Biotic versus Abiotic Controls on Bioavailable Soil Organic Carbon
Abstract
:1. Introduction
2. Materials and Methods
2.1. Site Description
2.2. Plant Removal
2.3. Laboratory Soil Incubations
2.3.1. Soil Sampling
2.3.2. Incubation Setup
2.3.3. Soil Measurements
2.4. Statistical Analyses
3. Results
3.1. Microbial Biomass
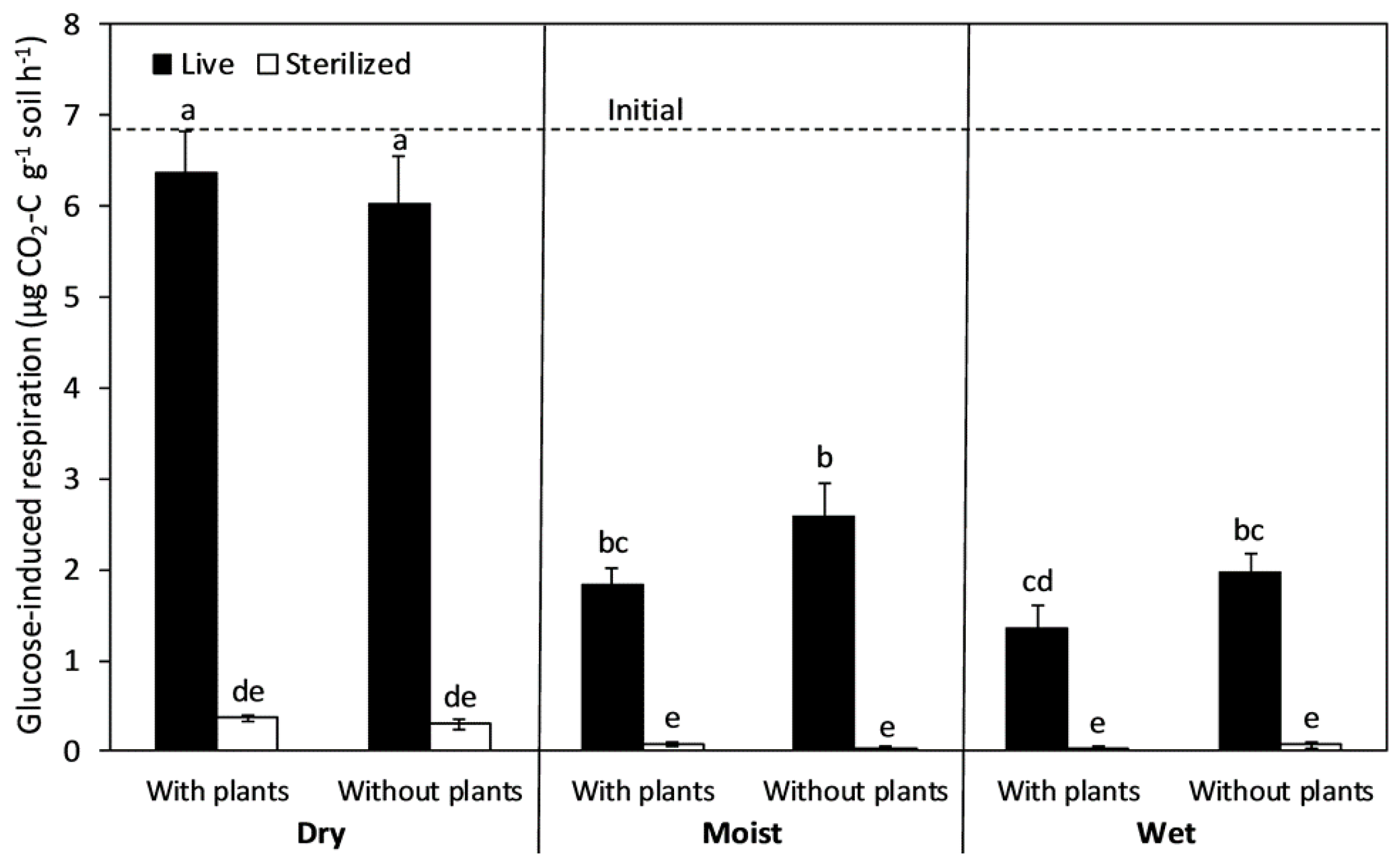
3.2. Cumulative CO2 Emission
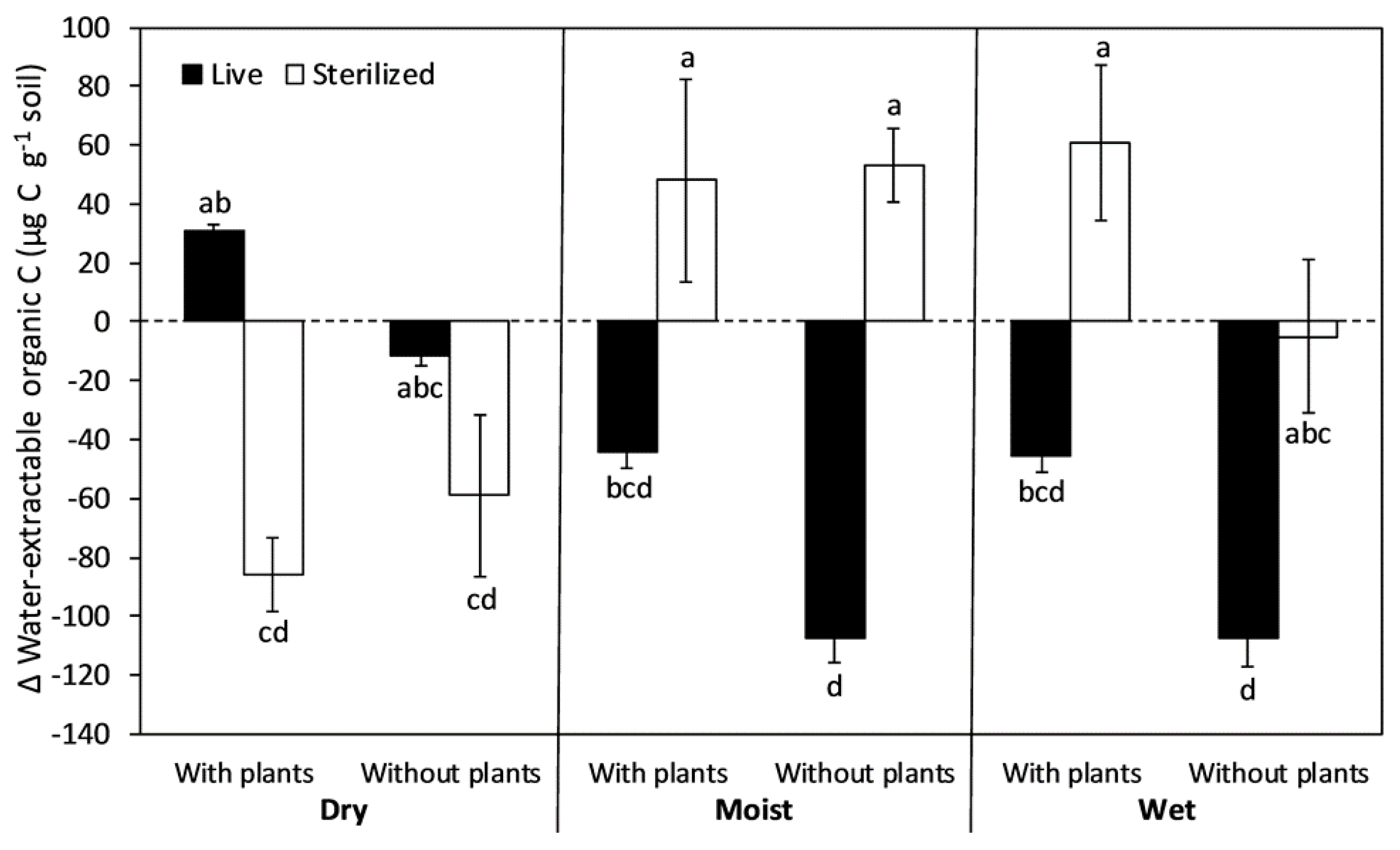
3.3. Water-Extractable Organic Carbon (WEOC)
4. Discussion
Acknowledgements
Author Contributions
Conflicts of Interest
References
- Marschner, B.; Kalbitz, K. Controls of bioavailability and biodegradability of dissolved organic matter in soils. Geoderma 2003, 113, 211–235. [Google Scholar] [CrossRef]
- Von Lützow, M.; Kögel-Knabner, I.; Ekschmitt, K.; Flessa, H.; Guggenberger, G.; Matzner, E.; Marschner, B. SOM fractionation methods: Relevance to functional pools and to stabilization mechanisms. Soil Biol. Biochem. 2007, 39, 2183–2207. [Google Scholar] [CrossRef]
- Marschner, B.; Noble, A.D. Chemical and biological processes leading to the neutralisation of acidity in soil incubated with litter materials. Soil Biol. Biochem. 2000, 32, 805–813. [Google Scholar] [CrossRef]
- Guo, X.; Drury, C.F.; Yang, X.; Reynolds, D.W.; Fan, R. The extent of soil drying and rewetting affects nitrous oxide emissions, denitrification, and nitrogen mineralization. Soil Sci. Soc. Am. J. 2014, 78, 194–204. [Google Scholar] [CrossRef]
- Qualls, R.G. Comparison of the behavior of soluble organic and inorganic nutrients in forest soils. For. Ecol. Manag. 2000, 138, 29–50. [Google Scholar] [CrossRef]
- Fierer, N.; Schimel, J.P. Effects of drying–rewetting frequency on soil carbon and nitrogen transformations. Soil Biol. Biochem. 2002, 34, 777–787. [Google Scholar] [CrossRef]
- Lawrence, C.R.; Neff, J.C.; Schimel, J.P. Does adding microbial mechanisms of decomposition improve soil organic matter models? A comparison of four models using data from a pulsed rewetting experiment. Soil Biol. Biochem. 2009, 41, 1923–1934. [Google Scholar] [CrossRef]
- Schimel, J.P.; Schaeffer, S.M. Microbial control over carbon cycling in soil. Front. Microbiol. 2012, 3, 348. [Google Scholar] [CrossRef] [PubMed]
- Dai, O.H.; David, M.B.; Vance, G.F. Characterization of solid and dissolved carbon in a spruce-fir Spodosol. Biogeochemistry 1996, 35, 339–365. [Google Scholar] [CrossRef]
