Fast and Simultaneous Determination of Soil Properties Using Laser-Induced Breakdown Spectroscopy (LIBS): A Case Study of Typical Farmland Soils in China
Abstract
:1. Introduction
2. Materials and Methods
2.1. Soil Sampling and Chemical Analysis
2.2. LIBS System and Spectra Acquisition
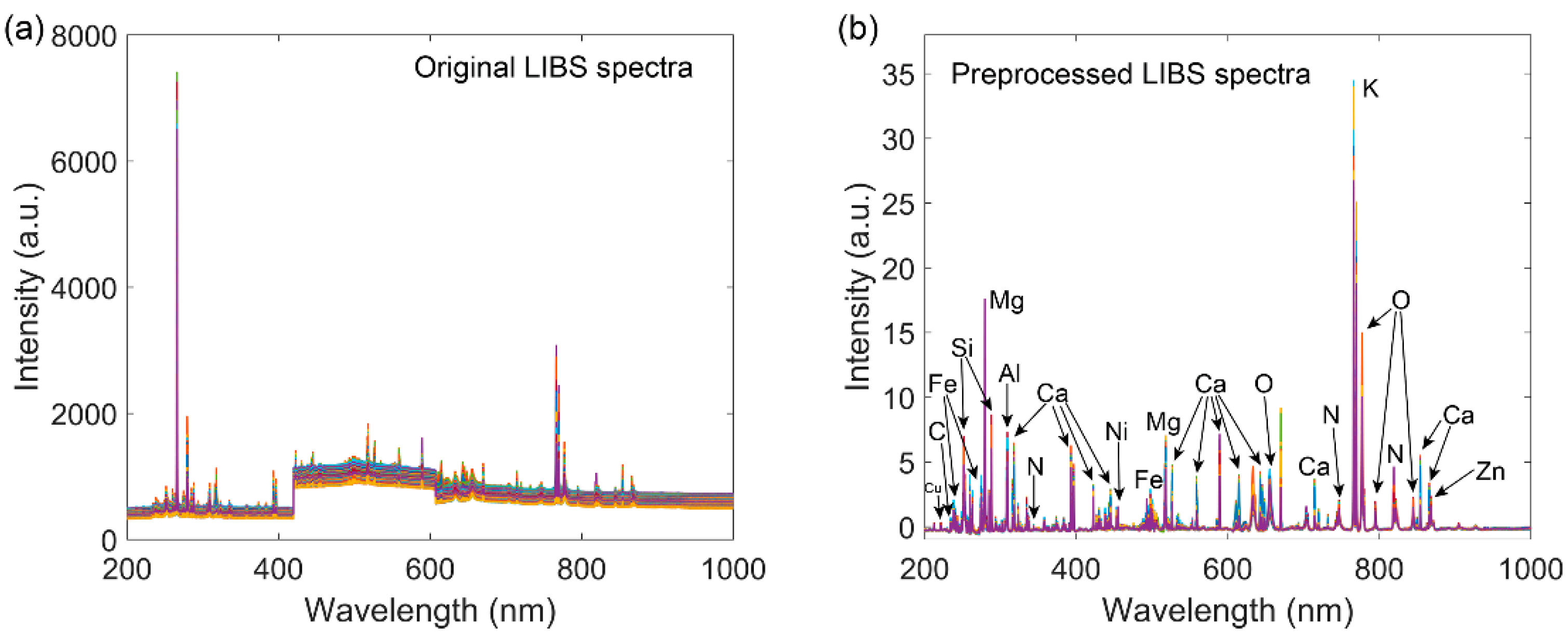
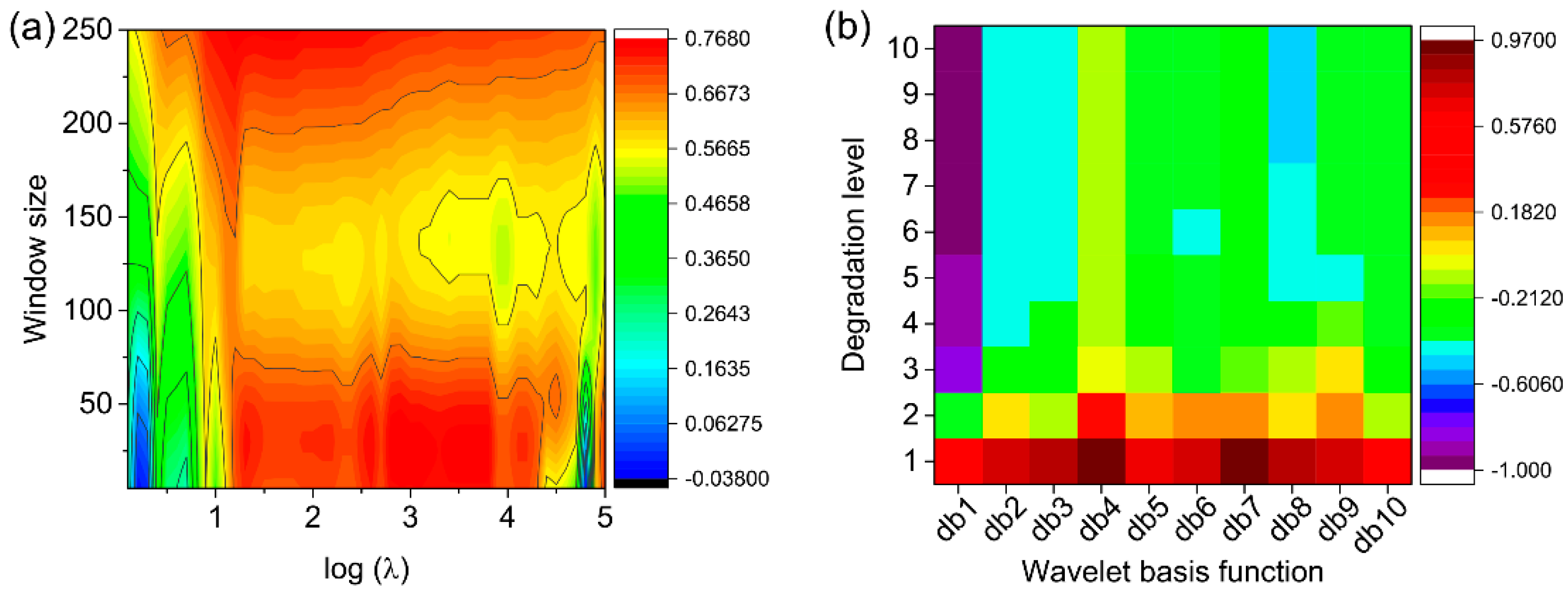
2.3. Spectra Preprocessing
2.4. Calibration and Validation
3. Results and Discussion
3.1. Soil Properties
3.2. Spectra Preprocessing
3.3. Investigation of Spectra
3.4. PCA of LIBS Spectra
3.5. Prediction of Soil Properties
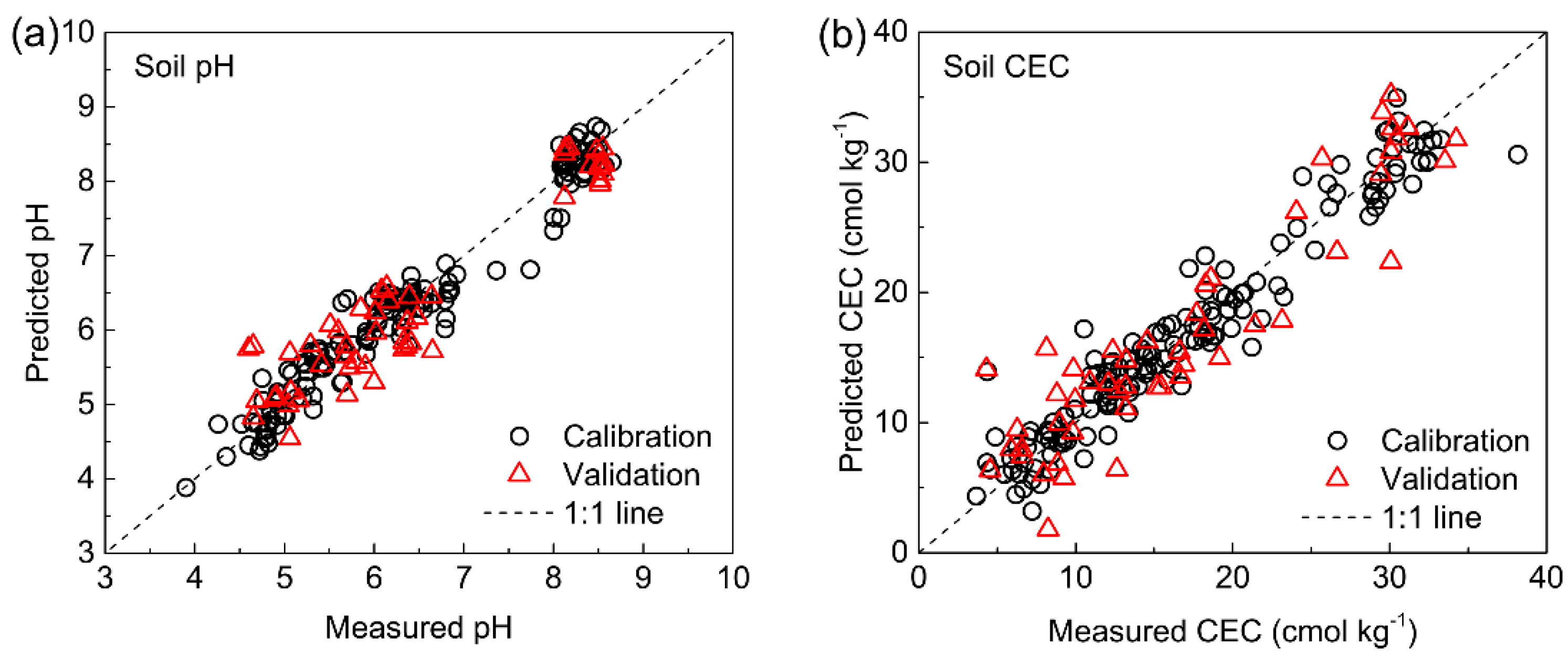
3.5.1. Soil pH and CEC
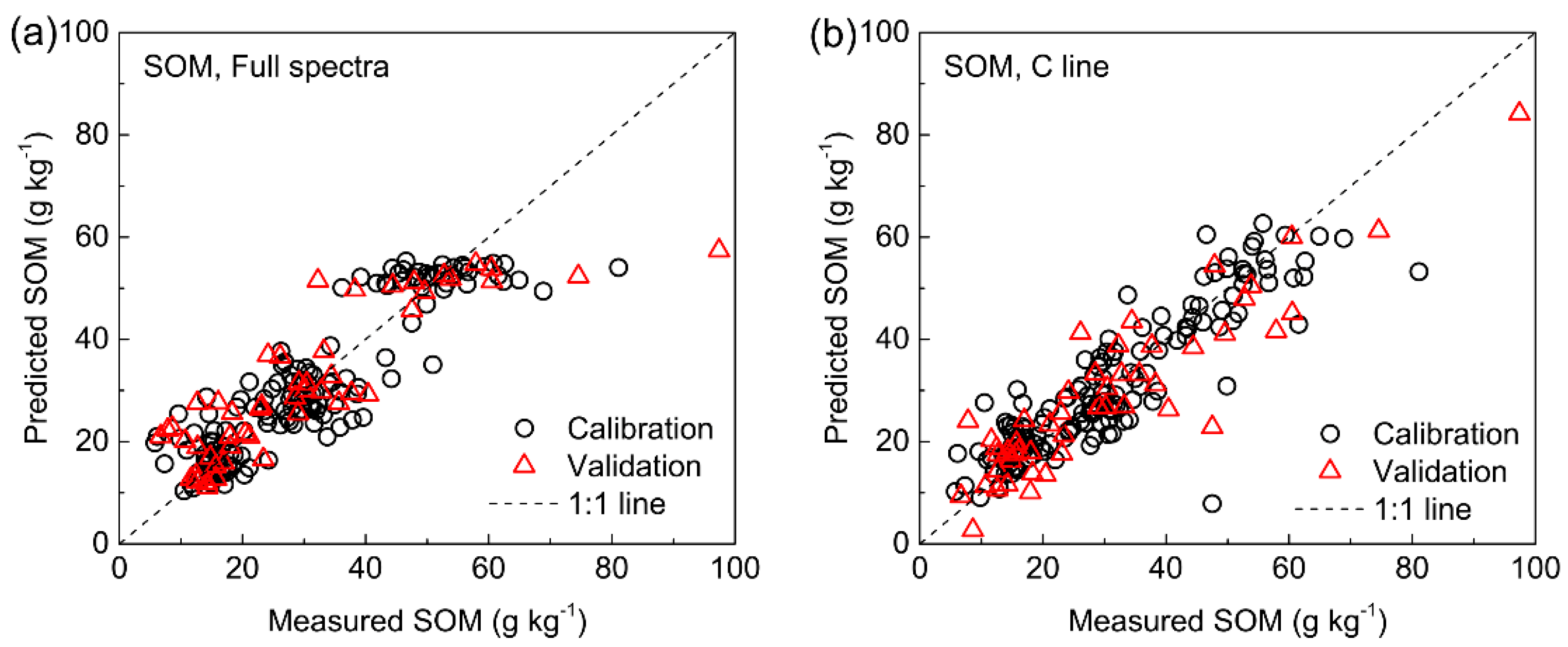
3.5.2. SOM
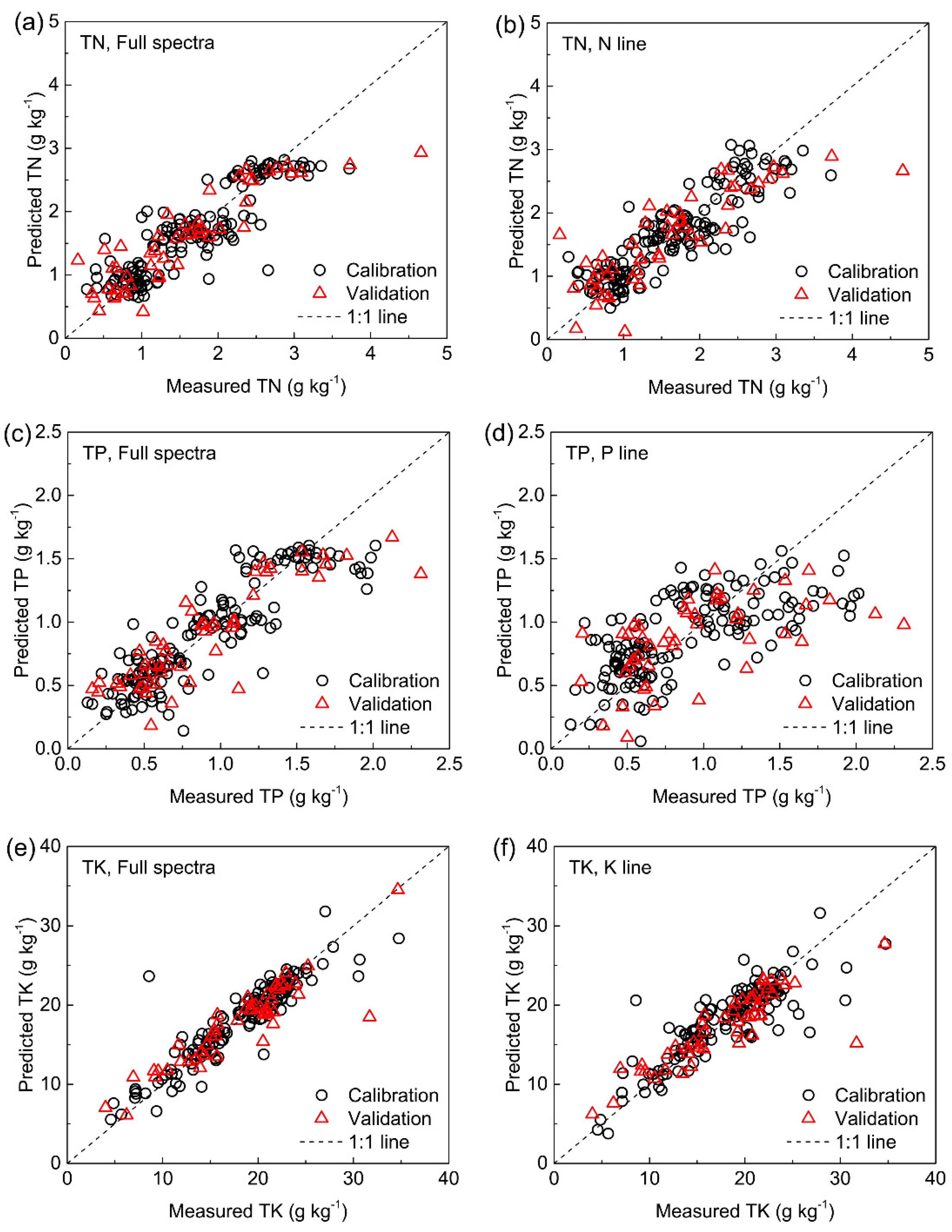
3.5.3. Soil TN, TP, and TK
3.5.4. Soil AP and AK
3.6. Simultaneous Determination of Soil Properties
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Demattê, J.A.M.; Ramirez-Lopez, L.; Marques, K.P.P.; Rodella, A.A. Chemometric soil analysis on the determination of specific bands for the detection of magnesium and potassium by spectroscopy. Geoderma 2017, 288, 8–22. [Google Scholar] [CrossRef]
- Capmourteres, V.; Adams, J.; Berg, A.; Fraser, E.; Swanton, C.; Anand, M. Precision conservation meets precision agriculture: A case study from southern Ontario. Agric. Syst. 2018, 167, 176–185. [Google Scholar] [CrossRef]
- Gebbers, R.; Adamchuk, V.I. Precision agriculture and food security. Science 2010, 327, 828–831. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Pray, C.; Rozelle, S. Enhancing the crops to feed the poor. Nature 2002, 418, 678–684. [Google Scholar] [CrossRef] [PubMed]
- Shaw, R.; Lark, R.M.; Williams, A.P.; Chadwick, D.R.; Jones, D.L. Characterising the within-field scale spatial variation of nitrogen in a grassland soil to inform the efficient design of in-situ nitrogen sensor networks for precision agriculture. Agric. Ecosyst. Environ. 2016, 230, 294–306. [Google Scholar] [CrossRef] [Green Version]
- Xing, Z.; Du, C.; Zeng, Y.; Ma, F.; Zhou, J. Characterizing typical farmland soils in China using Raman spectroscopy. Geoderma 2016, 268, 147–155. [Google Scholar] [CrossRef]
- Kaniu, M.I.; Angeyo, K.H. Challenges in rapid soil quality assessment and opportunities presented by multivariate chemometric energy dispersive X-ray fluorescence and scattering spectroscopy. Geoderma 2015, 241–242, 32–40. [Google Scholar] [CrossRef]
- De Lucia, F.C., Jr.; Gottfried, J.L. Rapid analysis of energetic and geo-materials using LIBS. Mater. Today 2011, 14, 274–281. [Google Scholar] [CrossRef]
- Miziolek, A.W.; Palleschi, V.; Schechter, I. Laser Induced Breakdown Spectroscopy (LIBS): Fundamentals and Applications; Cambridge University Press: Cambridge, UK, 2006. [Google Scholar]
- Radziemski, L.J.; Cremers, D.A. Handbook of Laser Induced Breakdown Spectroscopy; Wiley: New York, NY, USA, 2006. [Google Scholar]
- Han, D.; Joe, Y.J.; Ryu, J.-S.; Unno, T.; Kim, G.; Yamamoto, M.; Park, K.; Hur, H.-G.; Lee, J.-H.; Nam, S.-I. Application of laser-induced breakdown spectroscopy to Arctic sediments in the Chukchi Sea. Spectrochim. Acta Part B 2018, 146, 84–92. [Google Scholar] [CrossRef]
- Wang, T.; He, M.; Shen, T.; Liu, F.; He, Y.; Liu, X.; Qiu, Z. Multi-element analysis of heavy metal content in soils using laser-induced breakdown spectroscopy: A case study in eastern China. Spectrochim. Acta Part B 2018, 149, 300–312. [Google Scholar] [CrossRef]
