Influence of Soil Moisture Status on Soil Cadmium Phytoavailability and Accumulation in Plantain (Plantago lanceolata)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Pot Design
2.2. Soils
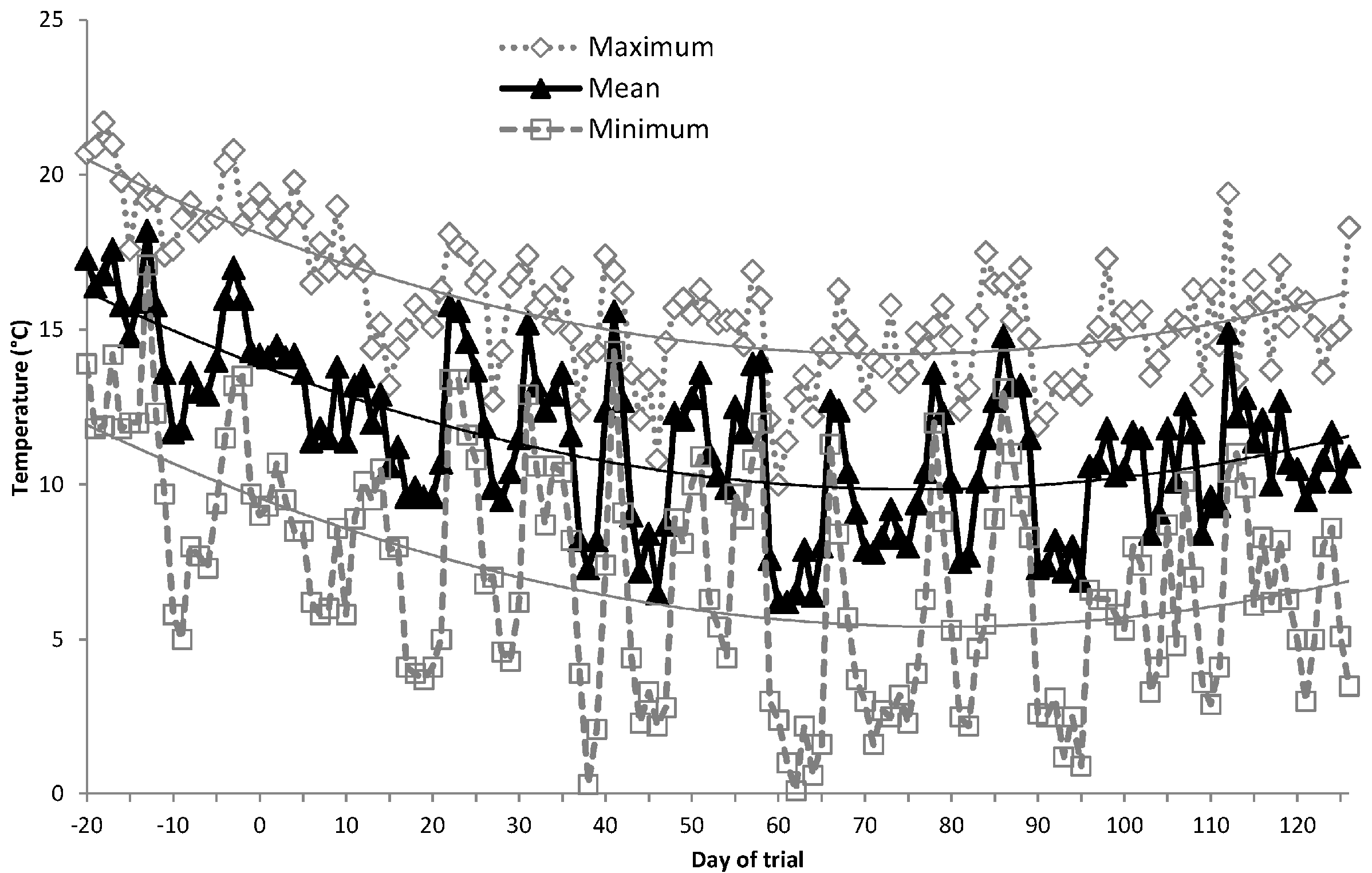
2.3. Experimental Layout and Management
2.4. Soil Sampling and Plant Harvest
2.5. Chemical Analysis
2.6. Quality Control
2.7. Statistical Analysis
3. Results
3.1. Soil Extractable Cd
Soil Extractable Cd over Time
3.2. Soil Solution pH
3.3. Plantain Tissue Cd Concentration, Yield, and Cd Uptake
4. Discussion
4.1. Effect of Fluctuating Soil Moisture on Soil Cd Phytoavailability
4.2. Change in Soil Extractable Cd as a Function of Time
4.3. Effect of Soil Type on Cd Phytoavailability and Uptake
4.4. Implication of These Findings for Cd Management in Agricultural Soils
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Adriano, D.C. Trace Elements in the Terrestrial Environment; Springer: New York, NY, USA, 1986; 533p. [Google Scholar]
- Smolders, E.; Mertens, J. Cadmium. In Heavy Metals in Soils: Trace Metals and Metalloids in Soils and Their Bioavailability; Alloway, B.J., Ed.; Springer: Dordrecht, The Netherlands, 2012; pp. 283–311. [Google Scholar]
- Williams, C.H.; David, D.J. Some effects of the distribution of cadmium and phosphate in the root zone on the cadmium content of plants. Aust. J. Soil Res. 1977, 15, 59–68. [Google Scholar] [CrossRef]
- Loganathan, P.; Vigneswaran, S.; Kandasamy, J.; Naidu, R. Cadmium Sorption and Desorption in Soils: A Review. Crit. Rev. Environ. Sci. Technol. 2012, 42, 489–533. [Google Scholar] [CrossRef]
- Young, S.D. Chemistry of heavy metals and metalloids in soils. In Heavy Metals in Soils: Trace Metals and Metalloids in Soils and Their Bioavailability; Alloway, B.J., Ed.; Springer: Dordrecht, The Netherlands, 2012; pp. 51–95. [Google Scholar]
- McLaughlin, M.J.; Tiller, K.G.; Naidu, R.; Stevens, D.P. Review: The behaviour and environmental impact of contaminants in fertilisers. Aust. J. Soil Res. 1996, 34, 1–54. [Google Scholar] [CrossRef]
- Tiller, K.G.; Gerth, J.; Brummer, G. The relative affinities of Cd, Ni and Zn for different soil clay fractions and goethite. Geoderma 1984, 34, 17–35. [Google Scholar] [CrossRef]
- McBride, M.B. Reactions controlling heavy metal solubility in soils. Adv. Soil Sci. 1989, 10, 1–56. [Google Scholar]
- McBride, M.; Sauve, S.; Hendershot, W. Solubility control of Cu, Zn, Cd and Pb in contaminated soils. Eur. J. Soil Sci. 1997, 48, 337–346. [Google Scholar] [CrossRef]