- Jandl, R.; Sollins, P. Water-extractable soil carbon in relation to the belowground carbon cycle. Biol. Fert. Soils 1997, 25, 196–201. [Google Scholar] [CrossRef]
- Wagai, R.; Sollins, P. Biodegradation and regeneration of water-soluble carbon in a forest soil: Leaching column study. Biol. Fert. Soils 2002, 35, 18–26. [Google Scholar] [CrossRef]
- Kalbitz, K.; Schmerwitz, J.; Schwesig, D.; Matzner, E. Biodegradation of soil-derived dissolved organic matter as related to its properties. Geoderma 2003, 113, 273–291. [Google Scholar] [CrossRef]
- Manzoni, S.; Schimel, J.P.; Porporato, A. Responses of soil microbial communities to water stress: Results from a meta-analysis. Ecology 2012, 93, 930–938. [Google Scholar] [CrossRef] [PubMed]
- Moore, T.R.; De Souza, W.; Koprivnjak, J.F. Controls on the sorption of dissolved organic carbon by soils. Soil Sci. 1992, 154, 120–129. [Google Scholar] [CrossRef]
- Kalbitz, K.; Solinger, S.; Park, J.H.; Michalzik, B.; Matzner, E. Controls on the dynamics of dissolved organic matter in soils: A review. Soil Sci. 2000, 165, 277–304. [Google Scholar] [CrossRef]
- Neff, J.C.; Asner, G.P. Dissolved organic carbon in terrestrial ecosystems: Synthesis and a model. Ecosystems 2001, 4, 29–48. [Google Scholar] [CrossRef]
- Chantigny, M.H. Dissolved and water-extractable organic matter in soils: A review on the influence of land use and management practices. Geoderma 2003, 113, 357–380. [Google Scholar] [CrossRef]
- Reid, I.D.; Abrams, G.D.; Pepper, J.M. Water-soluble products from the degradation of aspen lignin by Phanerochaete chrysosporium. Can. J. Bot. 1982, 60, 2357–2364. [Google Scholar] [CrossRef]
- Sinsabaugh, R.S. Enzymic analysis of microbial pattern and process. Biol. Fert. Soils 1994, 17, 69–74. [Google Scholar] [CrossRef]
- Wallenstein, M.D.; Weintraub, M.N. Emerging tools for measuring and modeling the in situ activity of soil extracellular enzymes. Soil Biol. Biochem. 2008, 40, 2098–2106. [Google Scholar] [CrossRef]
- Kleber, M.; Eusterhues, K.; Keiluweit, M.; Mikutta, C.; Mikutta, R.; Nico, P.S. Chapter one-mineral–organic associations: Formation, properties, and relevance in soil environments. Adv. Agron. 2015, 130, 1–140. [Google Scholar]
