Impact of Drought and Changing Water Sources on Water Use and Soil Salinity of Almond and Pistachio Orchards: 2. Modeling
Abstract
:1. Introduction
2. Materials and Methods
2.1. Field Sites
2.2. Model Setup
2.2.1. Water Flow
2.2.2. Initial and Boundary Conditions
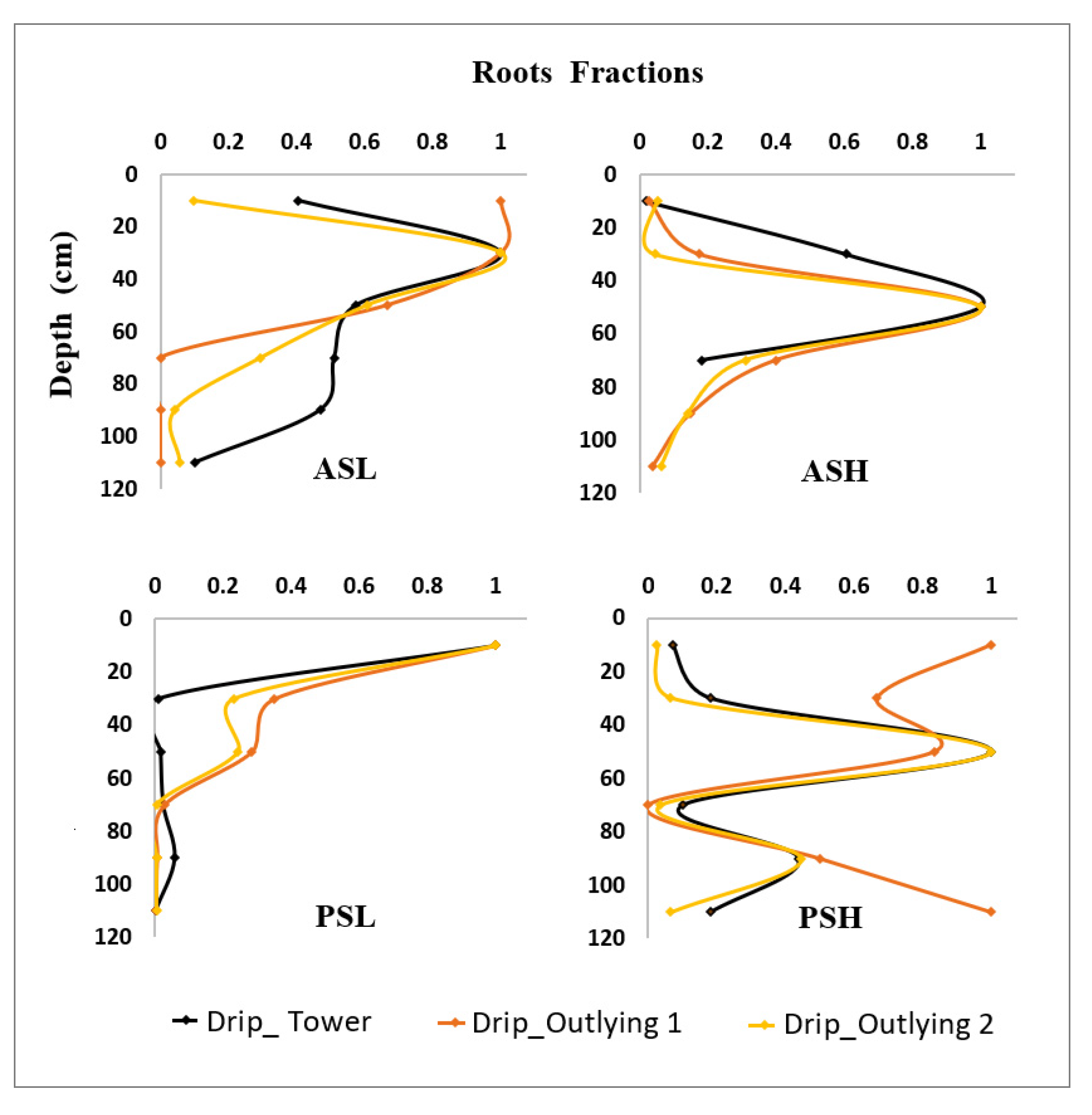
2.2.3. Root Water Uptake
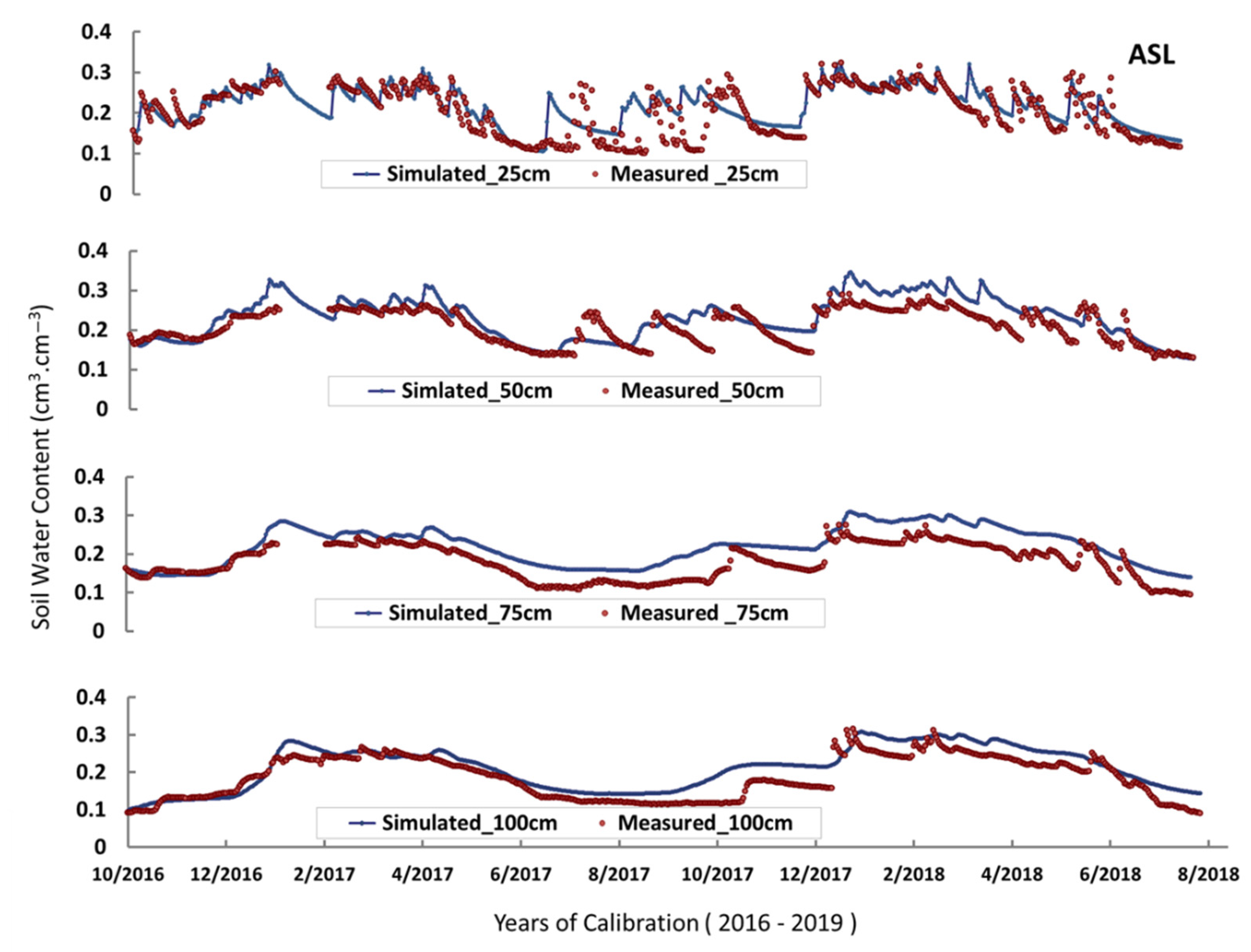
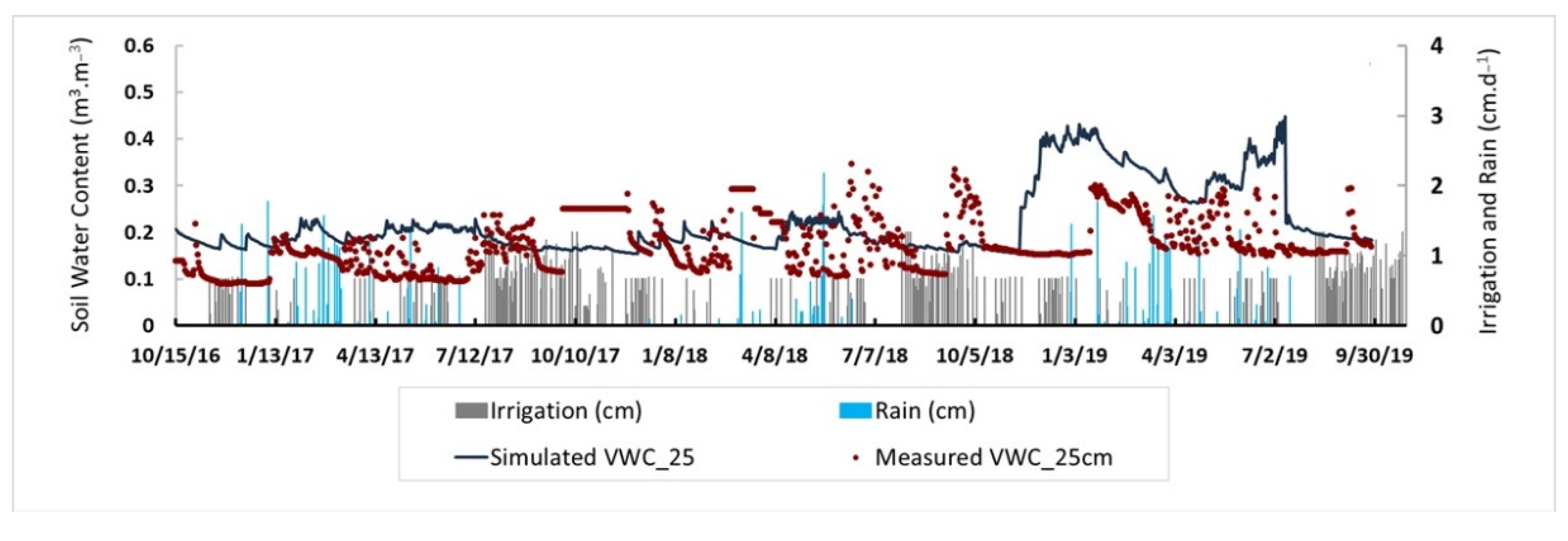
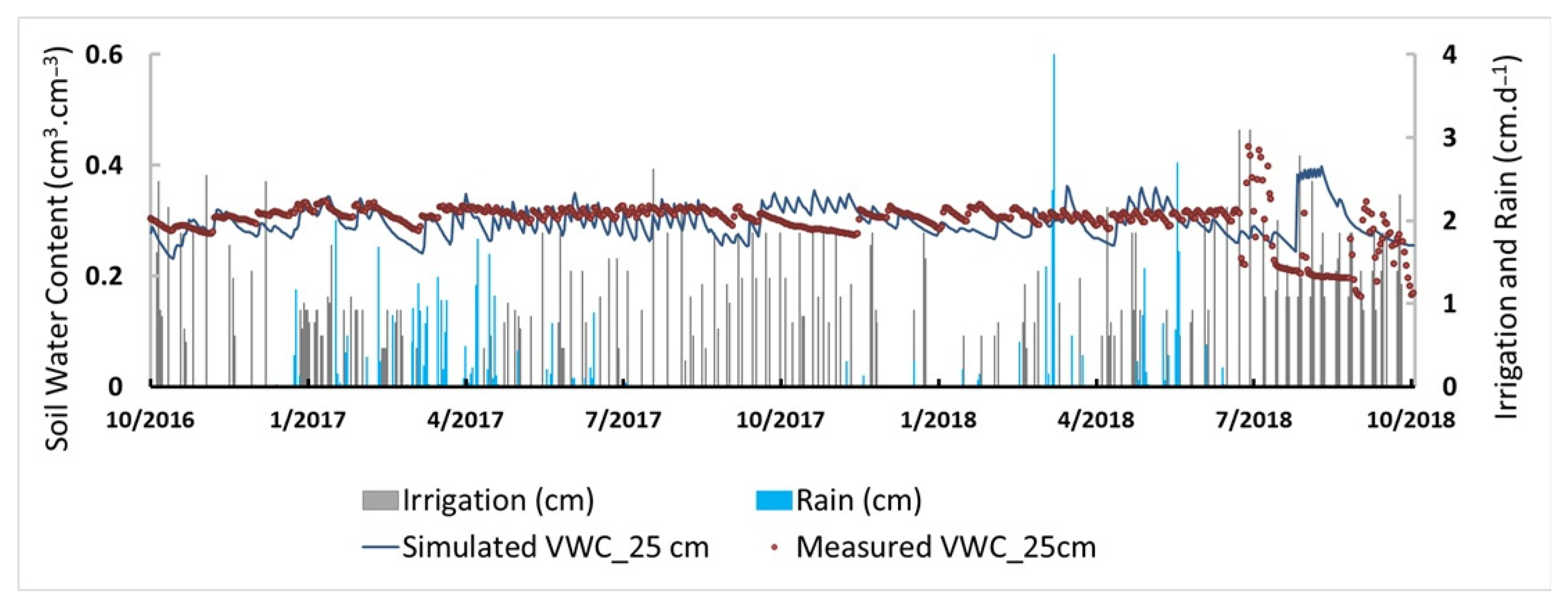
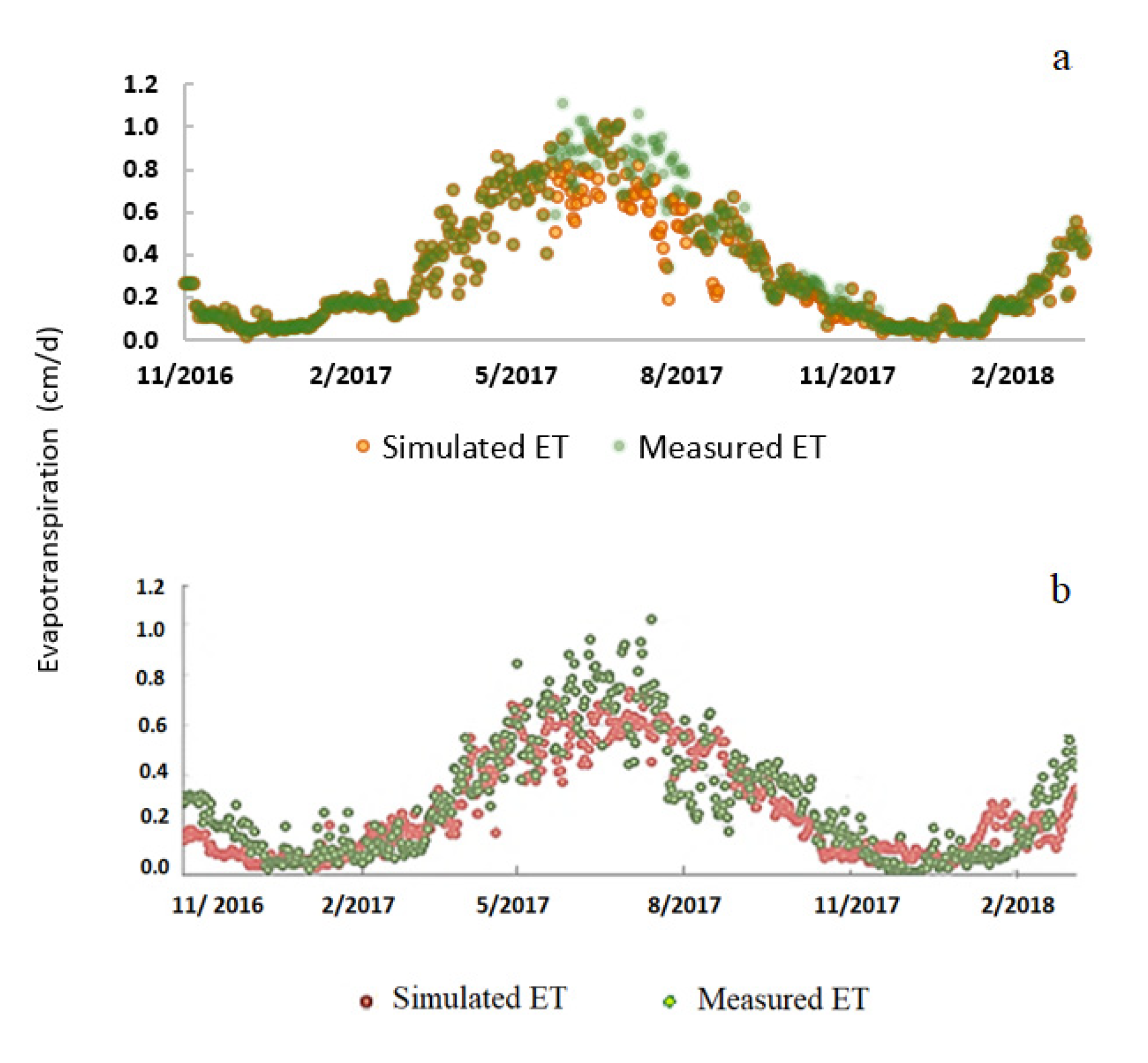
2.3. Model Calibration and Validation
3. Results
3.1. Root Zone Water Content
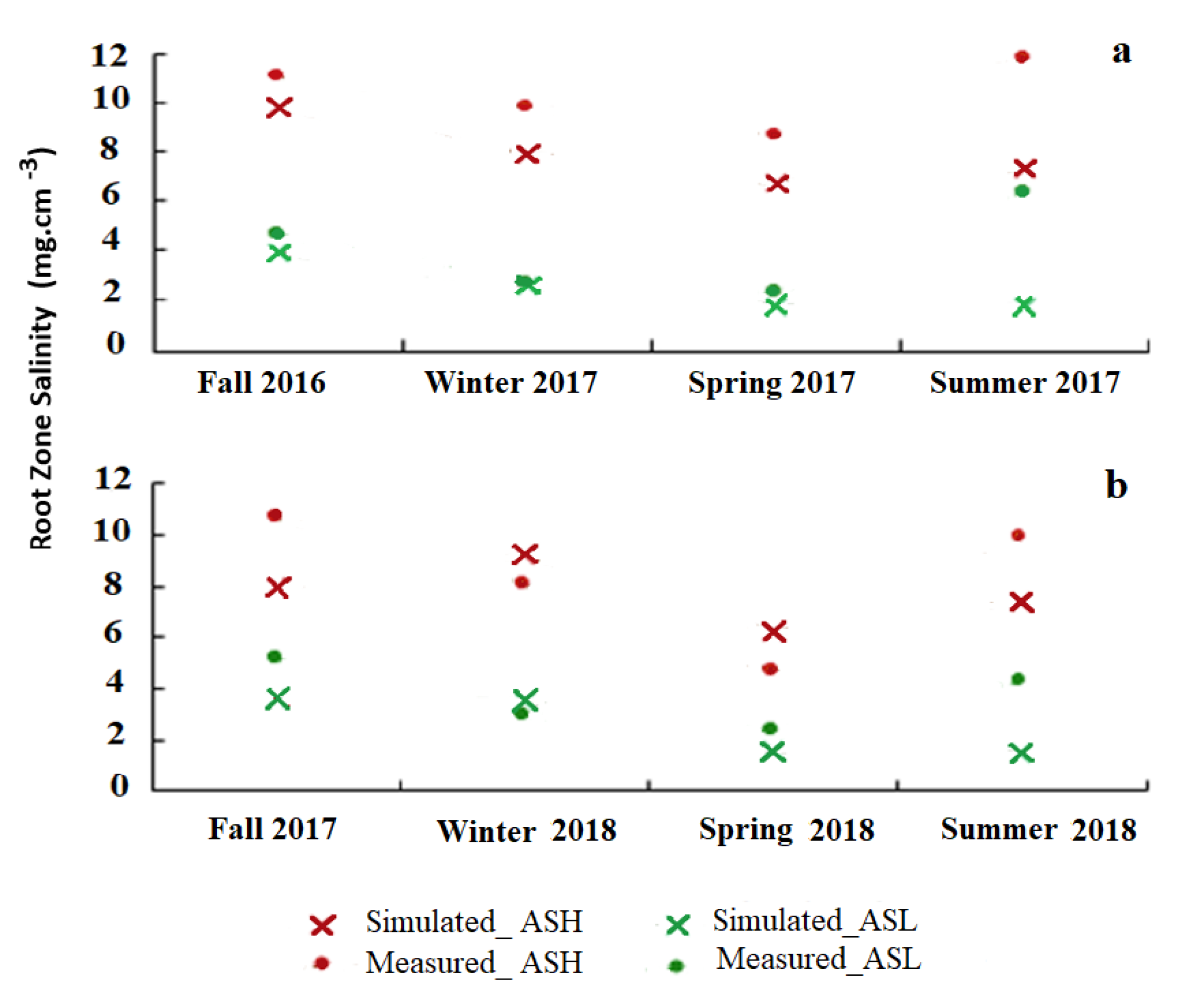
3.2. Root Zone Salinity
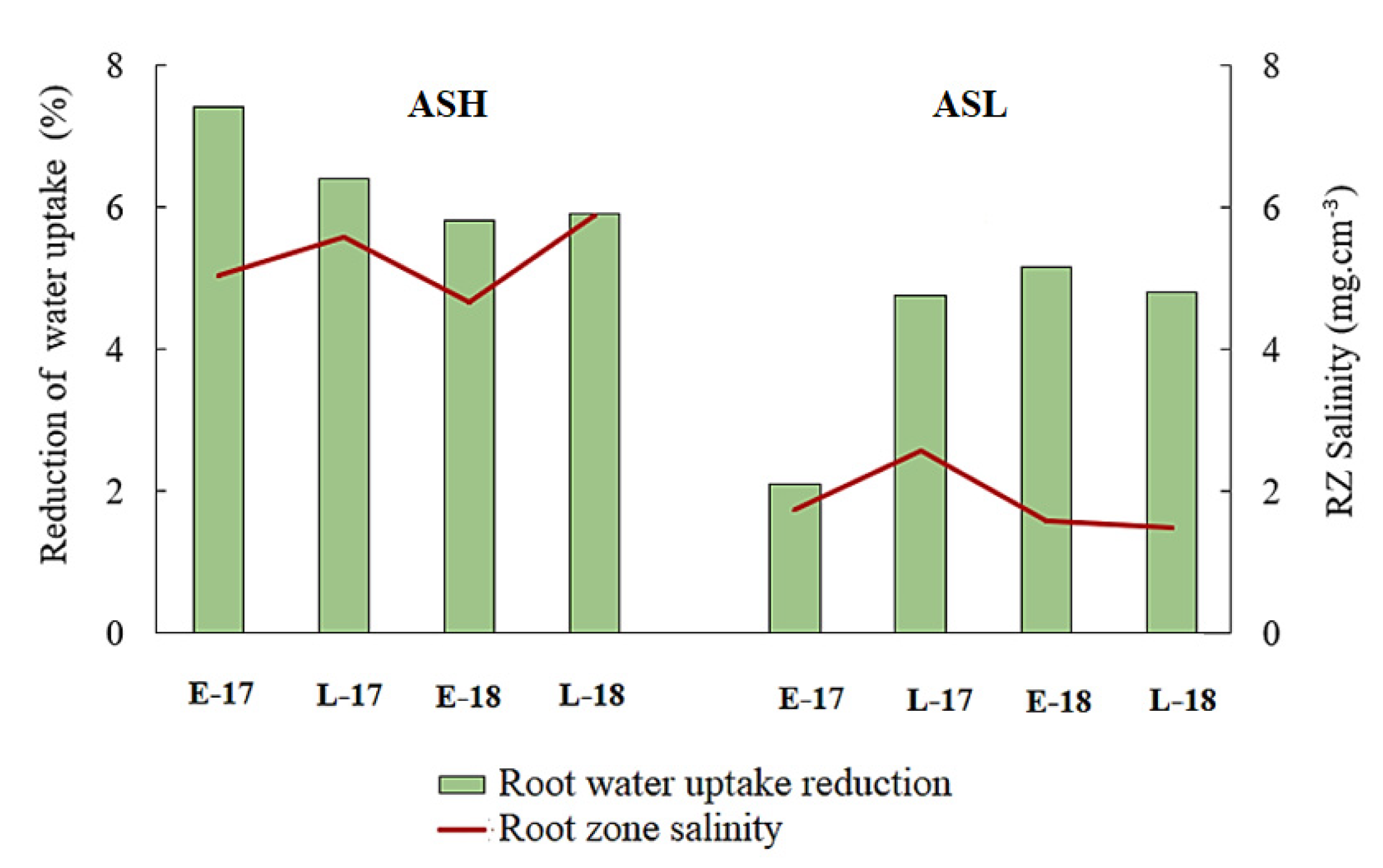
3.3. Salinity Impacts on Root Water Uptake
3.4. The Impact of Salt Leaching on Root Water Uptake
4. Summary and Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Green, T.R.; Ahuja, L.R.; Benjamin, J.G. Advances and Challenges in Predicting Agricultural Management Effects on Soil Hydraulic Properties. Geoderma 2003, 116, 3–27. [Google Scholar] [CrossRef]
- Schoups, G.; Hopmans, J.W.; Young, C.A.; Vrugt, J.A.; Wallender, W.W.; Tanji, K.K.; Panday, S. Sustainability of Irrigated Agriculture in the San Joaquin Valley, California. Proc. Natl. Acad. Sci. USA 2005, 102, 15352–15356. [Google Scholar] [CrossRef] [Green Version]
- Li, X.; Kang, Y.; Wan, S.; Chen, X.; Liu, S.; Xu, J. Response of a Salt-Sensitive Plant to Processes of Soil Reclamation in Two Saline-Sodic, Coastal Soils Using Drip Irrigation with Saline Water. Agric. Water Manag. 2016, 164, 223–234. [Google Scholar] [CrossRef]