- Christensen, T.H.; Haung, P.M. Solid phase cadmium and the reactions of aqueous cadmium with soil surfaces. In Cadmium in Soils and Plants; McLaughlin, M.J., Singh, B.R., Eds.; Kluwer Academic Publishers: Dordrecht, The Netherlands, 1999; pp. 65–96. [Google Scholar]
- Naidu, R.; Bolan, N.S.; Kookana, R.S.; Tiller, K.G. Ionic-strength and pH effects on the sorption of cadmium and the surface charge of soils. Eur. J. Soil Sci. 1994, 45, 419–429. [Google Scholar] [CrossRef]
- Naidu, R.; Kookana, R.S.; Sumner, M.E.; Harter, R.D.; Tiller, K.G. Cadmium sorption and transport in variable charge soils: A review. J. Environ. Qual. 1997, 26, 602–617. [Google Scholar] [CrossRef]
- Bolan, N.S.; Naidu, R.; Syers, J.K.; Tillman, R.W. Surface charge and solute interactions in soils. Adv. Agron. 1999, 67, 88–141. [Google Scholar]
- Iu, K.L.; Pulford, I.D.; Duncan, H.J. Influence of waterlogging and lime or organic matter additions on the distribution of trace metals in an acid soil—I. Manganese and iron. Plant Soil 1981, 59, 317–326. [Google Scholar] [CrossRef]
- Hernandez-Soriano, M.C.; Jimenez-Lopez, J.C. Effects of soil water content and organic matter addition on the speciation and bioavailability of heavy metals. Sci. Total Environ. 2012, 423, 55–61. [Google Scholar] [CrossRef] [PubMed]
- Charlatchka, R.; Cambier, P. Influence of reducing conditions on solubility of trace metals in contaminated soils. Water Air Soil Pollut. 2000, 118, 143–167. [Google Scholar] [CrossRef]
- Atta, S.K.; Mohammed, S.A.; Van Cleemput, O.; Zayed, A. Transformations of iron and manganese under controlled E(h), E(h)-pH conditions and addition of organic matter. Soil Technol. 1996, 9, 223–237. [Google Scholar] [CrossRef]
- Kashem, M.A.; Singh, B.R. Metal availability in contaminated soils: I. Effects of flooding and organic matter on changes in Eh, pH and solubility of Cd, Ni and Zn. Nutr. Cycl. Agroecosyst. 2001, 61, 247–255. [Google Scholar]
- Zheng, S.; Zheng, X.; Chen, C. Transformation of metal speciation in purple soil as affected by waterlogging. Int. J. Environ. Sci. Technol. 2013, 10, 351–358. [Google Scholar] [CrossRef]
- Zhang, C.; Ge, Y.; Yao, H.; Chen, X.; Hu, M. Iron oxidation-reduction and its impacts on cadmium bioavailability in paddy soils: A review. Front. Environ. Sci. Eng. 2012, 6, 509–517. [Google Scholar] [CrossRef]
- De Livera, J.; McLaughlin, M.J.; Hettiarachchi, G.M.; Kirby, J.K.; Beak, D.G. Cadmium solubility in paddy soils: Effects of soil oxidation, metal sulfides and competitive ions. Sci. Total Environ. 2011, 409, 1489–1497. [Google Scholar] [CrossRef] [PubMed]