- Blankinship, J.C.; Becerra, C.A.; Schaeffer, S.M.; Schimel, J.P. Separating cellular metabolism from exoenzyme activity in soil organic matter decomposition. Soil Biol. Biochem. 2014, 71, 68–75. [Google Scholar] [CrossRef]
- Blankinship, J.C.; Fonte, S.J.; Six, J.; Schimel, J.P. Plant versus microbial controls on soil aggregate stability in a seasonally dry ecosystem. Geoderma 2016, 272, 39–50. [Google Scholar] [CrossRef]
- Silver, W.L.; Lugo, A.E.; Keller, M. Soil oxygen availability and biogeochemistry along rainfall and topographic gradients in upland wet tropical forest soils. Biogeochemistry 1999, 44, 301–328. [Google Scholar] [CrossRef]
- Manzoni, S.; Katul, G. Invariant soil water potential at zero microbial respiration explained by hydrological discontinuity in dry soils. Geophys. Res. Lett. 2014, 41, 7151–7158. [Google Scholar] [CrossRef]
- Anderson, J.P.E.; Domsch, K.H. A physiological method for the quantitative measurement of microbial biomass in soils. Soil Biol. Biochem. 1978, 10, 215–221. [Google Scholar] [CrossRef]
- West, A.W.; Sparling, G.P. Modifications to the substrate-induced respiration method to permit measurement of microbial biomass in soils of differing water contents. J. Microbiol. Methods 1986, 5, 177–189. [Google Scholar] [CrossRef]
- Boyer, J.N.; Groffman, P.M. Bioavailability of water extractable organic carbon fractions in forest and agricultural soil profiles. Soil Biol. Biochem. 1996, 28, 783–790. [Google Scholar] [CrossRef]
- Rees, R.M.; Parker, J.P. Filtration increases the correlation between water extractable organic carbon and soil microbial activity. Soil Biol. Biochem. 2005, 37, 2240–2248. [Google Scholar] [CrossRef]
- Ren, C.; Zhao, F.; Shi, Z.; Chen, J.; Han, X.; Yang, G.; Feng, Y.; Ren, G. Differential responses of soil microbial biomass and carbon-degrading enzyme activities to altered precipitation. Soil Biol. Biochem. 2017, 115, 1–10. [Google Scholar] [CrossRef]
- Schaeffer, S.M.; Homyak, P.M.; Boot, C.M.; Roux-Michollet, D.; Schimel, J.P. Soil carbon and nitrogen dynamics throughout the summer drought in a California annual grassland. Soil Biol. Biochem. 2017, 115, 54–62. [Google Scholar] [CrossRef]
- Cheng, W.; Zhang, Q.; Coleman, D.C.; Carroll, C.R.; Hoffman, C.A. Is available carbon limiting microbial respiration in the rhizosphere? Soil Biol. Biochem. 1996, 28, 1283–1288. [Google Scholar] [CrossRef]