- Li, H.; Yi, J.; Zhang, J.; Zhao, Y.; Si, B.; Hill, R.L.; Cui, L.; Liu, X. Modeling of Soil Water and Salt Dynamics and Its Effects on Root Water Uptake in Heihe Arid Wetland, Gansu, China. Water 2015, 7, 2382–2401. [Google Scholar] [CrossRef] [Green Version]
- Phogat, V.; Pitt, T.; Cox, J.W.; Šimůnek, J.; Skewes, M.A. Soil Water and Salinity Dynamics under Sprinkler Irrigated Almond Exposed to a Varied Salinity Stress at Different Growth Stages. Agric. Water Manag. 2018, 201, 70–82. [Google Scholar] [CrossRef] [Green Version]
- Phogat, V.; Mallants, D.; Cox, J.W.; Šimůnek, J.; Oliver, D.P.; Awad, J. Management of Soil Salinity Associated with Irrigation of Protected Crops. Agric. Water Manag. 2020, 227. [Google Scholar] [CrossRef]
- Ramos, T.B.; Darouich, H.; Šimůnek, J.; Gonçalves, M.C.; Martins, J.C. Soil Salinization in Very High-Density Olive Orchards Grown in Southern Portugal: Current Risks and Possible Trends. Agric. Water Manag. 2019, 217. [Google Scholar] [CrossRef] [Green Version]
- Suarez, D.L. Modeling transient root zone salinity (SWS model). In Agricultural Salinity Assessment and Management; Wallender, W.W., Tanji, K.K., Eds.; American Society of Civil Engineers: Reston, VA, USA, 2011; pp. 856–897. ISBN 978-0-7844-1169-8. [Google Scholar]
- Minhas, P.S.; Ramos, T.B.; Ben-Gal, A.; Pereira, L.S. Coping with Salinity in Irrigated Agriculture: Crop Evapotranspiration and Water Management Issues. Agric. Water Manag. 2020, 227, 105832. [Google Scholar] [CrossRef]
