Kinetic Study of Pyrolysis of Coniferous Bark Wood and Modified Fir Bark Wood
Abstract
:1. Introduction
2. Materials and Methods
2.1. Arrhenius Model
2.2. Coats–Redfern Model
3. Results and Discussion
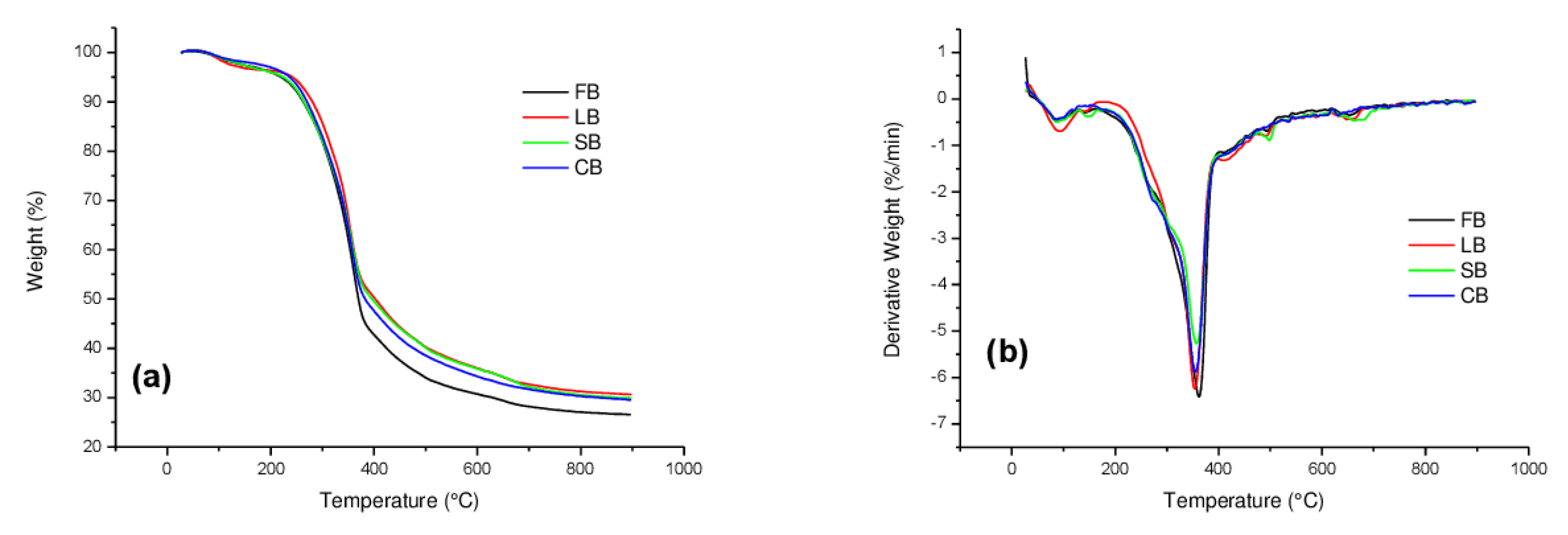
3.1. Thermogravimetry Analysis
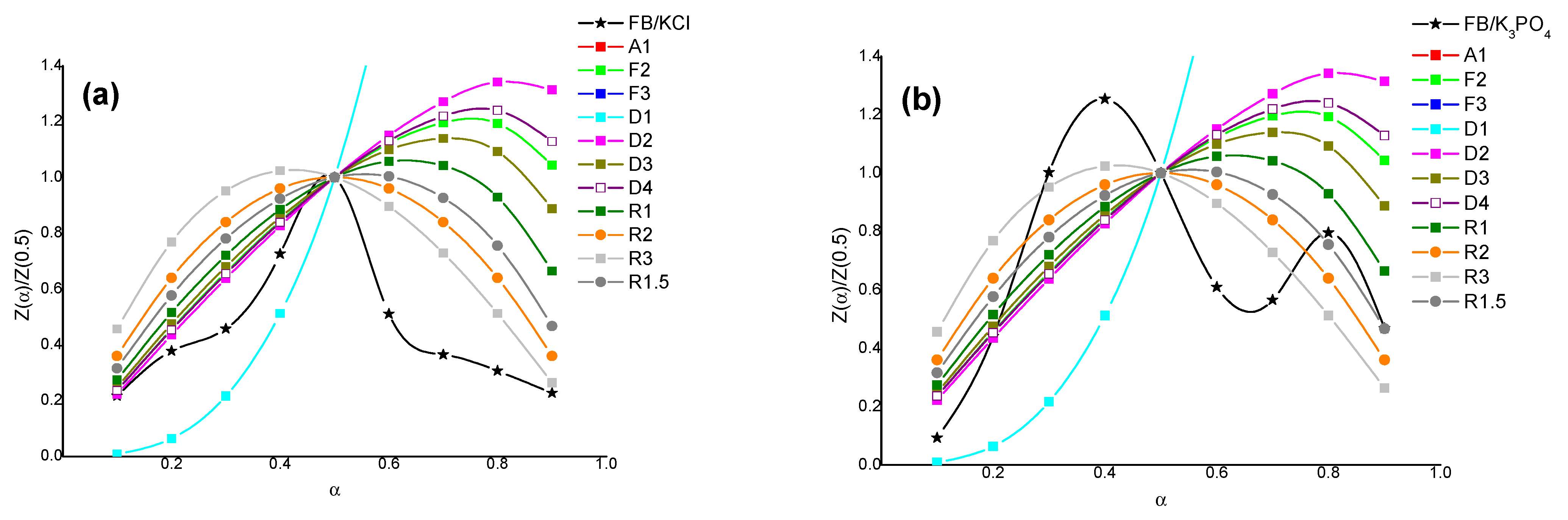
3.2. Activation of Fir Bark by Potassium Compounds
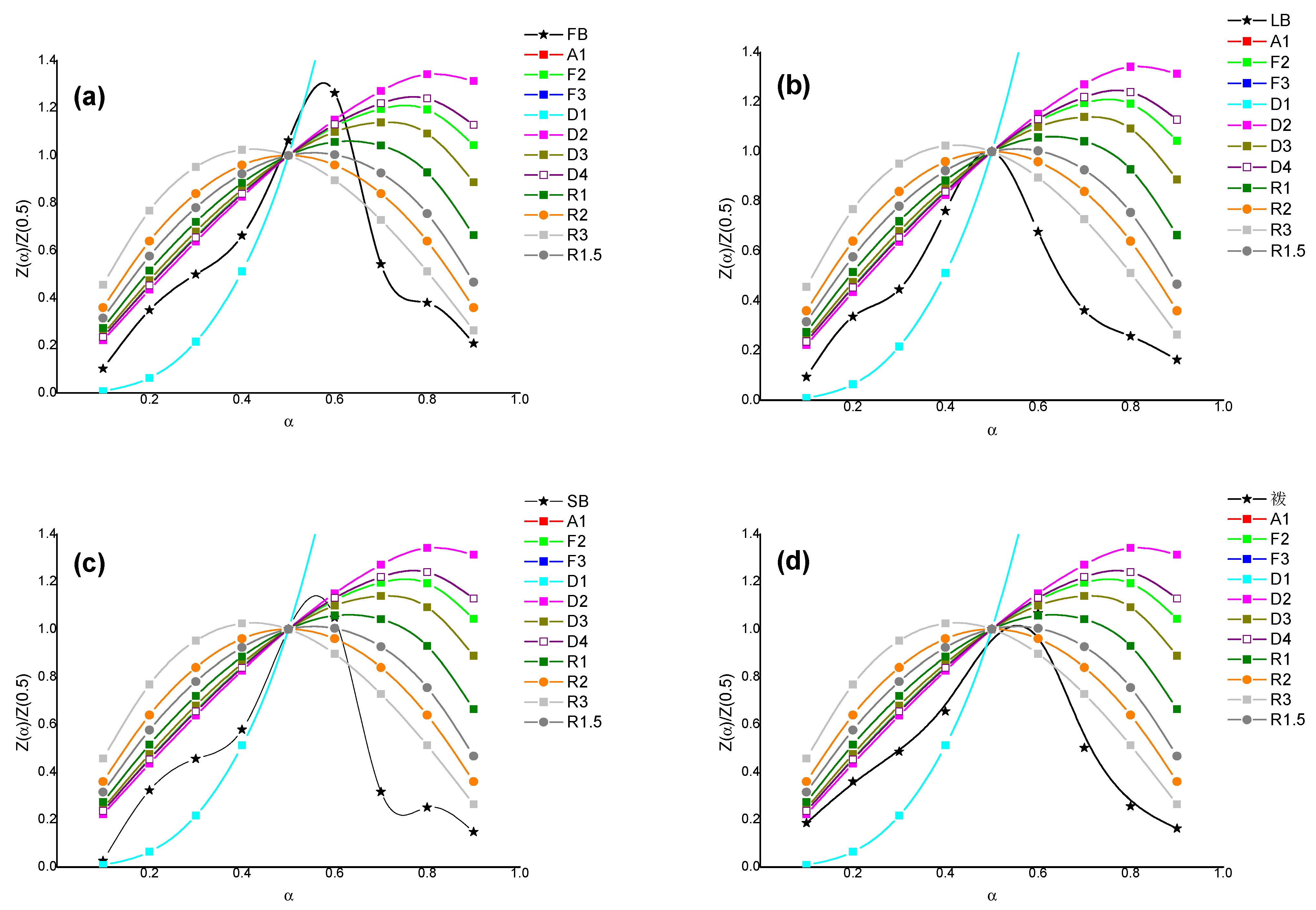
3.3. Results of the Kinetic Analysis
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ding, Y.; Huang, B.; Li, K.; Du, W.; Lu, K.; Zhang, Y. Thermal interaction analysis of isolated hemicellulose and cellulose by kinetic parameters during biomass pyrolysis. Energy 2020, 195, 117010. [Google Scholar] [CrossRef]
- Lin, B.; Zhou, J.; Qin, Q.; Song, X.; Luo, Z. Thermal behavior and gas evolution characteristics during co-pyrolysis of lignocellulosic biomass and coal. J. Anal. Appl. Pyrolysis 2019, 144, 104718. [Google Scholar] [CrossRef]
- Fermanelli, C.S.; Córdoba, A.; Pierella, L.B.; Saux, C. Pyrolysis and copyrolysis of three lignocellulosic biomass residues from the agro-food industry: A comparative study. Waste Manag. 2019, 102, 362–370. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Che, Q.; Li, S.; Liu, Z.; Yang, H.; Chen, Y.; Wang, X.; Shao, J.; Chen, H. Recent developments in lignocellulosic biomass catalytic fast pyrolysis: Strategies for the optimization of bio-oil quality and yield. Fuel Process. Technol. 2019, 196, 106180. [Google Scholar] [CrossRef]
- Mian, I.; Li, X.; Jian, Y.; Dacres, O.D.; Zhong, M.; Liu, J.; Ma, F.; Rahman, N. Kinetic study of biomass pellet pyrolysis by using distributed activation energy model and Coats Redfern methods and their comparison. Bioresour. Technol. 2019, 294, 122099. [Google Scholar] [CrossRef]
- Perander, M.; DeMartini, N.; Brink, A.; Kramb, J.; Karlström, O.; Hemming, J.; Moilanen, A.; Konttinen, J.; Hupa, M. Catalytic effect of Ca and K on CO2 gasification of spruce wood char. Fuel 2015, 150, 464–472. [Google Scholar] [CrossRef]
- Shah, M.; Dai, J.J.; Guo, Q.X.; Fu, Y. Products and production routes for the catalytic conversion of seed oil into fuel and chemicals: A comprehensive review. Sci. China Chem. 2015, 58, 1110–1121. [Google Scholar] [CrossRef]