- Kleber, M.; Sollins, P.; Sutton, R. A conceptual model of organo-mineral interactions in soils: Self-assembly of organic molecular fragments into zonal structures on mineral surfaces. Biogeochemistry 2007, 85, 9–24. [Google Scholar] [CrossRef]
- Guggenberger, G.; Kaiser, K.; Zech, W. Mobilization and immobilization of dissolved organic matter in forest soils. J. Plant Nutr. Soil Sci. 1998, 161, 401–408. [Google Scholar] [CrossRef]
- Kalbitz, K.; Schwesig, D.; Rethemeyer, J.; Matzner, E. Stabilization of dissolved organic matter by sorption to the mineral soil. Soil Biol. Biochem. 2005, 37, 1319–1331. [Google Scholar] [CrossRef]
- Keil, R.G.; Mayer, L.M. Mineral matrices and organic matter. Treatise Geochem. Org. Geochem. 2014, 12, 337–359. [Google Scholar]
- Schimel, J.; Becerra, C.A.; Blankinship, J. Estimating decay dynamics for enzyme activities in soils from different ecosystems. Soil Biol. Biochem. 2017, 114, 5–11. [Google Scholar] [CrossRef]
- Nakanishi, T.; Atarashi-Andoh, M.; Koarashi, J.; Saito-Kokubu, Y.; Hirai, K. Carbon isotopes of water-extractable organic carbon in a depth profile of forest soil imply a dynamic relationship with soil carbon. Eur. J. Soil Sci. 2012, 63, 495–500. [Google Scholar] [CrossRef]
- Six, J.; Conant, R.T.; Paul, E.A.; Paustian, K. Stabilization mechanisms of soil organic matter: Implications for C-saturation of soils. Plant Soil 2002, 241, 155–176. [Google Scholar] [CrossRef]
- Lal, R. Soil carbon sequestration impacts on global climate change and food security. Science 2004, 304, 1623–1627. [Google Scholar] [CrossRef] [PubMed]
- Paustian, K.; Lehmann, J.; Ogle, S.; Reay, D.; Robertson, G.P.; Smith, P. Climate-smart soils. Nature 2016, 532, 49–57. [Google Scholar] [CrossRef] [PubMed]
- Jackson, R.B.; Lajtha, K.; Crow, S.E.; Hugelius, G.; Kramer, M.G.; Piñeiro, G. The ecology of soil carbon: Pools, vulnerabilities, and biotic and abiotic controls. Annu. Rev. Ecol. Evol. Syst. 2017, 48, 419–445. [Google Scholar] [CrossRef]
- Ahrens, B.; Braakhekke, M.C.; Guggenberger, G.; Schrumpf, M.; Reichstein, M. Contribution of sorption, DOC transport and microbial interactions to the 14C age of a soil organic carbon profile: Insights from a calibrated process model. Soil Biol. Biochem. 2015, 88, 390–402. [Google Scholar] [CrossRef]