- Shrivastava, P.; Kumar, R. Soil Salinity: A Serious Environmental Issue and Plant Growth Promoting Bacteria as One of the Tools for Its Alleviation. Saudi J. Biol. Sci. 2015, 22, 123–131. [Google Scholar] [CrossRef] [Green Version]
- Lobell, D.B.; Burke, M.B. On the Use of Statistical Models to Predict Crop Yield Responses to Climate Change. Agric. For. Meteorol. 2010, 150, 1443–1452. [Google Scholar] [CrossRef]
- Rasouli, F.; Kiani Pouya, A.; Šimůnek, J. Modeling the Effects of Saline Water Use in Wheat-Cultivated Lands Using the UNSATCHEM Model. Irrig. Sci. 2013, 31, 1009–1024. [Google Scholar] [CrossRef] [Green Version]
- Kumar, R.; Shankar, V.; Jat, M.K. Evaluation of Root Water Uptake Models—A Review. ISH J. Hydraul. Eng. 2015, 21, 115–124. [Google Scholar] [CrossRef]
- Šimůnek, J.; Šejna, M.; Saito, H.; Sakai, M.; Van Genuchten, M. The HYDRUS-1D Software Package for Simulating the One-Dimensional Movement of Water, Heat, and Multiple Solutes in Variably-Saturated Media; Version 4.17, HYDRUS Software Series 3; Department of Environmental Sciences, University of California Riverside: Riverside, CA, USA, 2008. [Google Scholar]
- Feddes, R.A.; Raats, P.A.C. Parameterizing the soil–water–plant root system. In Unsaturated-Zone Modeling; Progress, Challenges and Applications; Feddes, R.A., de Rooij, G.H., van Dam, J.C., Eds.; Wageningen UR Frontis Series; Kluwer Academic Publishers: Dordrecht, The Netherlands, 2004; pp. 95–141. ISBN 978-1-4020-2918-9. [Google Scholar]