- Shen, Y.; Yoshikawa, K. Recent progresses in catalytic tar elimination during biomass gasification or pyrolysis-A review. Renew. Sustain. Energy Rev. 2013, 21, 371–392. [Google Scholar] [CrossRef]
- Dickerson, T.; Soria, J. Catalytic fast pyrolysis: A review. Energies 2013, 6, 514–538. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, Y.; Guo, F.; Li, X. Characterization of the gas releasing behaviors of catalytic pyrolysis of rice husk using potassium over a micro-fluidized bed reactor. Energy Convers. Manag. 2017, 136, 395–403. [Google Scholar] [CrossRef]
- Velasco, U.I.; Sierra, I.; Zudaire, L.; Ayastuy, J.L. Conversion of waste animal bones into porous hydroxyapatite by alkaline treatment: Effect of the impregnation ratio and investigation of the activation mechanism. J. Mater. Sci. 2015, 50, 7568–7582. [Google Scholar] [CrossRef]
- Ouédraogo, I.W.K.; Mouras, S.; Changotade, O.A.; Blin, J. Development of a new solid catalyst for biodiesel production using local vegetable resources, adapted to the contexts of the west african countries. Waste Biomass Valori. 2018, 9, 1893–1901. Available online: https://link.springer.com/article/10.1007/s12649-017-9964-3 (accessed on 1 February 2023).