- Dungait, J.A.; Hopkins, D.W.; Gregory, A.S.; Whitmore, A.P. Soil organic matter turnover is governed by accessibility not recalcitrance. Glob. Chang. Biol. 2012, 18, 1781–1796. [Google Scholar] [CrossRef]
- Zsolnay, A.; Görlitz, H. Water extractable organic matter in arable soils: Effects of drought and long-term fertilization. Soil Biol. Biochem. 1994, 26, 1257–1261. [Google Scholar] [CrossRef]
- Zhang, Q.; Zak, J.C. Effects of water and nitrogen amendment on soil microbial biomass and fine root production in a semi-arid environment in West Texas. Soil Biol. Biochem. 1998, 30, 39–45. [Google Scholar] [CrossRef]
- Parker, S.S.; Schimel, J.P. Soil nitrogen availability and transformations differ between the summer and the growing season in a California grassland. Appl. Soil Ecol. 2011, 48, 185–192. [Google Scholar] [CrossRef]
- Ma, L.; Guo, C.; Lü, X.; Yuan, S.; Wang, R. Soil moisture and land use are major determinants of soil microbial community composition and biomass at a regional scale in northeastern China. Biogeosciences 2015, 12, 2585–2596. [Google Scholar] [CrossRef]
- Insam, H. Are the soil microbial biomass and basal respiration governed by the climatic regime? Soil Biol. Biochem. 1990, 22, 525–532. [Google Scholar] [CrossRef]
- Blankinship, J.C.; Niklaus, P.A.; Hungate, B.A. A meta-analysis of responses of soil biota to global change. Oecologia 2011, 165, 553–565. [Google Scholar] [CrossRef] [PubMed]
- Ren, C.; Chen, J.; Lu, X.; Doughty, R.; Zhao, F.; Zhong, Z.; Han, X.; Yang, G.; Feng, Y.; Ren, G. Responses of soil total microbial biomass and community compositions to rainfall reductions. Soil Biol. Biochem. 2018, 116, 4–10. [Google Scholar] [CrossRef]
- Vargas, R.; Hattori, T. Protozoan predation of bacterial cells in soil aggregates. FEMS Microbiol. Lett. 1986, 38, 233–242. [Google Scholar] [CrossRef]
- Van Veen, J.A.; van Overbeek, L.S.; van Elsas, J.D. Fate and activity of microorganisms introduced into soil. Microbiol. Mol. Biol. Rev. 1997, 61, 121–135. [Google Scholar] [PubMed]
- Carson, J.K.; Gonzalez-Quiñones, V.; Murphy, D.V.; Hinz, C.; Shaw, J.A.; Gleeson, D.B. Low pore connectivity increases bacterial diversity in soil. Appl. Environ. Microbiol. 2010, 76, 3936–3942. [Google Scholar] [CrossRef] [PubMed]