- Oster, J.D.; Letey, J.; Vaughan, P.; Wu, L.; Qadir, M. Comparison of Transient State Models That Include Salinity and Matric Stress Effects on Plant Yield. Agric. Water Manag. 2012, 103, 167–175. [Google Scholar] [CrossRef]
- Gigante, V.; Iacobellis, V.; Manfreda, S.; Milella, P.; Portoghese, I. Influences of Leaf Area Index Estimations on Water Balance Modeling in a Mediterranean Semi-Arid Basin. Nat. Hazards Earth Syst. Sci. 2009, 9, 979–991. [Google Scholar] [CrossRef] [Green Version]
- Letey, J.; Hoffman, G.J.; Hopmans, J.W.; Grattan, S.R.; Suarez, D.; Corwin, D.L.; Oster, J.D.; Wu, L.; Amrhein, C. Evaluation of Soil Salinity Leaching Requirement Guidelines. Agric. Water Manag. 2011, 98, 502–506. [Google Scholar] [CrossRef] [Green Version]
- Kaledhonkar, M.J.; Keshari, A.K. Modelling the Effects of Saline Water Use in Agriculture. Irrig. Drain. 2006, 55, 177–190. [Google Scholar] [CrossRef]
- Kaledhonkar, M.J.; Sharma, D.R.; Tyagi, N.K.; Kumar, A.; Van Der Zee, S.E.A.T.M. Modeling for Conjunctive Use Irrigation Planning in Sodic Groundwater Areas. Agric. Water Manag. 2012, 107, 14–22. [Google Scholar] [CrossRef]
- Gonçalves, M.C.; Šimůnek, J.; Ramos, T.B.; Martins, J.C.; Neves, M.J.; Pires, F.P. Multicomponent Solute Transport in Soil Lysimeters Irrigated with Waters of Different Quality. Water Resour. Res. 2006, 42. [Google Scholar] [CrossRef] [Green Version]
- Ramos, T.B.; Šimůnek, J.; Gonçalves, M.C.; Martins, J.C.; Prazeres, A.; Castanheira, N.L.; Pereira, L.S. Field Evaluation of a Multicomponent Solute Transport Model in Soils Irrigated with Saline Waters. J. Hydrol. 2011, 407, 129–144. [Google Scholar] [CrossRef]
