Study on Pyrolysis Characteristics of Chinese Fir under Different Natural Aging Times
Abstract
:1. Introduction
2. Materials and Experimental Procedure
2.1. Materials
2.2. Experimental
3. Results and Discussion
3.1. Thermal Degradation
3.2. Estimation of Activation Energy
3.3. Determination of Reaction Mechanism
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Nomenclature
| Flame growth factor (kW/s²) | |
| A | Refers to prefactor |
| C | Empirical constants, |
| Material thickness (m) | |
| Flame diameter (m) | |
| Reaction activation energy (kJ/mol) | |
| EHC | Effective heat of combustion, ratio of heat release rate to mass loss rate (MJ/kg) |
| Gravitational acceleration (m/s2) | |
| HRR | Heat release rate, combustion release rate per unit area of material (kW/m2) |
| MLR | Mass loss rate (%/K) |
| MLRpeak | Peak mass loss rate (%/K) |
| n | Number of Reaction Levels |
| Power of combustion source (kW) | |
| R | Universal gas contants 8.314 × 10−3 J/(K·mol) |
| Flame spread duration (s) | |
| T5% | 5%Conversion rate corresponding to temperature (K) |
| T | Time (s) |
| Tpeak | Temperature corresponding to peak mass loss rate (K) |
References
- Li, H.Q.; Yu, Y.; Yu, X. On Fire Protection Problems and its Countermeasures about Chinese Ancient Architecture. Appl. Mech. Mater. 2012, 204–208, 3365–3368. [Google Scholar] [CrossRef]
- Wang, G.; Li, W.; Li, B.; Chen, H. TG study on pyrolysis of biomass and its three components under syngas. Fuel 2008, 87, 552–558. [Google Scholar] [CrossRef]
- Ding, Y.; Ezekoye, O.A.; Lu, S.; Wang, C.; Zhou, R. Comparative pyrolysis behaviors and reaction mechanisms of hardwood and softwood. Energy Convers. Manag. 2016, 132, 102–109. [Google Scholar] [CrossRef]
- Martin-Gullon, I.; Esperanza, M.; Font, R. Kinetic model for the pyrolysis and combustion of poly-(ethylene terephthalate) (PET). J. Anal. Appl. Pyrolysis 2001, 58–59, 635–650. [Google Scholar] [CrossRef]
- Liu, H.; Wang, C.; Zhao, W.; Yang, S.; Hou, X. Pyrolysis characteristics and kinetic modeling of Artemisia apiacea by thermogravimetric analysis. J. Therm. Anal. 2017, 131, 1783–1792. [Google Scholar] [CrossRef]
- Huang, R.; Teng, Z.; Li, S. Gaussian model analysis and thermal decomposition kinetics of nature fibers. J. Clean. Prod. 2022, 357, 131784. [Google Scholar] [CrossRef]
- Yao, F.; Wu, Q.; Lei, Y.; Guo, W.; Xu, Y. Thermal decomposition kinetics of natural fibers: Activation energy with dynamic thermogravimetric analysis. Polym. Degrad. Stab. 2008, 93, 90–98. [Google Scholar] [CrossRef]
- Wang, Z.; Wei, R.; Ning, X.; Xie, T.; Wang, J. Thermal degradation properties of LDPE insulation for new and aged fine wires. J. Therm. Anal. 2018, 137, 461–471. [Google Scholar] [CrossRef]
- Liu, H.-P.; Liang, W.-X.; Qin, H.; Wang, Q. Synergy in co-combustion of oil shale semi-coke with torrefied cornstalk. Appl. Therm. Eng. 2016, 109, 653–662. [Google Scholar] [CrossRef]
- Duan, L.; Chen, J.; Jiang, Y.; Li, X.; Longhurst, P.; Lei, M. Experimental and kinetic study of thermal decomposition behaviour of phytoremediation derived Pteris vittata. J. Therm. Anal. 2016, 128, 1207–1216. [Google Scholar] [CrossRef]
- Kránitz, K.; Sonderegger, W.; Bues, C.-T.; Niemz, P. Effects of aging on wood: A literature review. Wood Sci. Technol. 2015, 50, 7–22. [Google Scholar] [CrossRef]