- McLaren, R.G.; Cameron, K.C. Soil Science: Sustainable Production and Environmental Protection, 2nd ed.; Oxford University Press: Auckland, New Zealand, 1996; p. 304. [Google Scholar]
- Rumball, P.J. Pasture Plant, Water-Table Interactions on Manawatu Sandy Country. In Proceedings of the New Zealand Grassland Association 39, Palmerston North, New Zealand, 1977; Available online: https://www.grassland.org.nz/publications/nzgrassland_publication_1394.pdf (accessed on 9 February 2018).
- Hindersmann, I.; Mansfeldt, T. Trace element solubility in a multimetal-contaminated soil as affected by redox conditions. Water Air Soil Pollut. 2014, 225, 1–20. [Google Scholar] [CrossRef]
- Cornu, S.; Cattle, J.A.; Samouelian, A.; Laveuf, C.; Guilherme, L.R.G.; Alberic, P. Impact of redox cycles on manganese, iron, cobalt, and lead in nodules. Soil Sci. Soc. Am. J. 2009, 73, 1231–1241. [Google Scholar] [CrossRef] [Green Version]
- Backes, C.A.; McLaren, R.G.; Rate, A.W.; Swift, R.S. Kinetics of cadmium and cobalt desorption from iron and manganese oxides. Soil Sci. Soc. Am. J. 1995, 59, 778–785. [Google Scholar] [CrossRef]
- Kim, N.D.; Fergusson, J.E. Adsorption of cadmium by an aquent New Zealand soil and its components. Aust. J. Soil Res. 1992, 30, 159–167. [Google Scholar] [CrossRef]
- Gray, C.W.; McLaren, R.G.; Roberts, A.H.C.; Condron, L.M. Solubility, sorption and desorption of native and added cadmium in relation to properties of soils in New Zealand. Eur. J. Soil Sci. 1999, 50, 127–137. [Google Scholar] [CrossRef]
- Ponnamperuma, F.N. The chemistry of submerged soils. In Advances in Agronomy; Academic Press: Cambridge, MA, USA, 1972; Volume 24, pp. 29–96. [Google Scholar]
- Bhatti, S.M.; Anderson, C.W.N.; Stewart, R.B.; Robinson, B.H. Risk assessment of vegetables irrigated with arsenic-contaminated water. Environ. Sci. Process. Impacts 2013, 15, 1866–1875. [Google Scholar] [CrossRef] [PubMed]
- Stafford, A.D.; Anderson, C.W.N.; Hedley, M.J.; McDowell, R.W. Cadmium accumulation by forage species used in New Zealand livestock grazing systems. Geoderma Reg. 2016, 7, 11–18. [Google Scholar] [CrossRef]
- Powell, A.M.; Kemp, P.D.; Jaya, I.K.D.; Osborne, M.A. Establishment, Growth and Development of Plantain and Chicory under Grazing. In Proceedings of the New Zealand Grassland Association 69, Wairakei, New Zealand, 2007; Available online: https://www.grassland.org.nz/publications/nzgrassland_publication_144.pdf (accessed on 9 December 2017).