- Zeng, Z.; Wang, T.; Zhou, F.; Ciais, P.; Mao, J.; Shi, X.; Piao, S. A Worldwide Analysis of Spatiotemporal Changes in Water Balance-Based Evapotranspiration from 1982 to 2009. J. Geophys. Res. Atmos. 2014, 119, 1186–1202. [Google Scholar] [CrossRef]
- Suarez, D.L.; Wood, J.D.; Lesch, S.M. Effect of SAR on Water Infiltration under a Sequential Rain–Irrigation Management System. Agric. Water Manag. 2006, 86, 150–164. [Google Scholar] [CrossRef]
- Haj-Amor, Z.; Ibrahimi, M.-K.; Feki, N.; Lhomme, J.-P.; Bouri, S. Soil Salinisation and Irrigation Management of Date Palms in a Saharan Environment. Environ. Monit. Assess. 2016, 188, 497. [Google Scholar] [CrossRef] [PubMed]
- Ramos, T.B.; Šimůnek, J.; Gonçalves, M.C.; Martins, J.C.; Prazeres, A.; Pereira, L.S. Two-Dimensional Modeling of Water and Nitrogen Fate from Sweet Sorghum Irrigated with Fresh and Blended Saline Waters. Agric. Water Manag. 2012, 111, 87–104. [Google Scholar] [CrossRef]
- Wang, J.; Bai, Z.; Yang, P. Mechanism and Numerical Simulation of Multicomponent Solute Transport in Sodic Soils Reclaimed by Calcium Sulfate. Environ. Earth Sci. 2014, 72, 157–169. [Google Scholar] [CrossRef]
- Schaap, M.G.; Leij, F.J.; van Genuchten, M.T. Rosetta: A Computer Program for Estimating Soil Hydraulic Parameters with Hierarchical Pedotransfer Functions. J. Hydrol. 2001, 251, 163–176. [Google Scholar] [CrossRef]