- Ryczkowski, R.; Niewiadomski, M.; Michalkiewicz, B.; Skiba, E.; Ruppert, A.M. Effect of alkali and alkaline earth metals addition on Ni/ZrO2 catalyst activity in cellulose conversion. J. Therm. Anal. Calorim. 2016, 126, 103–110. [Google Scholar] [CrossRef]
- Zhang, Y.; Gong, X.; Zhang, B. Potassium catalytic hydrogen production in sorption enhanced gasification of biomass with steam. Int. J. Hydrog. Energy 2014, 39, 4234–4243. [Google Scholar] [CrossRef]
- Ferreiro, A.I.; Rabaçal, M.; Costa, M.; Giudicianni, P.; Grottola, C.M.; Ragucci, R. Modeling the impact of the presence of KCl on the slow pyrolysis of cellulose. Fuel 2018, 215, 57–65. [Google Scholar] [CrossRef]
- Shah, M.H.; Deng, L.; Bennadji, H.; Fisher, E.M. Pyrolysis of potassium-doped wood at the centimeter and submillimeter scales. Energy Fuels 2015, 29, 7350–7357. [Google Scholar] [CrossRef]
- Chen, M.-Q.; Wang, J.; Zhang, M.-X.; Chen, M.-G. Catalytic effects of eight inorganic additives on pyrolysis of pine wood sawdust by microwave heating. J. Anal. Appl. Pyrol. 2008, 82, 145–150. [Google Scholar] [CrossRef]
- Hwang, H.; Oh, S.; Cho, I.G.; Choi, J.W. Fast pyrolysis of potassium impregnated poplar wood and characterization of its influence on the formation as well as properties of pyrolysis products. Bioresour. Technol. 2013, 150, 359–366. [Google Scholar] [CrossRef]
- Zhou, W.; Bai, B.; Chen, G.; Ma, L.; Jing, D.; Yan, B. Study on catalytic properties of potassium carbonate during the process of sawdust pyrolysis. Int. J. Hydrog. Energy 2018, 43, 13829–13841. [Google Scholar] [CrossRef]
- Barneto, A.G.; Vila, C.; Ariza, J.; Vidal, T. Thermogravimetric measurement of amorphous cellulose content in flax fibre and flax pulp. Cellulose 2011, 18, 17–31. [Google Scholar] [CrossRef]
- Fetisova, O.Y.; Mikova, N.M.; Taran, O.P. Evaluation of the validity of model-fitting and model-free methods for kinetic analysis of nonisothermal pyrolysis of Siberian fir bark. Kinet. Catal. 2020, 61, 846–853. [Google Scholar] [CrossRef]
- Doyle, C.D. Techniques and Methods of “Polymer Evaluation”; Marsel-Dekker: New York, NY, USA, 1966; Chapter 4. [Google Scholar]
- Pásztory, Z.; Mohácsiné, I.R.; Gorbacheva, G.; Börcsök, Z. The utilization of tree bark. BioResources 2016, 11, 7859–7888. [Google Scholar]
- Sluiter, A.; Hames, B.; Ruiz, R.; Scarlata, C.; Sluiter, J.; Templeton, D.; Crocker, D. Determination of Structural Carbohydrates and Lignin in Biomass: Laboratory Analytical Procedure (LAP); National Renewable Energy Laboratory: Golden, CO, USA, 2008. [Google Scholar]
- Kürschner, K.; Hanak, A. Zur Bestimmung der sog. Rohfaser. Ein neues Verfahren der Bestimmung der Rohcellulose in Kakao. Z. Für Unters. Der Lebensmittel. 1930, 59, 484–494. [Google Scholar] [CrossRef]
- Ognyanov, M.; Remoroza, C.A.; Schols, H.A.; Petkova, N.T.; Georgiev, Y.N. Structural study of a pectic polysaccharide fraction isolated from “mountain tea” (Sideritis scardica Griseb.). Carbohydr. Polym. 2021, 260, 117798. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Prasad Chakraborty, J.; Kumar Mondal, M. Intrinsic kinetics, thermodynamic parameters and reaction mechanism of non-isothermal degradation of torrefied Acacia nilotica using isoconversional methods. Fuel 2020, 259, 116263. [Google Scholar] [CrossRef]