- Li, Z.; McLaren, R.G.; Metherell, A.K. Cobalt and manganese relationships in New Zealand soils. N. Z. J. Agric. Res. 2001, 44, 191–200. [Google Scholar] [CrossRef]
- Jeyakumar, P.; Loganathan, P.; Anderson, C.N.; Sivakumaran, S.; McLaren, R. Comparative tolerance of Pinus radiata and microbial activity to copper and zinc in a soil treated with metal-amended biosolids. Environ. Sci. Pollut. Res. 2014, 21, 3254–3263. [Google Scholar] [CrossRef] [PubMed]
- Tukey, J.W. Exploratory Data Analysis; Addison-Wesley: Reading, PA, USA, 1977; 688p. [Google Scholar]
- Gotoh, S.; Patrick, W.H. Transformations of manganese in a waterlogged soil as affected by redox potential and pH. Soil Sci. Soc. Am. J. 1972, 36, 738–742. [Google Scholar] [CrossRef]
- DeLaune, R.D.; Reddy, K.R. Redox Potential. In Encyclopedia of Soils in the Environment; Hillel, D., Ed.; Academic Press: Amsterdam, The Netherlands, 2005; pp. 366–371. [Google Scholar]
- Narteh, L.T.; Sahrawat, K.L. Influence of flooding on electrochemical and chemical properties of West African soils. Geoderma 1999, 87, 179–207. [Google Scholar] [CrossRef]
- Gotoh, S.; Patrick, W.H. Transformations of iron in a waterlogged soil as influenced by redox potential and pH. Soil Sci. Soc. Am. J. 1974, 38, 66–71. [Google Scholar] [CrossRef]
- Cornu, J.Y.; Denaix, L.; Schneider, A.; Pellerin, S. Temporal evolution of redox processes and free Cd dynamics in a metal-contaminated soil after rewetting. Chemosphere 2007, 70, 306–314. [Google Scholar] [CrossRef] [PubMed]
- Gray, C.W.; McLaren, R.G.; Roberts, A.H.C.; Condron, L.M. Fractionation of soil cadmium from some New Zealand soils. Commun. Soil Sci. Plant Anal. 2000, 31, 1261–1273. [Google Scholar] [CrossRef]
- Yamane, I.; Sato, K. Effect of temperature on the decomposition of organic substances in flooded soil. Soil Sci. Plant Nutr. 1967, 13, 94–100. [Google Scholar] [CrossRef]
- Lovley, D.R. Microbial reduction of iron, manganese, and other metals. Adv. Agron. 1995, 54, 175–231. [Google Scholar]
- Loganathan, P.; Hedley, M.J.; Gregg, P.E.H.; Currie, L.D. Effect of phosphate fertiliser type on the accumulation and plant availability of cadmium in grassland soils. Nutr. Cycl. Agroecosyst. 1997, 47, 169–178. [Google Scholar] [CrossRef]
- Mortvedt, J.J.; Mays, D.A.; Osborn, G. Uptake by wheat of cadmium and other heavy metal contaminants in phosphate fertilizers. J. Environ. Qual. 1981, 10, 193–197. [Google Scholar] [CrossRef]
- Roberts, A.H.C.; Longhurst, R.D. Cadmium cycling in sheep-grazed hill-country pastures. N. Z. J. Agric. Res. 2002, 45, 103–112. [Google Scholar] [CrossRef]
- Tan, K.H. Soil Sampling, Preparation, and Analysis, 2nd ed.; Taylor & Francis: Boca Raton, FL, USA, 2005; 672p. [Google Scholar]
- Somasiri, S.C.; Kenyon, P.R.; Kemp, P.D.; Morel, P.C.H.; Morris, S.T. Growth performance and carcass characteristics of lambs grazing forage mixes inclusive of plantain (Plantago lanceolata L.) and chicory (Cichorium intybus L.). Small Rumin. Res. 2015, 127, 20–27. [Google Scholar] [CrossRef]
- Bolan, N.S.; Adriano, D.C.; Mani, P.A.; Duraisamy, A. Immobilization and phytoavailability of cadmium in variable charge soils. II. Effect of lime addition. Plant Soil 2003, 251, 187–198. [Google Scholar]


| Soil Type | Kereone Fine Sandy Loam | Topehaehae Sandy Clay Loam |
|---|---|---|