- Juvé, V.; Portelli, R.; Boueri, M.; Baudelet, M.; Yu, J. Space-resolved analysis of trace elements in fresh vegetables using ultraviolet nanosecond laser-induced breakdown spectroscopy. Spectrochim. Acta Part B 2008, 63, 1047–1053. [Google Scholar] [CrossRef]
- Dell’Aglio, M.; López-Claros, M.; Laserna, J.J.; Longo, S.; De Giacomo, A. Stand-off laser induced breakdown spectroscopy on meteorites: Calibration-free approach. Spectrochim. Acta Part B 2018, 147, 87–92. [Google Scholar] [CrossRef]
- Moros, J.; ElFaham, M.M.; Laserna, J.J. Dual-spectroscopy platform for the surveillance of Mars mineralogy using a decisions fusion architecture on simultaneous LIBS-Raman data. Anal. Chem. 2018, 90, 2079–2087. [Google Scholar] [CrossRef]
- Mezzacappa, A.; Melikechi, N.; Cousin, A.; Wiens, R.C.; Lasue, J.; Clegg, S.M.; Tokar, R.; Bender, S.; Lanza, N.L.; Maurice, S.; et al. Application of distance correction to ChemCam laser-induced breakdown spectroscopy measurements. Spectrochim. Acta Part B 2016, 120, 19–29. [Google Scholar] [CrossRef]
- Hu, Y.; Li, Z.; Lü, T. Determination of elemental concentration in geological samples using nanosecond laser-induced breakdown spectroscopy. J. Anal. At. Spectrom. 2017, 32, 2263–2270. [Google Scholar] [CrossRef]
- Lü, T.; Hu, Y.; Li, Z.; Meng, J.; Zhang, C.; Hu, Z.; Liu, Y. Elemental fractionation and quantification of geological standard samples by nanosecond-laser ablation. Spectrochim. Acta Part B 2018, 143, 55–62. [Google Scholar] [CrossRef]
- García-Escárzaga, A.; Clarke, L.J.; Gutiérrez-Zugasti, I.; González-Morales, M.R.; Martinez, M.; López-Higuera, J.-M.; Cobo, A. Mg/Ca profiles within archaeological mollusc (Patella vulgata) shells: Laser-induced breakdown spectroscopy compared to inductively coupled plasma-optical emission spectrometry. Spectrochim. Acta Part B 2018, 148, 8–15. [Google Scholar] [CrossRef]
- Gaudiuso, R.; Dell’Aglio, M.; De Pascale, O.; Loperfido, S.; Mangone, A.; De Giacomo, A. Laser-induced breakdown spectroscopy of archaeological findings with calibration-free inverse method: Comparison with classical laser-induced breakdown spectroscopy and conventional techniques. Anal. Chim. Acta 2014, 813, 15–24. [Google Scholar] [CrossRef]
- Qi, J.; Zhang, T.; Tang, H.; Li, H. Rapid classification of archaeological ceramics via laser-induced breakdown spectroscopy coupled with random forest. Spectrochim. Acta Part B 2018, 149, 288–293. [Google Scholar] [CrossRef]
- Yu, X.; Li, Y.; Gu, X.; Bao, J.; Yang, H.; Sun, L. Laser-induced breakdown spectroscopy application in environmental monitoring of water quality: A review. Environ. Monit. Assess. 2014, 186, 8969–8980. [Google Scholar] [CrossRef]
- Ramli, M.; Khumaeni, A.; Kurniawan, K.H.; Tjia, M.O.; Kagawa, K. Spectrochemical analysis of Cs in water and soil using low pressure laser induced breakdown spectroscopy. Spectrochim. Acta Part B 2017, 132, 8–12. [Google Scholar] [CrossRef]
- Meng, D.; Zhao, N.; Wang, Y.; Ma, M.; Fang, L.; Gu, Y.; Jia, Y.; Liu, J. On-line/on-site analysis of heavy metals in water and soils by laser induced breakdown spectroscopy. Spectrochim. Acta Part B 2017, 137, 39–45. [Google Scholar] [CrossRef]
- Yi, R.; Yang, X.; Zhou, R.; Li, J.; Yu, H.; Hao, Z.; Guo, L.; Li, X.; Lu, Y.; Zeng, X. Determination of trace available heavy metals in soil using laser-induced breakdown spectroscopy assisted with phase transformation method. Anal. Chem. 2018, 90, 7080–7085. [Google Scholar] [CrossRef]