- Mallick, D.; Poddar, M.K.; Mahanta, P.; Moholkar, V.S. Discernment of synergism in pyrolysis of biomass blends using thermogravimetric analysis. Bioresour. Technol. 2018, 261, 294–305. [Google Scholar] [CrossRef]
- Criado, J.M. Kinetic analysis of DTG data from master curves. Thermochim. Acta 1978, 24, 186–189. [Google Scholar] [CrossRef]
- Nhuchhen, D.R.; Basu, P. Experimental investigation of mildly pressurized torrefaction in air and nitrogen. Energy Fuels 2014, 28, 3110–3121. [Google Scholar] [CrossRef]
- Maiti, S.; Purakayastha, S.; Ghosh, B. Thermal characterization of mustard straw and stalk in nitrogen at different heating rates. Fuel 2007, 86, 1513–1518. [Google Scholar] [CrossRef]
- Damartzis, T.; Vamvuka, D.; Sfakiotakis, S.; Zabaniotou, A. Thermal degradation studies and kinetic modeling of cardoon (Cynara cardunculus) pyrolysis using thermogravimetric analysis (TGA). Bioresour. Technol. 2011, 102, 6230–6238. [Google Scholar] [CrossRef]
- Patwardhan, P.R.; Satrio, J.A.; Brown, R.C.; Shanks, B.H. Influence of inorganic salts on the primary pyrolysis products of cellulose. Bioresour. Technol. 2010, 101, 4646–4655. [Google Scholar] [CrossRef] [PubMed]
- Peng, C.-V.; Lin, H.C. Japanese cedar (Cryptomeria japonica) ash as a natural activating agent for preparing activated carbon. J. Wood Sci. 2015, 61, 316–325. [Google Scholar] [CrossRef]
- Lu, Q.; Zhang, Z.B.; Yang, X.C.; Dong, C.Q.; Zhu, X.F. Catalytic fast pyrolysis of biomass impregnated with K3PO4 to produce phenolic compounds: Analytical Py-GC/MS study. J. Anal. Appl. Pyrolysis 2013, 104, 139–145. [Google Scholar] [CrossRef]
- Chen, C.; Luo, Z.; Yu, C.; Wang, T.; Zhang, H. Transformation behavior of potassium during pyrolysis of biomass. RSC Adv. 2017, 7, 31319–31326. [Google Scholar] [CrossRef]
- Criado, J.M.; Málek, J.; Ortega, A. Applicability of the master plots in kinetic analysis of non-isothermal data. Thermochim. Acta 1989, 147, 377–385. [Google Scholar] [CrossRef]
- Wu, Y.; Dollimore, D. Kinetic studies of thermal degradation of natural cellulosic materials. Thermochim. Acta 1998, 324, 49–57. [Google Scholar] [CrossRef]
- Bianchi, O.; Dal Castel, C.; Olivera, R.V.B.; Bertuoli, P.T.; Hillig, E. Nonisothermal degradation of wood using thermogravimetric measurements. Polímeros 2010, 20, 395–400. [Google Scholar] [CrossRef]
- Poletto, M.; Pistor, V.; Zeni, M.; Zattera, A.J. Crystalline properties and decomposition kinetics of cellulose fibers in wood pulp obtained by two pulping processes. Polym. Degrad. Stab. 2011, 96, 679–685. [Google Scholar] [CrossRef]
- Ding, Y.; Ezekoye, O.A.; Lu, S.; Wang, C.; Zhou, R. Comparative pyrolysis behaviors and reaction mechanisms of hardwood and softwood. Energy Convers. Manag. 2017, 132, 102–109. [Google Scholar] [CrossRef]
- Khawam, A.; Flanagan, D.R. Solid-state kinetic models: Basics and mathematical fundamentals. J. Phys. Chem. B 2006, 110, 17315–17328. [Google Scholar] [CrossRef]
- Borovikov, A.M.; Ugolev, B.N. Handbook of Wood; Forest Industry: Moscow, Russia, 1989; 244p. [Google Scholar]