- van Genuchten, M.T. A Closed-Form Equation for Predicting the Hydraulic Conductivity of Unsaturated Soils. Soil Sci. Soc. Am. J. 1980, 44, 892–898. [Google Scholar] [CrossRef] [Green Version]
- Jacques, D.; Šimůnek, J.; Timmerman, A.; Feyen, J. Calibration of Richards’ and Convection–Dispersion Equations to Field-Scale Water Flow and Solute Transport under Rainfall Conditions. J. Hydrol. 2002, 259, 15–31. [Google Scholar] [CrossRef]
- Helalia, S.A.; Anderson, R.G.; Skaggs, T.H.; Jenerette, G.D.; Wang, D.; Šimůnek, J. Impact of Drought and Changing Water Sources on Water Use and Soil Salinity of Almond and Pistachio Orchards: 1. Observations. Soil Syst. 2021, 5, 50. [Google Scholar] [CrossRef]
- Anderson, R. AmeriFlux US-ASH USSL San Joaquin Valley Almond High Salinity. 2020. Available online: https://ameriflux.lbl.gov/sites/siteinfo/US-ASH (accessed on 1 September 2020).
- Anderson, R. AmeriFlux US-ASM USSL San Joaquin Valley Almond Medium Salinity. 2020. Available online: https://ameriflux.lbl.gov/sites/siteinfo/US-ASM (accessed on 1 September 2020).
- Anderson, R. AmeriFlux US-ASL USSL San Joaquin Valley Almond Low Salinity. 2020. Available online: https://ameriflux.lbl.gov/sites/siteinfo/US-ASL (accessed on 1 September 2020).
- Anderson, R. AmeriFlux US-PSH USSL San Joaquin Valley Pistachio High. 2020. Available online: https://ameriflux.lbl.gov/sites/siteinfo/US-PSH (accessed on 1 September 2020).
- Anderson, R. AmeriFlux US-PSL USSL San Joaquin Valley Pistachio Low. 2020. Available online: https://ameriflux.lbl.gov/sites/siteinfo/US-PSL (accessed on 1 September 2020).