- Zou, L.; Kassim, B.; Smith, J.P.; Ormes, J.D.; Liu, Y.; Tu, Q.; Bu, X. In situ analytical characterization and chemical imaging of tablet coatings using laser induced breakdown spectroscopy (LIBS). Analyst 2018, 143, 5000–5007. [Google Scholar] [CrossRef]
- Serrano, J.; Cabalín, L.M.; Moros, J.; Laserna, J.J. Potential of laser-induced breakdown spectroscopy for discrimination of nano-sized carbon materials. Insights on the optical characterization of graphene. Spectrochim. Acta Part B 2014, 97, 105–112. [Google Scholar] [CrossRef]
- Liang, D.; Du, C.; Ma, F.; Shen, Y.; Wu, K.; Zhou, J. Characterization of nano FeIII-tannic acid modified polyacrylate in controlled-release coated urea by Fourier transform infrared photoacoustic spectroscopy and laser-induced breakdown spectroscopy. Polym. Test. 2017, 64, 101–108. [Google Scholar] [CrossRef]
- Markiewicz-Keszycka, M.; Cama-Moncunill, X.; Casado-Gavalda, M.P.; Dixit, Y.; Cama-Moncunill, R.; Cullen, P.J.; Sullivan, C. Laser-induced breakdown spectroscopy (LIBS) for food analysis: A review. Trends Food Sci. Technol. 2017, 65, 80–93. [Google Scholar] [CrossRef]
- Bilge, G.; Sezer, B.; Eseller, K.E.; Berberoglu, H.; Topcu, A.; Boyaci, I.H. Determination of whey adulteration in milk powder by using laser induced breakdown spectroscopy. Food Chem. 2016, 212, 183–188. [Google Scholar] [CrossRef]
- Casado-Gavalda, M.P.; Dixit, Y.; Geulen, D.; Cama-Moncunill, R.; Cama-Moncunill, X.; Markiewicz-Keszycka, M.; Cullen, P.J.; Sullivan, C. Quantification of copper content with laser induced breakdown spectroscopy as a potential indicator of offal adulteration in beef. Talanta 2017, 169, 123–129. [Google Scholar] [CrossRef]
- Peng, J.; Liu, F.; Zhou, F.; Song, K.; Zhang, C.; Ye, L.; He, Y. Challenging applications for multi-element analysis by laser-induced breakdown spectroscopy in agriculture: A review. TrAC Trends Anal. Chem. 2016, 85, 260–272. [Google Scholar] [CrossRef]
- Nicolodelli, G.; Senesi, G.S.; Ranulfi, A.C.; Marangoni, B.S.; Watanabe, A.; de Melo Benites, V.; de Oliveira, P.P.A.; Villas-Boas, P.; Milori, D.M.B.P. Double-pulse laser induced breakdown spectroscopy in orthogonal beam geometry to enhance line emission intensity from agricultural samples. Microchem. J. 2017, 133, 272–278. [Google Scholar] [CrossRef] [Green Version]
- Jull, H.; Künnemeyer, R.; Schaare, P. Nutrient quantification in fresh and dried mixtures of ryegrass and clover leaves using laser-induced breakdown spectroscopy. Precis. Agric. 2018, 19, 823–839. [Google Scholar] [CrossRef]
- Noll, R.; Fricke-Begemann, C.; Brunk, M.; Connemann, S.; Meinhardt, C.; Scharun, M.; Sturm, V.; Makowe, J.; Gehlen, C. Laser-induced breakdown spectroscopy expands into industrial applications. Spectrochim. Acta Part B 2014, 93, 41–51. [Google Scholar] [CrossRef]
- Sarkar, A.; Karki, V.; Aggarwal, S.K.; Maurya, G.S.; Kumar, R.; Rai, A.K.; Mao, X.; Russo, R.E. Evaluation of the prediction precision capability of partial least squares regression approach for analysis of high alloy steel by laser induced breakdown spectroscopy. Spectrochim. Acta Part B 2015, 108, 8–14. [Google Scholar] [CrossRef]
