, K. Antioxidant Activity and Total Phenolic Content of Nine Plants from Côte d’Ivoire (West Africa). J. Appl. Pharm. Sci. 2014, 4, 36–41. [Google Scholar] [CrossRef]
- Nantongo, J.S.; Odoi, J.B.; Abigaba, G.; Gwali, S. Variability of Phenolic and Alkaloid Content in Different Plant Parts of Carissa Edulis Vahl and Zanthoxylum Chalybeum Engl. BMC Res. Notes 2018, 11, 125. [Google Scholar] [CrossRef]
- Meragelman, K.M.; McKee, T.C.; Boyd, M.R. Anti-HIV Prenylated Flavonoids from Monotes Africanus. J. Nat. Prod. 2001, 64, 546–548. [Google Scholar] [CrossRef]
- Jere, J.; Chimbayo, E.; Maiden, P.; Kampira, E.; Lampiao, F. Assessing the Cytotoxic Effects of the Anti-HIV Drug, Garani MW1. SMU Med. J. 2016, 3, 346–351. [Google Scholar]
- Imad, H.H.; Hussein, J.H.; Muhanned, A.K.; Nidaa, S.H. Identification of Five Newly Described Bioactive Chemical Compounds in Methanolic Extract of Mentha Viridis by Using Gas Chromatography—Mass Spectrometry (GC-MS). J. Pharm. Phytother. 2015, 7, 107–125. [Google Scholar] [CrossRef]
- Tembo, D.T.; Holmes, M.J.; Marshall, L.J. Effect of Thermal Treatment and Storage on Bioactive Compounds, Organic Acids and Antioxidant Activity of Baobab Fruit (Adansonia digitata) Pulp from Malawi. J. Food Comp. Anal. 2017, 58, 40–51. [Google Scholar] [CrossRef]
- Msiska, T.; Mwakikunga, A.; Tembo, D.; Lampiao, F. A Phytochemical Analysis and in Vivo Effects of an Herbal Aphrodisiac Newtonia Hildebrandtii on Male Wistar Rat Reproductive System. Pharm. Res. 2020, 12, 8. [Google Scholar]
- Santos, E.S.; Luís, Â.; Alagbe, J.O.; Rosado, T.; Pereira, L.; Gallardo, E.; Duarte, A.P. Julbernardia Paniculata and Pterocarpus Angolensis: From Ethnobotanical Surveys to Phytochemical Characterization and Bioactivities Evaluation. Molecules 2020, 25, 1828. [Google Scholar] [CrossRef]
- Chigayo, K.; Mojapelo, P.E.L.; Mnyakeni-Moleele, S.; Misihairabgwi, J.M. Phytochemical and Antioxidant Properties of Different Solvent Extracts of Kirkia Wilmsii Tubers. Asian Pac. J. Trop. Biomed. 2016, 6, 1037–1043. [Google Scholar] [CrossRef]
- Anwar, F.; Przybylski, R. Effect of Solvents Extraction on Total Phenolics and Antioxidant Activity of Extracts from Flaxseed (Linum usitatissimum L.). Acta Sci. Pol. Technol. Aliment. 2012, 11, 293–301. [Google Scholar]
- Che Sulaiman, I.S.; Basri, M.; Fard Masoumi, H.R.; Chee, W.J.; Ashari, S.E.; Ismail, M. Effects of Temperature, Time, and Solvent Ratio on the Extraction of Phenolic Compounds and the Anti-Radical Activity of Clinacanthus Nutans Lindau Leaves by Response Surface Methodology. Chem. Cent. J. 2017, 11, 54. [Google Scholar] [CrossRef]
- Cotton, F.A.; Wilknson, G.; Murillo, C.A.; Bochamann, W. Advanced Inorganic Chemistry, 6th ed.; John Wiley & Sons: New York, NY, USA, 1999. [Google Scholar]
- Ahamed, A.; Panneerselvam, A.; Alaklabi, A.; Arif, I.A.; Ambikapathy, V.; Thajuddin, N. Molecular Perspective and Anticancer Activity of Medicinal Plants. Saudi J. Biol. Sci. 2020, 27, 666–675. [Google Scholar] [CrossRef]
- Chiribagula, V.B.; Bakari, A.S.; Ndjolo, P.O.; Kahumba, B.J.; Simbi, J.L. Phytochemical Screening and Antioxidant Activity of Methanolic Extracts of 53 Antimalarial Plants from Bagira in Eastern DR Congo. GSC Biol. Pharm. Sci. 2020, 12, 099–118. [Google Scholar] [CrossRef]
- Molyneux, P. The Use of the Stable Free Radical Diphenylpicrylhydrazyl (DPPH) for Estimating Antioxidant Activity. Songklanakarin J. Sci. Technol. 2004, 26, 211–219. [Google Scholar]