| New Zealand Soil Classification | Typic Orthic Allophanic | Typic Recent Gley |
| USDA Taxonony | Udand | Aquept |
| Total Cd (mg kg−1 DW) | 0.79 | 0.61 |
| Total P (mg kg−1 DW) | 1577 | 1132 |
| Total Mn (mg kg−1 DW) | 1840 | 710 |
| Total Fe (%) | 2.1 | 2.4 |
| Total C (%) | 5.7 | 3.2 |
| pH | 6.3 | 5.6 |
| Cation Exchange Capacity (meq 100 g−1) | 26 | 17 |
| P retention (%) | 69 | 34 |
| Measure | Treatment | p-Value | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| KPN | KUN | KPF | KUF | TPN | TUN | TPF | TUF | |||
| Soil extractable Cd (µg kg−1) | (i) Overall mean | 20.9 (a) | 21.1 (a) | 20.0 (a) | 18.5 (a) | 84.4 (b) | 89.3 (b) | 88.9 (b) | 86.1 (b) | *** |
| Std. Error | 0.7 | 0.7 | 0.8 | 0.8 | 3.4 | 3.4 | 3.5 | 3.4 | ||
| n | 88 | 87 | 88 | 84 | 86 | 88 | 87 | 84 | ||
| (ii) Pre-irrigation mean | 19.3 (a) | 19.4 (a) | 17.9 (a) | 16.1 (a) | 83.0 (b) | 87.6 (b) | 85.4 (b) | 81.4 (b) | *** | |
| Std. Error | 1.0 | 1.1 | 1.0 | 1.1 | 5.4 | 5.2 | 4.9 | 5.0 | ||
| n | 44 | 43 | 45 | 41 | 44 | 44 | 44 | 40 | ||
| (iii) +3 day mean | 22.6 (a) | 22.7 (a) | 22.1 (a) | 20.7 (a) | 86.0 (b) | 91.0 (b) | 92.5 (b) | 90.4 (b) | *** | |
| Std. Error | 1.0 | 0.9 | 1.2 | 1.1 | 4.1 | 4.3 | 4.9 | 4.6 | ||
| n | 44 | 44 | 43 | 43 | 42 | 44 | 43 | 44 | ||
| p-value (pre-irrigation vs. +3 day) | * | * | ** | ** | n.s. | n.s. | n.s. | n.s. | ||
| (iv) Plant tissue Cd (mg kg−1 DM) | Mean | 0.208 (a) | 0.384 (ab) | 0.650 (c) | 0.604 (bc) | *** | ||||
| Std. Error | 0.059 | 0.077 | 0.054 | 0.068 | ||||||
| n | 5 | 5 | 5 | 5 | ||||||
| Main effect—Soil type | *** | |||||||||
| Main effect—Irrigation regime | n.s. | |||||||||
| (v) Plant yield (g DM pot−1) | Mean | 5.40 (c) | 2.70 (ab) | 4.02 (bc) | 1.51 (a) | *** | ||||
| Std. Error | 0.57 | 0.29 | 0.30 | 0.16 | ||||||
| Main effect—Soil type | n.s. | |||||||||
| Main effect—Irrigation regime | *** | |||||||||
| (vi) Plant Cd uptake (µg Cd pot−1) | Mean | 1.001 (a) | 1.020 (a) | 2.602 (b) | 0.880 (a) | *** | ||||
| Std. Error | 0.224 | 0.182 | 0.280 | 0.056 | ||||||
| Main effect—Soil type | * | |||||||||
| Main effect—Irrigation regime | * | |||||||||
| Irrigation Treatment | Day of Sampling | p-Value | |||
|---|---|---|---|---|---|
| 0 | 28 | 84 | 112 | ||
| KPN | 4.04 | 4.09 (ab) | 4.29 | 4.44 | |
| KPF | 3.94 | 4.28 (b) | 4.38 | 4.27 | |
| TPN | 3.97 | 4.00 (a) | 4.17 | 4.23 | |
| TPF | 3.92 | 4.10 (ab) | 4.13 | 4.12 | |
| p-value | 0.150 | 0.042 | 0.294 | 0.144 | |
| Sampling mean | 3.97 (A) | 4.11 (AB) | 4.24 (B) | 4.27 (B) | <0.001 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stafford, A.; Jeyakumar, P.; Hedley, M.; Anderson, C. Influence of Soil Moisture Status on Soil Cadmium Phytoavailability and Accumulation in Plantain (Plantago lanceolata). Soil Syst. 2018, 2, 9. https://doi.org/10.3390/soils2010009
Stafford A, Jeyakumar P, Hedley M, Anderson C. Influence of Soil Moisture Status on Soil Cadmium Phytoavailability and Accumulation in Plantain (Plantago lanceolata). Soil Systems. 2018; 2(1):9. https://doi.org/10.3390/soils2010009
Chicago/Turabian StyleStafford, Aaron, Paramsothy Jeyakumar, Michael Hedley, and Christopher Anderson. 2018. "Influence of Soil Moisture Status on Soil Cadmium Phytoavailability and Accumulation in Plantain (Plantago lanceolata)" Soil Systems 2, no. 1: 9. https://doi.org/10.3390/soils2010009