- Cremers, D.A.; Ebinger, M.H.; Breshears, D.D.; Unkefer, P.J.; Kammerdiener, S.A.; Ferris, M.J.; Catlett, K.M.; Brown, J.R. Measuring total soil carbon with laser-induced breakdown spectroscopy (LIBS). J. Environ. Qual. 2001, 30, 2202–2206. [Google Scholar] [CrossRef]
- Martin, M.Z.; Wullschleger, S.D.; Garten, C.T.; Palumbo, A.V. Laser-induced breakdown spectroscopy for the environmental determination of total carbon and nitrogen in soils. Appl. Opt. 2003, 42, 2072–2077. [Google Scholar] [CrossRef]
- Bricklemyer, R.S.; Brown, D.J.; Barefield, J.E.; Clegg, S.M. Intact soil core total, inorganic, and organic carbon measurement using laser-induced breakdown spectroscopy. Soil Sci. Soc. Am. J. 2011, 75, 1006–1018. [Google Scholar] [CrossRef]
- Senesi, G.S.; Senesi, N. Laser-induced breakdown spectroscopy (LIBS) to measure quantitatively soil carbon with emphasis on soil organic carbon. A review. Anal. Chim. Acta 2016, 938, 7–17. [Google Scholar] [CrossRef]
- Yan, X.T.; Donaldson, K.M.; Davidson, C.M.; Gao, Y.; Wu, H.; Houston, A.M.; Kisdi, A. Effects of sample pretreatment and particle size on the determination of nitrogen in soil by portable LIBS and potential use on robotic-borne remote Martian and agricultural soil analysis systems. RSC Adv. 2018, 8, 36886–36894. [Google Scholar] [CrossRef] [Green Version]
- He, Y.; Liu, X.; Lv, Y.; Liu, F.; Peng, J.; Shen, T.; Zhao, Y.; Tang, Y.; Luo, S. Quantitative analysis of nutrient elements in soil using single and double-pulse laser-induced breakdown spectroscopy. Sensors 2018, 18, 1526. [Google Scholar] [CrossRef]
- Yongcheng, J.; Wen, S.; Baohua, Z.; Dong, L. Quantitative analysis of magnesium in soil by laser-induced breakdown spectroscopy coupled with nonlinear multivariate calibration. J. Appl. Spectrosc. 2017, 84, 731–737. [Google Scholar] [CrossRef]
- Zhang, G.; Song, H.; Liu, Y.; Zhao, Z.; Li, S.; Ren, Z. Optimization of experimental parameters about laser induced breakdown and measurement of soil elements. Optik 2018, 165, 87–93. [Google Scholar] [CrossRef]
- Kim, E.-A.; Choi, J.H. Changes in the mineral element compositions of soil colloidal matter caused by a controlled freeze-thaw event. Geoderma 2018, 318, 160–166. [Google Scholar] [CrossRef]
- Senesi, G.S. Laser-Induced Breakdown Spectroscopy (LIBS) applied to terrestrial and extraterrestrial analogue geomaterials with emphasis to minerals and rocks. Earth-Sci. Rev. 2014, 139, 231–267. [Google Scholar] [CrossRef]
- Takahashi, T.; Thornton, B. Quantitative methods for compensation of matrix effects and self-absorption in laser induced breakdown spectroscopy signals of solids. Spectrochim. Acta Part B 2017, 138, 31–42. [Google Scholar] [CrossRef]
- Zaytsev, S.M.; Krylov, I.N.; Popov, A.M.; Zorov, N.B.; Labutin, T.A. Accuracy enhancement of a multivariate calibration for lead determination in soils by laser induced breakdown spectroscopy. Spectrochim. Acta Part B 2018, 140, 65–72. [Google Scholar] [CrossRef]
- Senesi, G.S.; Cabral, J.; Menegatti, C.R.; Marangoni, B.; Nicolodelli, G. Recent advances and future trends in LIBS applications to agricultural materials and their food derivatives: An overview of developments in the last decade (2010–2019). Part II. Crop plants and their food derivatives. TrAC Trends Anal. Chem. 2019, 118, 453–469. [Google Scholar] [CrossRef]
- Sumner, M.E.; Miller, W.P. Cation exchange capacity and exchange coefficients. In Methods of Soil Analysis Part 3—Chemical Methods; Bartels, J.M., Bigham, J.M., Eds.; Soil Science Society of America, Inc.: Madison, WI, USA, 1996; pp. 1201–1229. [Google Scholar]