- Holm, L.; Podger, D.; Harader, S.; Fernandez, P.; Ullrey, R.; Li, X.; McCarthy, M.; Larsen, K.; Burau, J.; Orloff, L.; et al. Conceptual Model For Salinity in the Central Valley and Sacramento-San Joaquin Delta; CALFED Bay-Delta Program: Sacramento, CA, USA, 2007; p. 65. [Google Scholar]
- Almond Board of California. Almond Almanc; Almond Board of California: Modesto, CA, USA, 2018; p. 25. [Google Scholar]
- Šimůnek, J.; van Genuchten, M.T.; Šejna, M. Recent Developments and Applications of the HYDRUS Computer Software Packages. Vadose Zone J. 2016, 15. [Google Scholar] [CrossRef] [Green Version]
- Richards, L.A. Capillary conduction of liquids through porous mediums. Physics 1931, 1, 318–333. [Google Scholar] [CrossRef]
- Allen, R.G.; Pereira, L.S.; Raes, D.; Smith, M. Crop Evapotranspiration: Guidelines for Computing Crop Water Requirements; Food and Agriculture Organization of the United Nations: Rome, Italy, 1998; ISBN 92-5-104219-5. [Google Scholar]
- Zheng, C.; Lu, Y.; Guo, X.; Li, H.; Sai, J.; Liu, X. Application of HYDRUS-1D Model for Research on Irrigation Infiltration Characteristics in Arid Oasis of Northwest China. Environ. Earth Sci. 2017, 76, 785. [Google Scholar] [CrossRef]
- Raz-Yaseef, N.; Yakir, D.; Schiller, G.; Cohen, S. Dynamics of Evapotranspiration Partitioning in a Semi-Arid Forest as Affected by Temporal Rainfall Patterns. Agric. For. Meteorol. 2012, 157, 77–85. [Google Scholar] [CrossRef]
- Melton, F.S.; Johnson, L.F.; Lund, C.P.; Pierce, L.L.; Michaelis, A.R.; Hiatt, S.H.; Guzman, A.; Adhikari, D.D.; Purdy, A.J.; Rosevelt, C.; et al. Satellite Irrigation Management Support With the Terrestrial Observation and Prediction System: A Framework for Integration of Satellite and Surface Observations to Support Improvements in Agricultural Water Resource Management. IEEE J. Sel. Top. Appl. Earth Obs. Remote Sens. 2012, 5, 1709–1721. [Google Scholar] [CrossRef]
- van Genuchten, M.T.; Hoffman, G.J.; Hanks, R.J.; Meiri, A.; Shalhevet, J.; Kafkafi, U. Management Aspect for Crop Production. In Soil Salinity under Irrigation; Shainberg, I., Shalhevet, J., Eds.; Springer Berlin Heidelberg: Berlin/Heidelberg, Germany, 1984; pp. 258–338. ISBN 978-3-642-69836-1. [Google Scholar]
- Skaggs, T.H.; van Genuchten, M.T.; Shouse, P.J.; Poss, J.A. Macroscopic Approaches to Root Water Uptake as a Function of Water and Salinity Stress. Agric. Water Manag. 2006, 86, 140–149. [Google Scholar] [CrossRef]
- Grismer, M.E. Leaching Fraction, Soil Salinity, and Drainage Efficiency. Calif. Agric. 1990, 44, 24–26. [Google Scholar] [CrossRef]
- Maas, E.V.; Hoffman, G. Crop Salt Tolerance—Current Assessment. J. Irrig. Drain. Div.-ASCE 1977, 103, 115–134. [Google Scholar] [CrossRef]
- Maas, E.V. Crop salt tolerance. In Agricultural Salinity Assessment and Management; Tanji, K.K., Ed.; ASCE manuals and reports on engineering practice; American Society of Civil Engineers: New York, NY, USA, 1990; pp. 262–304. ISBN 978-0-87262-762-8. [Google Scholar]
- Sanden, B.L.; Ferguson, L.; Reyes, H.C.; Grattan, S.R. Effect of salinity on evapotranspiration and yield of San Joaquin Valley pistachios. In Proceedings of the Acta Horticulturae; International Society for Horticultural Science (ISHS): Leuven, Belgium, 2004; pp. 583–589. [Google Scholar]
- Sepaskhah, A.R.; Maftoun, M. Relative Salt Tolerance of Pistachio Cultivars. J. Hortic. Sci. 1988, 63, 157–162. [Google Scholar] [CrossRef]
- Hanson, B.R.; Šimůnek, J.; Hopmans, J.W. Evaluation of Urea–Ammonium–Nitrate Fertigation with Drip Irrigation Using Numerical Modeling. Agric. Water Manag. 2006, 86, 102–113. [Google Scholar] [CrossRef]
- Gärdenäs, A.I.; Hopmans, J.W.; Hanson, B.R.; Šimůnek, J. Two-Dimensional Modeling of Nitrate Leaching for Various Fertigation Scenarios under Micro-Irrigation. Agric. Water Manag. 2005, 74, 219–242. [Google Scholar] [CrossRef]
- Weihermüller, L.; Kasteel, R.; Vanderborght, J.; Pütz, T.; Vereecken, H. Soil Water Extraction with a Suction Cup: Results of Numerical Simulations. Vadose Zone J. 2005, 4, 899–907. [Google Scholar] [CrossRef]
- METER Group. GS3 Manual; METER Group: Pullman, DC, USA, 2019. [Google Scholar]
- US Salinity Laboratory. Diagnosis and Improvement of Saline and Alkali Soils; Richards, L.A., Ed.; Handbook; Soil and Water Conservative Research Branch, Agricultural Research Service, U.S. Dept. of Agriculture: Washington, DC, USA, 1954.