- Vance, E.D.; Chapin, F.S. Substrate limitations to microbial activity in taiga forest floors. Soil Biol. Biochem. 2001, 33, 173–188. [Google Scholar] [CrossRef]
- Schimel, J.P.; Weintraub, M.N. The implications of exoenzyme activity on microbial carbon and nitrogen limitation in soil: A theoretical model. Soil Biol. Biochem. 2003, 35, 549–563. [Google Scholar] [CrossRef]
- Brooks, P.D.; McKnight, D.; Elder, K. Carbon limitation of soil respiration under winter snowpacks: Potential feedbacks between growing season and winter carbon fluxes. Glob. Chang. Biol. 2005, 11, 231–238. [Google Scholar] [CrossRef]
- Maire, V.; Alvarez, G.; Colombet, J.; Comby, A.; Despinasse, R.; Dubreucq, E.; Joly, M.; Lehours, A.-C.; Perrier, V.; Shahzad, T.; et al. An unknown oxidative metabolism substantially contributes to soil CO2 emissions. Biogeosciences 2013, 10, 1155–1167. [Google Scholar] [CrossRef]
- Kéraval, B.; Lehours, A.C.; Colombet, J.; Amblard, C.; Alvarez, G.; Fontaine, S. Soil carbon dioxide emissions controlled by an extracellular oxidative metabolism identifiable by its isotope signature. Biogeosciences 2016, 13, 6353–6362. [Google Scholar] [CrossRef]
- Wang, B.; Lerdau, M.; He, Y. Widespread production of nonmicrobial greenhouse gases in soils. Glob. Chang. Biol. 2017, 23, 4472–4482. [Google Scholar] [CrossRef] [PubMed]
- Feng, S.; Fu, Q. Expansion of global drylands under a warming climate. Atmos. Chem. Phys. 2013, 13, 10081–10094. [Google Scholar] [CrossRef]
- Jenkinson, D.S.; Powlson, D.S. The effects of biocidal treatments on metabolism in soil—I. fumigation with chloroform. Soil Biol. Biochem. 1976, 8, 167–177. [Google Scholar] [CrossRef]
- Zelles, L.; Palojärvi, A.; Kandeler, E.; von Lützow, M.; Winter, K.; Bai, Q.Y. Changes in soil microbial properties and phospholipid fatty acid fractions after chloroform fumigation. Soil Biol. Biochem. 1997, 29, 1325–1336. [Google Scholar] [CrossRef]
- Gärdenäs, A.I.; Ågren, G.I.; Bird, J.A.; Clarholm, M.; Hallin, S.; Ineson, P.; Kätterer, T.; Knicker, H.; Nilsson, S.I.; Näsholm, T.; et al. Knowledge gaps in soil carbon and nitrogen interactions–from molecular to global scale. Soil Biol. Biochem. 2011, 43, 702–717. [Google Scholar] [CrossRef]
- Vereecken, H.; Schnepf, A.; Hopmans, J.W.; Javaux, M.; Or, D.; Roose, T.; Vanderborght, J.; Young, M.H.; Amelung, W.; Aitkenhead, M.; et al. Modeling soil processes: Review, key challenges, and new perspectives. Vadose Zone J. 2016, 15, 57. [Google Scholar] [CrossRef] [Green Version]