- Walkley, A.; Black, I.A. An examination of Degtjareff method for determining soil organic matter, and a proposed modification of the chromic acid titration method. Soil Sci. 1934, 37, 29–38. [Google Scholar] [CrossRef]
- Pansu, M.; Gautheyrou, J. Handbook of Soil Analysis; Springer-Verlag: Heidelberg/Germany, Germany, 2007. [Google Scholar]
- Murphy, J.; Riley, J.P. A modified single solution method for the determination of phosphate in natural waters. Anal. Chim. Acta 1962, 27, 31–36. [Google Scholar] [CrossRef]
- Hanway, J.J.; Heidel, H. Soil analysis methods as used in Iowa State College Soil Testing Laboratory. Iowa Agric. 1952, 57, 1–31. [Google Scholar]
- Olsen, S.R.; Cole, C.V.; Watanabe, F.S.; Dean, L.A. Estimation of Available Phosphorus in Soils by Extraction with Sodium Bicarbonate; USDA Circular: Washington, DC, USA, 1954; Volume 939.
- Bray, R.H.; Kurtz, L.T. Determination of total, organic, and available forms of phosphorus in soils. Soil Sci. 1945, 59, 39–46. [Google Scholar] [CrossRef]
- Li, Z.; Zhan, D.J.; Wang, J.J.; Huang, J.; Xu, Q.S.; Zhang, Z.M.; Zheng, Y.B.; Liang, Y.Z.; Wang, H. Morphological weighted penalized least squares for background correction. Analyst 2013, 138, 4483–4492. [Google Scholar] [CrossRef]
- Shetty, N.; Gislum, R. Quantification of fructan concentration in grasses using NIR spectroscopy and PLSR. Field Crops Res. 2011, 120, 31–37. [Google Scholar] [CrossRef]
- Guo, L.; Zhao, C.; Zhang, H.; Chen, Y.; Linderman, M.; Zhang, Q.; Liu, Y. Comparisons of spatial and non-spatial models for predicting soil carbon content based on visible and near-infrared spectral technology. Geoderma 2017, 285, 280–292. [Google Scholar] [CrossRef]
- Wilding, L.P. Spatial Variability: Its Documentation, Accommodation and Implication to Soil Surveys; Pudoc: Wageningen, The Netherlands, 1985; pp. 166–194. [Google Scholar]
- Kramida, A.; Ralchenko, Y.; Reader, J.; NIST ASD Team. NIST Atomic Spectra Database (Ver. 5.6); National Institute of Standards and Technology: Gaithersburg, MD, USA, 2012. [Google Scholar]
- Ebinger, M.H.; Norfleet, M.L.; Breshears, D.D.; Cremers, D.A.; Ferris, M.J.; Unkefer, P.J.; Lamb, M.S.; Goddard, K.L.; Meyer, C.W. Extending the applicability of laser-induced breakdown spectroscopy for total soil carbon measurement. Soil Sci. Soc. Am. J. 2003, 67, 1616–1619. [Google Scholar] [CrossRef]
- Ji, Z.G.; Xi, J.H.; Mao, Q.N. Determination of oxygen concentration in heavily doped silicon wafer by laser induced breakdown spectroscopy. J. Inorg. Mater. 2010, 25, 893–895. [Google Scholar] [CrossRef]
- Harris, R.D.; Cremers, D.A.; Ebinger, M.H.; Bluhm, B.K. Determination of nitrogen in sand using laser-induced breakdown spectroscopy. Appl. Spectrosc. 2004, 58, 770–775. [Google Scholar] [CrossRef]
- Dong, D.M.; Zhao, C.J.; Zheng, W.G.; Zhao, X.D.; Jiao, L.Z. Spectral characterization of nitrogen in farmland soil by laser-induced breakdown spectroscopy. Spectrosc. Lett. 2013, 46, 421–426. [Google Scholar] [CrossRef]
- De Lucia, F.C., Jr.; Gottfried, J.L. Characterization of a series of nitrogen-rich molecules using laser induced breakdown spectroscopy. Propellants. Explos. Pyrotech. 2010, 35, 268–277. [Google Scholar] [CrossRef]