- Masalu, R.J.; Hosea, K.M.; Malendeja, S. Free Radical Scavenging Activity of Some Fungi Indigenous to Tanzania. Tanzan. J. Health Res. 2012, 14, 1–8. [Google Scholar] [CrossRef]
- Mishra, P.; Sohrab, S.; Mishra, S.K. A review on the phytochemical and pharmacological properties of Hyptis suaveolens (L.) Poit. Future J. Pharm. Sci. 2021, 7, 1. [Google Scholar] [CrossRef]
- Rohmah, M.; Saragih, B.; Amaliah, N.; Kristopal, K.; Putra, Y.H.E.; Rahmadi, A. Determination of Moisture, Ash, Protein, Polyphenolic, Flavonoids, and Amino Acid Contents and Antioxidant Capacity of Dried Mekai (Pycnarrhena tumefacta Miers) Leaf as Potential Herbal Flavor Enhancers. In Proceedings of the International Conference on Tropical Agrifood, Feed and Fuel (ICTAFF 2021), Online, 7 September 2021; Atlantis Press: Samarinda, Indonesia, 2022. [Google Scholar] [CrossRef]
- Bergman, R. Drying and Contols of Moisture Content and Dimensional Changes; Forest Products Laboratory, The United States Depatment of Agriculture, University of Wisconsin: Madison, WI, USA, 2021; pp. 13–19. [Google Scholar]
- Kamanula, J.F.; Belmain, S.R.; Hall, D.R.; Farman, D.I.; Goyder, D.J.; Mvumi, B.M.; Masumbu, F.F.; Stevenson, P.C. Chemical Variation and Insecticidal Activity of Lippia Javanica (Burm. f.) Spreng Essential Oil against Sitophilus Zeamais Motschulsky. Ind. Crops Prod. 2017, 110, 75–82. [Google Scholar] [CrossRef]
- Surveswaran, S.; Cai, Y.-Z.; Corke, H.; Mei, S. Systematic Evaluation of Natural Phenolic Antioxidants from 133 Indian Medicinal Plants. Food Chem. 2007, 102, 938–953. [Google Scholar] [CrossRef]
- Taha, M.M.; Abdelwahab, S.I.; Mariod, A.; Abdulla, M.A.; Nordin, N.; Jayapalan, J.J.; Hashim, O.H. Stem bark methanolic extract of Bauhinia thonningii (Schum) demonstrates gastroprotective properties against in vivo ethanol-induced injury. Funct. Food Sci. 2023, 3, 26–40. [Google Scholar] [CrossRef]
- Vicente, S.J.V.; Queiroz, Y.S.; Gotlieb, S.L.D.; Torres, E.A.F.S. Stability of Phenolic Compounds and Antioxidant Capacity of Regular and Decaffeinated Coffees. Braz. Arch. Biol. Technol. 2014, 57, 110–118. [Google Scholar] [CrossRef]
- Bhandari, K.; Singla, R.K.; De, B.; Ghosh, B.C.; Katakam, P.; Khushwaha, D.P.; Gundamaraju, R.; Sen, G.; Saha, G.; Mitra, A.; et al. Chemometrics Based Extraction of Polyphenolics from Fresh Tea Leaves and Processed Tea Showing In- Silico Docking and Antioxidative Theronostic Dietary Adjuvant in Alzheimer. IGJPS 2015, 5, 171–191. [Google Scholar] [CrossRef]
- Vichapong, J.; Sookserm, M.; Srijesdaruk, V.; Swatsitang, P.; Srijaranai, S. High Performance Liquid Chromatographic Analysis of Phenolic Compounds and Their Antioxidant Activities in Rice Varieties. LWT 2010, 43, 1325–1330. [Google Scholar] [CrossRef]
- Sombie, E.N.; Tibiri, A.; N’do, J.Y.; Traore, T.K.; Ouedraogo, N.; Hilou, A.; Guissou, P.I.; Nacoulma, O.G. Ethnobotanical Study and Antioxidant Activity of Anti-Hepatitis Plants Extracts of the COMOE Province, Burkina Faso. Int. J. Biol. Chem. Sci. 2018, 12, 1308. [Google Scholar] [CrossRef]
- Olafadehan, O.A.; Oluwafemi, R.A.; Alagbe, J.O. Performance, Haemato-Biochemical Parameters of Broiler Chicks Administered Rolfe (Daniellia oliveri) Leaf Extract as an Antibiotic Alternative. Drug Discov. 2020, 14, 135–145. [Google Scholar]
- Oluwafemi, R.A.; Olawale, A.I.; Alagbe, J.O. Recent trends in the utilization of medicinal plants as growth promoters in poultry nutrition-A review. Res. Agri. Vet. Sci. 2020, 4, 5–11. [Google Scholar]