- Mikova, N.M.; Fetisova, O.Y.; Ivanov, I.P.; Chesnokov, N.V. Study of the thermochemical transformation of fir bark under conditions of its activation by potassium compounds. Bull. Tomsk State Univ. Chemistry. 2021, 23, 18–29. [Google Scholar] [CrossRef]

| Reaction Mechanism | f(α) | g(α) |
|---|---|---|
| Diffusion models | ||
| D1 diffusion (1D) | 1/2α | α2 |
| D2 diffusion, Valensi (2D) | [−ln(1 − α)]−1 | α + (1 − α)ln(1 − α) |
| D3 diffusion, Jander (3D) | (3/2) (1 − α)2/3/[1 − (1 − α)1/3] | [1 − (1 − α)1/3]2 |
| D4 diffusion, Ginstling (3D) | (3/2)/[(1 − α)−1/3 − 1 | 1 − 2α/3 − (1 − α)2/3 |
| Random nucleation and nuclei growth | ||
| A1 Avrami–Erofeev | 3/2(1 − α)[−ln(1 − α)]1/3 | [−ln(1 − α)]2/3 |
| Geometrical contraction models | ||
| Phase boundary-controlled reaction (contracting area) (F2) | 2(1 − α)1/2 | 1−(1 − α)1/2 |
| Phase boundary-controlled reaction (contracting volume) (F3) | 3(1 − α)2/3 | 1−(1 − α)1/3 |
| Reaction order | ||
| R1 First order | 1 − α | −ln(1 − α) |
| R2 Second order | (1 − α)2 | (1−α)−1 − 1 |
| R3 Third order | (1 − α)3 | [(1 − α)−2 − 1]/2 |
| R1.5 One and half order | (1 − α)3/2 | 2[(1 − α)−1/2 − 1] |
| Sample | Chemical Composition (%) | Ash (%) | ||
|---|---|---|---|---|
| Cellulose | Lignin | Hemicelluloses * | ||
| FB | 29.4 | 35.5 | 32.7 | 2.4 |
| LB | 29.3 | 38.8 | 29.3 | 2.5 |
| CB | 28.2 | 43.9 | 25.1 | 2.8 |
| SB | 22.0 | 42.3 | 31.8 | 3.8 |
| Sample | Interval, °C | Vmax, %/min | Tmax, °C | Δm |
|---|---|---|---|---|
| FB | 140–280 | 1.01 | ~260 | 14.30 |
| 280–400 | 3.93 | 357.5 | 38.60 | |
| 400–520 | 1.08 | 460.0 | 12.60 | |
| FB/KCl | 200–280 | 1.25 | 263.0 | 6.90 |
| 280–380 | 2.74 | 340.9 | 16.41 | |
| 380–500 | 0.58 | - | 7.00 | |
| FB/K3PO4 | 117–240 | 0.60 | - | 7.20 |
| 240–360 | 2.72 | 277.8 | 23.05 | |
| 360–520 | 1.12 | 440.4 | 13.25 |
| Sample | Temperature Range, °C | Coates–Redfern | Arrhenius | ||
|---|---|---|---|---|---|
| Mechanism | Ea | Mechanism | Ea | ||
| FB | 261–346 | D2 | 101 | D2 | 99 |
| 346–456 | R3 | 87 | R1.5 | 81 | |
| LB | 277–352 | D2 | 114 | D2 | 110 |
| 352–487 | R3 | 90 | R3 | 88 | |
| SB | 262–347 | D2 | 112 | D1 | 115 |
| 347–482 | R3 | 73 | R3 | 69 | |
| CB | 262–347 | D2 | 101 | D1 | 97 |
| 347–477 | R3 | 76 | R3 | 70 | |
| FB/KCl | 237–337 | D3 | 82 | D2 | 80 |
| 337–617 | R3 | 43 | R3 | 47 | |
| FB/K3PO4 | 152–237 | D2 | 23 | D2 | 20 |
| 267–392 | R3 | 44 | R3 | 45 | |
| 442–497 | R1.5 | 34 | R1.5 | 38 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fetisova, O.Y.; Mikova, N.M.; Chudina, A.I.; Kazachenko, A.S. Kinetic Study of Pyrolysis of Coniferous Bark Wood and Modified Fir Bark Wood. Fire 2023, 6, 59. https://doi.org/10.3390/fire6020059
Fetisova OY, Mikova NM, Chudina AI, Kazachenko AS. Kinetic Study of Pyrolysis of Coniferous Bark Wood and Modified Fir Bark Wood. Fire. 2023; 6(2):59. https://doi.org/10.3390/fire6020059
Chicago/Turabian StyleFetisova, Olga Yu., Nadezhda M. Mikova, Anna I. Chudina, and Aleksandr S. Kazachenko. 2023. "Kinetic Study of Pyrolysis of Coniferous Bark Wood and Modified Fir Bark Wood" Fire 6, no. 2: 59. https://doi.org/10.3390/fire6020059