- Aras, N.; Yalçın, Ş. Rapid identification of phosphorus containing proteins in electrophoresis gel spots by laser-induced breakdown spectroscopy, LIBS. J. Anal. At. Spectrom. 2014, 29, 545–552. [Google Scholar] [CrossRef]
- Mansoori, A.; Roshanzadeh, B.; Khalaji, M.; Tavassoli, S.H. Quantitative analysis of cement powder by laser induced breakdown spectroscopy. Opt. Lasers Eng. 2011, 49, 318–323. [Google Scholar] [CrossRef]
- Sallé, B.; Cremers, D.A.; Maurice, S.; Wiens, R.C.; Fichet, P. Evaluation of a compact spectrograph for in-situ and stand-off laser-induced breakdown spectroscopy analyses of geological samples on Mars missions. Spectrochim. Acta Part B 2005, 60, 805–815. [Google Scholar] [CrossRef]
- Lu, C.; Wang, L.; Hu, H.; Zhuang, Z.; Wang, Y.; Wang, R.; Song, L. Analysis of total nitrogen and total phosphorus in soil using laser-induced breakdownspectroscopy. Chin. Opt. Lett. 2013, 11, 053004. [Google Scholar]
- Slessarev, E.W.; Lin, Y.; Bingham, N.L.; Johnson, J.E.; Dai, Y.; Schimel, J.P.; Chadwick, O.A. Water balance creates a threshold in soil pH at the global scale. Nature 2016, 540, 567. [Google Scholar] [CrossRef]
- Fierer, N.; Jackson, R.B. The diversity and biogeography of soil bacterial communities. Proc. Natl. Acad. Sci. USA 2006, 103, 626–631. [Google Scholar] [CrossRef] [Green Version]
- Lauber, C.L.; Strickland, M.S.; Bradford, M.A.; Fierer, N. The influence of soil properties on the structure of bacterial and fungal communities across land-use types. Soil Biol. Biochem. 2008, 40, 2407–2415. [Google Scholar] [CrossRef]
- Likar, M.; Vogel-Mikuš, K.; Potisek, M.; Hančević, K.; Radić, T.; Nečemer, M.; Regvar, M. Importance of soil and vineyard management in the determination of grapevine mineral composition. Sci. Total Environ. 2015, 505, 724–731. [Google Scholar] [CrossRef]
- Van Breemen, N.; Mulder, J.; Driscoll, C.T. Acidification and alkalinization of soils. Plant Soil 1983, 75, 283–308. [Google Scholar] [CrossRef]
- Sposito, G. The Chemistry of Soils; Oxford University Press: New York, NY, USA, 1989. [Google Scholar]
- Sharma, A.; Weindorf, D.C.; Wang, D.; Chakraborty, S. Characterizing soils via portable X-ray fluorescence spectrometer: 4. Cation exchange capacity (CEC). Geoderma 2015, 239–240, 130–134. [Google Scholar] [CrossRef]
- Shekofteh, H.; Ramazani, F.; Shirani, H. Optimal feature selection for predicting soil CEC: Comparing the hybrid of ant colony organization algorithm and adaptive network-based fuzzy system with multiple linear regression. Geoderma 2017, 298, 27–34. [Google Scholar] [CrossRef]
- Faber, N.M.; Rajkó, R. How to avoid over-fitting in multivariate calibration—The conventional validation approach and an alternative. Anal. Chim. Acta 2007, 595, 98–106. [Google Scholar] [CrossRef]
- Deng, B.-C.; Yun, Y.-H.; Liang, Y.-Z.; Cao, D.-S.; Xu, Q.-S.; Yi, L.-Z.; Huang, X. A new strategy to prevent over-fitting in partial least squares models based on model population analysis. Anal. Chim. Acta 2015, 880, 32–41. [Google Scholar] [CrossRef]
- Xing, Z.; Du, C.; Tian, K.; Ma, F.; Shen, Y.; Zhou, J. Application of FTIR-PAS and Raman spectroscopies for the determination of organic matter in farmland soils. Talanta 2016, 158, 262–269. [Google Scholar] [CrossRef]
- Shi, Z.; Ji, W.; Viscarra Rossel, R.A.; Chen, S.; Zhou, Y. Prediction of soil organic matter using a spatially constrained local partial least squares regression and the Chinese vis–NIR spectral library. Eur. J. Soil Sci. 2015, 66, 679–687. [Google Scholar] [CrossRef]