- Burt, C.M.; Isbell, B. Leaching of Accumulated Soil Salinity under Drip Irrigation. Trans. ASAE 2005, 48, 2115–2121. [Google Scholar] [CrossRef]
- Corwin, D.L.; Grattan, S.R. Are Existing Irrigation Salinity Leaching Requirement Guidelines Overly Conservative or Obsolete? J. Irrig. Drain. Eng. 2018, 144, 02518001. [Google Scholar] [CrossRef] [Green Version]





| Site Name | Ameri Flux Designation | Soil Type | Mean Annual Rain (mm) | Initial Salinity (dS/m) | Initial SAR (mmol/L)0.5 |
|---|---|---|---|---|---|
| Almond Salinity High | US-ASH | Clay Loam | <200 | 2.2–4.1 | 7–25 |
| Almond Salinity Medium | US-ASM | Silty Clay Loam | <200 | 1.8–3.5 | 7–20 |
| Pistachio Salinity High | US-PSH | Silty Clay | <200 | 2.3–4.7 | 5–15 |
| Almond Salinity Low | US-ASL | Sandy Loam | >250 | 1.3–1.9 | 10–25 |
| Pistachio Salinity Low | US-PSL | Sandy Loam | >250 | 1.1–2.1 | 3–10 |
| Sites | Day of Water Collection | ECiw (dS·m−1) | SAR | pH | Ca2+ | Mg2+ | Na+ | K+ |
|---|---|---|---|---|---|---|---|---|
| (mmolc/L)0.5 | (mmolc·L−1) | |||||||
| PSL | 30 March 2017 | 0.05 | 0.48 | 7.64 | 0.1 | 0.02 | 0.19 | 0.05 |
| 30 March 2017 | 0.04 | 0.6 | 7.6 | 0.08 | 0.01 | 0.13 | 0.04 | |
| 5 July 2017 | 0.24 | 7.8 | 0.49 | 0.24 | 1.15 | 0.07 | ||
| 17 May 2018 | 0.04 | 7.2 | 0.08 | 0.02 | 0.19 | 0.04 | ||
| 2 August 2018 | 0.05 | 8.06 | 0.07 | 0.01 | 0.15 | 0.04 | ||
| ASH | 30 May 2017 | 0.3 | 7.78 | 0.32 | 0.32 | 1.29 | 0.17 | |
| 11 August 2017 | 0.24 | 1.4 | 7.14 | 0.35 | 0.35 | 1.54 | 0.09 | |
| 6 July 2018 | 0.43 | 7.6 | 1.04 | 0.51 | 2.24 | 0.1 | ||
| 19 September 2018 | 0.54 | 6.95 | 0.31 | 0.29 | 1.09 | 0.07 | ||
| 20 January 2019 | 0.79 | 7.8 | 1.1 | 1.16 | 4.76 | 0.19 | ||
| ASM | 30 May 2017 | 0.28 | 1.7 | 7.7 | 0.33 | 0.33 | 1.47 | 0.08 |
| 17 May 2018 | 0.43 | 7.69 | 0.87 | 0.58 | 2.47 | 0.13 | ||
| 6 July 2018 | 0.54 | 1.8 | 7.72 | 0.43 | 0.61 | 3.26 | 0.12 | |
| 6 July 2018 | 0.53 | 1.8 | 8 | 1.11 | 1.2 | 4.92 | 0.24 | |
| 19 September 2018 | 0.51 | 7.89 | 0.46 | 0.52 | 2.31 | 0.11 | ||
| 5 January 2018 | 0.48 | 7.8 | 0.91 | 0.69 | 5.98 | 0.14 | ||
| PSH | 20 February 2018 | 0.44 | 7.57 | 0.52 | 0.59 | 2.86 | 0.16 | |
| 31 August 2018 | 0.89 | 4.1 | 7.72 | 0.51 | 0.56 | 2.54 | 0.12 | |
| 19 September 2018 | 0.48 | 3 | 7.93 | 0.44 | 0.57 | 3.03 | 0.11 | |
| 30 January 2019 | 0.9 | 7.77 | 1.22 | 0.74 | 5.89 | 0.09 | ||
| Crop | Growing Season | Early Season | Late Season |
|---|---|---|---|
| Almond | February–August | February–March | July–August |
| Pistachio | April–September | April–May | August–September |
| Sites | Depth (cm) | θr (cm3·cm−3) | θs (cm3·cm−3) | α (cm−1) | n (-) | Ks (cm·d−1) |
|---|---|---|---|---|---|---|
| ASL | 25 | 0.039 | 0.395 | 0.020 | 1.49 | 29.2 |
| 50 | 0.041 | 0.464 | 0.020 | 1.51 | 17.7 | |
| 75 | 0.047 | 0.404 | 0.014 | 1.48 | 21.8 | |
| 100 | 0.036 | 0.349 | 0.017 | 1.53 | 27.8 | |
| ASH | 25 | 0.072 | 0.343 | 0.009 | 1.30 | 16.2 |
| 50 | 0.098 | 0.433 | 0.002 | 1.71 | 5.6 | |
| 75 | 0.117 | 0.847 | 0.003 | 1.75 | 0.5 | |
| 100 | 0.116 | 0.935 | 0.003 | 1.66 | 2.1 | |
| ASM | 25 | 0.098 | 0.503 | 0.014 | 1.39 | 19.8 |
| 50 | 0.094 | 0.486 | 0.013 | 1.31 | 18.3 | |
| 75 | 0.092 | 0.490 | 0.010 | 1.48 | 20.6 | |
| 100 | 0.092 | 0.490 | 0.010 | 1.48 | 20.6 | |
| PSL | 25 | 0.038 | 0.523 | 0.040 | 1.55 | 51.0 |
| 50 | 0.035 | 0.423 | 0.024 | 1.26 | 30.4 | |
| 75 | 0.040 | 0.550 | 0.036 | 1.50 | 30.3 | |
| 100 | 0.035 | 0.376 | 0.041 | 1.52 | 13.8 | |
| PSH | 25 | 0.101 | 0.480 | 0.016 | 1.33 | 21.9 |
| 50 | 0.101 | 0.503 | 0.016 | 1.33 | 7.03 | |
| 75 | 0.099 | 0.494 | 0.015 | 1.35 | 7.03 | |
| 100 | 0.093 | 0.478 | 0.013 | 1.40 | 13.6 |
| ASH, West SJV, CA | ASL, East SJV, CA | ||||||
|---|---|---|---|---|---|---|---|
| 0–25 cm | 25–50 cm | 50–75 cm | 75–100 cm | 0–25 cm | 25–50 cm | 50–75 cm | 75–100 cm |
| 0.054 | 0.15 | 0.28 | 0.25 | 0.056 | 0.059 | 0.063 | 0.033 |
| ASH, West SJV, CA | ASL, East SJV, CA | ||||||
|---|---|---|---|---|---|---|---|
| Wet 2017 | Dry 2018 | Wet 2017 | Dry 2018 | ||||
| Early | Late | Early | Late | Early | Late | Early | Late |
| −0.912 ** | 0.236 | 0.236 | 0.955 ** | 0.419 ** | −0.949 ** | −0.933 ** | −0.436 ** |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Helalia, S.A.; Anderson, R.G.; Skaggs, T.H.; Šimůnek, J. Impact of Drought and Changing Water Sources on Water Use and Soil Salinity of Almond and Pistachio Orchards: 2. Modeling. Soil Syst. 2021, 5, 58. https://doi.org/10.3390/soilsystems5040058
Helalia SA, Anderson RG, Skaggs TH, Šimůnek J. Impact of Drought and Changing Water Sources on Water Use and Soil Salinity of Almond and Pistachio Orchards: 2. Modeling. Soil Systems. 2021; 5(4):58. https://doi.org/10.3390/soilsystems5040058
Chicago/Turabian StyleHelalia, Sarah A., Ray G. Anderson, Todd H. Skaggs, and Jirka Šimůnek. 2021. "Impact of Drought and Changing Water Sources on Water Use and Soil Salinity of Almond and Pistachio Orchards: 2. Modeling" Soil Systems 5, no. 4: 58. https://doi.org/10.3390/soilsystems5040058