- Alsiede, M.M.S.A.; Abddrahman, M.A.; Saeed, A.E.M. Phytochemical Screening, Total Phenolics Content and Antioxidants Activity of Cassia singueana. J. Med. Plants Stud. 2015, 3, 160–165. [Google Scholar]
- Saleem, R.; Ahmed, M.; Ahmed, S.I.; Azeem, M.; Khan, R.A.; Rasool, N.; Saleem, H.; Noor, F.; Faizi, S. Hypotensive Activity and Toxicology of Constituents from Root Bark OfPolyalthia Longifolia Var.Pendula. Phytother. Res. 2005, 19, 881–884. [Google Scholar] [CrossRef]
- Chanda, S.; Nagani, K. In vitro and in vivo Methods for Anticancer Activity Evaluation and Some Indian Medicinal Plants Possessing Anticancer Properties: An Overview. J. Pharmacogn. Phytochem. 2013, 2, 13. [Google Scholar]
- Olajuyigbe, O.O.; Afolayan, A.J. Phenolic Content and Antioxidant Property of the Bark Extracts of Ziziphus Mucronata Willd. Subsp. Mucronata Willd. BMC Complement. Altern. Med. 2011, 11, 130. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.H. Potential Synergy of Phytochemicals in Cancer Prevention: Mechanism of Action 1. Am. Soc. Nut. Sci. 2004, 134, 3479–3485. [Google Scholar] [CrossRef]
- Larbat, R.; Le Bot, J.; Bourgaud, F.; Robin, C.; Adamowicz, S. Organ-Specific Responses of Tomato Growth and Phenolic Metabolism to Nitrate Limitation: Organ-Specific Responses to Nitrate Limitation. Plant Biol. 2012, 14, 760–769. [Google Scholar] [CrossRef] [PubMed]
- Larbat, R.; Paris, C.; Le Bot, J.; Adamowicz, S. Phenolic Characterization and Variability in Leaves, Stems and Roots of Micro-Tom and Patio Tomatoes, in Response to Nitrogen Limitation. Plant Sci. 2014, 224, 62–73. [Google Scholar] [CrossRef] [PubMed]
- War, A.R.; Paulraj, M.G.; Ahmad, T.; Buhroo, A.A.; Hussain, B.; Ignacimuthu, S.; Sharma, H.C. Mechanisms of Plant Defense against Insect Herbivores. Plant Signal. Behav. 2012, 7, 1306–1320. [Google Scholar] [CrossRef] [PubMed]
| Plant | P. thonningii (Schumach) Milne-Redh | P. febrifugum Spach | I. glomerata Oliv. and Hiern | Z. chalybeum Eng. | M. africanus A.DC |
|---|---|---|---|---|---|
| % Moisture % Yield | 17.62 ± 0.21 a 49 ± 0.15% | 16.67 ± 0.14 b 45 ± 0.10% | 17.40 ± 0.19 a 6 ± 0.03% | 14.57 ±0.11 d 20 ± 0.05% | 15.72 ± 0.13 c 19 ± 0.03% |
| Reference | 39% [5] | 30.8% [24] | 8.5% [8] | N/A | N/A |
| Medicinal Plants | FRAP (mg TEAC/g DW) | SA50 (DPPH) (μg/mL) |
|---|---|---|
| P. thonningii (Schumach.) Milne-Redh | 687.28 ± 0.71 a | 0.09 ± 0.01 a |
| P. febrifugum Spach | 401.11 ± 0.41 b | 0.21 ± 0.01 a |
| I. glomerata Oliv. and Hiern | 120.23 ± 0.12 e | 0.14 ± 0.01 a |
| Z. chalybeum Engl. | 82.15 ± 0.07 c | 1.57 ± 0.01 c |
| M. africanus A.DC Trolox | 123.86 ± 0.14 d | 1.29 ± 0.02 b 0.05 ± 0.01 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Masumbu, F.F.F.; Kamanula, J.F.; Mwakikunga, A.; Mwamatope, B.; Tembo, D. Antioxidant Activity of Selected Medicinal Plants Used by Traditional Herbal Practitioners to Treat Cancer in Malawi. J 2023, 6, 592-604. https://doi.org/10.3390/j6040039
Masumbu FFF, Kamanula JF, Mwakikunga A, Mwamatope B, Tembo D. Antioxidant Activity of Selected Medicinal Plants Used by Traditional Herbal Practitioners to Treat Cancer in Malawi. J. 2023; 6(4):592-604. https://doi.org/10.3390/j6040039
Chicago/Turabian StyleMasumbu, Friday Fosta Fred, John Finias Kamanula, Anthony Mwakikunga, Bonface Mwamatope, and David Tembo. 2023. "Antioxidant Activity of Selected Medicinal Plants Used by Traditional Herbal Practitioners to Treat Cancer in Malawi" J 6, no. 4: 592-604. https://doi.org/10.3390/j6040039