- Christy, C.D. Real-time measurement of soil attributes using on-the-go near infrared reflectance spectroscopy. Comput. Electron. Agric. 2008, 61, 10–19. [Google Scholar] [CrossRef]
- Li, S.; Shi, Z.; Chen, S.; Ji, W.; Zhou, L.; Yu, W.; Webster, R. In situ measurements of organic carbon in soil profiles using vis-NIR spectroscopy on the Qinghai–Tibet plateau. Environ. Sci. Technol. 2015, 49, 4980–4987. [Google Scholar] [CrossRef]
- Bricklemyer, R.S.; Brown, D.J.; Turk, P.J.; Clegg, S. Comparing vis–NIRS, LIBS, and combined vis–NIRS-LIBS for intact soil core soil carbon measurement. Soil Sci. Soc. Am. J. 2018, 82, 1482–1496. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, X.; Huang, C. Spatial variability of soil total nitrogen and soil total phosphorus under different land uses in a small watershed on the Loess Plateau, China. Geoderma 2009, 150, 141–149. [Google Scholar] [CrossRef]
- Yang, H.; Kuang, B.; Mouazen, A.M. Quantitative analysis of soil nitrogen and carbon at a farm scale using visible and near infrared spectroscopy coupled with wavelength reduction. Eur. J. Soil Sci. 2012, 63, 410–420. [Google Scholar] [CrossRef]
- Viscarra Rossel, R.A.; Walvoort, D.J.J.; McBratney, A.B.; Janik, L.J.; Skjemstad, J.O. Visible, near infrared, mid infrared or combined diffuse reflectance spectroscopy for simultaneous assessment of various soil properties. Geoderma 2006, 131, 59–75. [Google Scholar] [CrossRef]
- Janik, L.J.; Merry, R.H.; Skjemstad, J.O. Can mid infrared diffuse reflectance analysis replace soil extractions? Aust. J. Exp. Agric. 1998, 38, 681–696. [Google Scholar] [CrossRef]
- Soriano-Disla, J.M.; Janik, L.J.; Viscarra Rossel, R.A.; Macdonald, L.M.; McLaughlin, M.J. The performance of visible, near-, and mid-infrared reflectance spectroscopy for prediction of soil physical, chemical, and biological properties. Appl. Spectrosc. Rev. 2014, 49, 139–186. [Google Scholar] [CrossRef]
- Chang, C.-W.; Laird, D.A.; Mausbach, M.J.; Hurburgh, C.R. Near-infrared reflectance spectroscopy–principal components regression analyses of soil properties. Soil Sci. Soc. Am. J. 2001, 65, 480–490. [Google Scholar] [CrossRef]
- Towett, E.K.; Shepherd, K.D.; Sila, A.; Aynekulu, E.; Cadisch, G. Mid-infrared and total X-ray fluorescence spectroscopy complementarity for assessment of soil properties. Soil Sci. Soc. Am. J. 2015, 79, 1375–1385. [Google Scholar] [CrossRef]
- Ferreira, E.C.; Anzano, J.M.; Milori, D.M.B.P.; Ferreira, E.J.; Lasheras, R.J.; Bonilla, B.; Montull-Ibor, B.; Casas, J.; Neto, L.M. Multiple response optimization of laser-induced breakdown spectroscopy parameters for multi-element analysis of soil samples. Appl. Spectrosc. 2009, 63, 1081–1088. [Google Scholar] [CrossRef]
- Ferreira, E.C.; Ferreira, E.J.; Villas-Boas, P.R.; Senesi, G.S.; Carvalho, C.M.; Romano, R.A.; Martin-Neto, L.; Milori, D.M.B.P. Novel estimation of the humification degree of soil organic matter by laser-induced breakdown spectroscopy. Spectrochim. Acta Part B 2014, 99, 76–81. [Google Scholar] [CrossRef]
- Ferreira, E.C.; Neto, J.A.G.; Milori, D.M.B.P.; Ferreira, E.J.; Anzano, J.M. Laser-induced breakdown spectroscopy: Extending its application to soil pH measurements. Spectrochim. Acta Part B 2015, 110, 96–99. [Google Scholar] [CrossRef] [Green Version]