- Holden, P.A.; Ritz, K.; Young, I. How do the microhabitats framed by soil structure impact soil bacteria and the processes that they regulate. In The Architecture and Biology of Soils: Life in Inner Space; CABI: Cambridge, MA, USA, 2011; p. 118. [Google Scholar]
- Manzoni, S.; Porporato, A. Soil carbon and nitrogen mineralization: Theory and models across scales. Soil Biol. Biochem. 2009, 41, 1355–1379. [Google Scholar] [CrossRef]
- Wieder, W.R.; Allison, S.D.; Davidson, E.A.; Georgiou, K.; Hararuk, O.; He, Y.; Hopkins, F.; Luo, Y.; Smith, M.J.; Sulman, B.N.; et al. Explicitly representing soil microbial processes in Earth system models. Glob. Biogeochem. Cycles 2015, 29, 1782–1800. [Google Scholar] [CrossRef]
- Kallenbach, C.M.; Frey, S.D.; Grandy, A.S. Direct evidence for microbial-derived soil organic matter formation and its ecophysiological controls. Nat. Commun. 2016, 7, 13630. [Google Scholar] [CrossRef] [PubMed]

| Treatment | df | Microbial Biomass | Cumulative CO2 Emission | WEOC | |||
|---|---|---|---|---|---|---|---|
| F stat | p-value | F stat | p-value | F stat | p-value | ||
| Ster | 1 | 485.45 | <0.0001 * | 719.51 | <0.0001 * | 25.06 | <0.0001 * |
| Plant | 1 | 1.14 | 0.29 | 12.03 | 0.0010 * | 11.41 | 0.0014 * |
| H2O | 2 | 109.42 | <0.0001 * | 206.33 | <0.0001 * | 1.19 | 0.31 |
| Ster × Plant | 1 | 1.46 | 0.23 | 11.47 | 0.0013 | 5.04 | 0.029 * |
| Ster × H2O | 2 | 84.54 | <0.0001 * | 190.46 | <0.0001 * | 44.77 | <0.0001 * |
| Plant × H2O | 2 | 1.61 | 0.21 | 3.14 | 0.051 * | 2.83 | 0.068 |
| Ster × Plant × H2O | 2 | 1.29 | 0.28 | 2.83 | 0.067 | 1.55 | 0.22 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Blankinship, J.C.; Schimel, J.P. Biotic versus Abiotic Controls on Bioavailable Soil Organic Carbon. Soil Syst. 2018, 2, 10. https://doi.org/10.3390/soilsystems2010010
Blankinship JC, Schimel JP. Biotic versus Abiotic Controls on Bioavailable Soil Organic Carbon. Soil Systems. 2018; 2(1):10. https://doi.org/10.3390/soilsystems2010010
Chicago/Turabian StyleBlankinship, Joseph C., and Joshua P. Schimel. 2018. "Biotic versus Abiotic Controls on Bioavailable Soil Organic Carbon" Soil Systems 2, no. 1: 10. https://doi.org/10.3390/soilsystems2010010