- Lin, Z.-X.; Liu, L.-M.; Liu, L.-W. Validation of the solidifying soil process using laser-induced breakdown spectroscopy. Opt. Laser Technol. 2016, 83, 13–15. [Google Scholar] [CrossRef]


| Index | Dataset | NS | Min | Max | Mean | SD | CV (%) |
|---|---|---|---|---|---|---|---|
| pH | Calibration | 150 | 3.90 | 8.65 | 6.40 | 1.31 | 20.44 |
| Validation | 50 | 4.60 | 8.56 | 6.39 | 1.30 | 20.42 | |
| CEC (cmol kg−1) | Calibration | 150 | 3.68 | 38.15 | 16.90 | 8.33 | 49.29 |
| Validation | 50 | 4.31 | 34.24 | 16.82 | 8.75 | 52.02 | |
| SOM (g kg−1) | Calibration | 150 | 5.74 | 81.06 | 30.72 | 15.39 | 50.11 |
| Validation | 50 | 6.72 | 97.41 | 29.97 | 18.69 | 62.38 | |
| TN (g kg−1) | Calibration | 150 | 0.28 | 3.72 | 1.65 | 0.76 | 45.85 |
| Validation | 50 | 0.16 | 4.66 | 1.56 | 0.94 | 60.46 | |
| TP (g kg−1) | Calibration | 150 | 0.13 | 2.02 | 0.89 | 0.46 | 51.91 |
| Validation | 50 | 0.16 | 2.31 | 0.90 | 0.50 | 55.21 | |
| TK (g kg−1) | Calibration | 150 | 4.58 | 34.75 | 18.15 | 5.34 | 29.43 |
| Validation | 50 | 4.01 | 34.65 | 17.88 | 5.97 | 33.37 | |
| AP (mg kg−1) | Calibration | 150 | 0.20 | 123.63 | 13.36 | 14.85 | 111.16 |
| Validation | 50 | 0.00 | 126.25 | 16.04 | 20.81 | 129.73 | |
| AK (mg kg−1) | Calibration | 150 | 33.10 | 424.05 | 118.38 | 60.42 | 51.04 |
| Validation | 50 | 18.34 | 307.88 | 118.24 | 70.90 | 59.96 |
| Properties | Variables Range | nLVs | Calibration Set | Validation Set | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| RMSECV | RCV2 | RMSEC | RC2 | RPDC | RMSEV | RV2 | RPDV | |||
| pH | 200–1000 nm | 10 | 0.444 | 0.885 | 0.280 | 0.954 | 4.691 | 0.444 | 0.886 | 2.940 |
| CEC(cmol kg−1) | 200–1000 nm | 10 | 3.157 | 0.857 | 2.185 | 0.931 | 3.826 | 3.492 | 0.849 | 2.507 |
| SOM (g kg−1) | 200–1000 nm | 5 | 7.071 | 0.789 | 6.046 | 0.846 | 2.554 | 8.297 | 0.812 | 2.253 |
| 247.2–248.0 nm | 3 | 7.431 | 0.767 | 7.081 | 0.788 | 2.181 | 7.878 | 0.833 | 2.373 | |
| TN (g kg−1) | 200–1000 nm | 5 | 0.437 | 0.709 | 0.366 | 0.766 | 2.073 | 0.442 | 0.797 | 2.130 |
| 459.8–460.9, 739.4–755.3 nm | 6 | 0.451 | 0.645 | 0.369 | 0.763 | 2.059 | 0.510 | 0.710 | 1.849 | |
| TP (g kg−1) | 200–1000 nm | 4 | 0.223 | 0.769 | 0.222 | 0.771 | 2.097 | 0.249 | 0.756 | 1.993 |
| 253.1–254.5 nm | 3 | 0.360 | 0.395 | 0.338 | 0.468 | 1.376 | 0.413 | 0.364 | 1.201 | |
| TK (g kg−1) | 200–1000 nm | 6 | 2.563 | 0.770 | 2.152 | 0.838 | 2.490 | 2.568 | 0.821 | 2.323 |
| 765.4–770.8 nm | 5 | 2.863 | 0.713 | 2.667 | 0.751 | 2.010 | 3.180 | 0.737 | 1.876 | |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, X.; Du, C.; Ma, F.; Shen, Y.; Zhou, J. Fast and Simultaneous Determination of Soil Properties Using Laser-Induced Breakdown Spectroscopy (LIBS): A Case Study of Typical Farmland Soils in China. Soil Syst. 2019, 3, 66. https://doi.org/10.3390/soilsystems3040066
Xu X, Du C, Ma F, Shen Y, Zhou J. Fast and Simultaneous Determination of Soil Properties Using Laser-Induced Breakdown Spectroscopy (LIBS): A Case Study of Typical Farmland Soils in China. Soil Systems. 2019; 3(4):66. https://doi.org/10.3390/soilsystems3040066
Chicago/Turabian StyleXu, Xuebin, Changwen Du, Fei Ma, Yazhen Shen, and Jianmin Zhou. 2019. "Fast and Simultaneous Determination of Soil Properties Using Laser-Induced Breakdown Spectroscopy (LIBS): A Case Study of Typical Farmland Soils in China" Soil Systems 3, no. 4: 66. https://doi.org/10.3390/soilsystems3040066