- Wadhwani, R.; Sutherland, D.; Moinuddin, K.A.M.; Joseph, P. Kinetics of pyrolysis of litter materials from pine and eucalyptus forests. J. Therm. Anal. 2017, 130, 2035–2046. [Google Scholar] [CrossRef] [Green Version]
- Yang, P.; He, X.; Zhang, W.; Qiao, Y.; Wang, F.; Tang, K. Study on thermal degradation of cattlehide collagen fibers by simultaneous TG–MS–FTIR. J. Therm. Anal. 2016, 127, 2005–2012. [Google Scholar] [CrossRef]
- Lenth, C.A.; Kamke, F.A. Moisture dependent softening behavior of wood. Wood Fiber Sci. 2001, 33, 492–507. [Google Scholar]
- Conesa, J.A.; Marcilla, A.; Font, R.; Caballero, J. Thermogravimetric studies on the thermal decomposition of polyethylene. J. Anal. Appl. Pyrolysis 1996, 36, 1–15. [Google Scholar] [CrossRef]
- Park, J.W.; Oh, S.C.; Lee, H.P.; Kim, H.T.; Yoo, K.O. A kinetic analysis of thermal degradation of polymers using a dynamic method. Polym. Degrad. Stab. 2000, 67, 535–540. [Google Scholar] [CrossRef]
- Yang, J.; Miranda, R.; Roy, C. Using the DTG curve fitting method to determine the apparent kinetic parameters of thermal decomposition of polymers. Polym. Degrad. Stab. 2001, 73, 455–461. [Google Scholar] [CrossRef]
- Yang, H.; Yan, R.; Chen, H.; Lee, D.H.; Zheng, C. Characteristics of hemicellulose, cellulose and lignin pyrolysis. Fuel 2007, 86, 1781–1788. [Google Scholar] [CrossRef]
- Flynn, J.H.; Wall, L.A. General treatment of the thermogravimetry of polymers, Journal of research of the National Bureau of Standards. Section A. Phys. Chem. 1966, 70, 487. [Google Scholar]
- Flynn, J.H.; Wall, L.A. A quick, direct method for the determination of activation energy from thermogravimetric data. J. Polym. Sci. Part B Polym. Lett. 1966, 4, 323–328. [Google Scholar] [CrossRef]
- Ozawa, T. A new method of analyzing thermogravimetric data. Bull. Chem. Soc. Jan. 1965, 38, 1881–1886. [Google Scholar] [CrossRef] [Green Version]
- Kissinger, H.E. Variation of peak temperature with heating rate in differential thermal analysis. J. Res. Natl. Bur. Stand. 1956, 57, 217. [Google Scholar] [CrossRef]
- Kissinger, H.E. Reaction Kinetics in Differential Thermal Analysis. Anal. Chem. 1957, 29, 1702–1706. [Google Scholar] [CrossRef]
- Oh, S.C.; Jun, H.C.; Kim, H.T. Thermogravimetric Evaluation for Pyrolysis Kinetics of Styrene-Butadiene Rubber. J. Chem. Eng. Jpn. 2003, 36, 1016–1022. [Google Scholar] [CrossRef]
- Chen, J.; Fan, X.; Jiang, B.; Mu, L.; Yao, P.; Yin, H.; Song, X. Pyrolysis of oil-plant wastes in a TGA and a fixed-bed reactor: Thermochemical behaviors, kinetics, and products characterization. Bioresour. Technol. 2015, 192, 592–602. [Google Scholar] [CrossRef]
- Maurya, R.; Ghosh, T.; Saravaia, H.; Paliwal, C.; Ghosh, A.; Mishra, S. Non-isothermal pyrolysis of de-oiled microalgal biomass: Kinetics and evolved gas analysis. Bioresour. Technol. 2016, 221, 251–261. [Google Scholar] [CrossRef]
- Vyazovkin, S.; Sbirrazzuoli, N. Isoconversional Kinetic Analysis of Thermally Stimulated Processes in Polymers. Macromol. Rapid Commun. 2006, 27, 1515–1532. [Google Scholar] [CrossRef]
- Naktiyok, J.; Bayrakçeken, H.; Özer, A.K.; Gülaboğlu, M.Ş. Investigation of combustion kinetics of Umutbaca-lignite by thermal analysis technique. J. Therm. Anal. Calorim. 2017, 129, 531–539. [Google Scholar] [CrossRef]
- Martín-Lara, M.A.; Blázquez, G.; Zamora, M.C.; Calero, M. Kinetic modelling of torrefaction of olive tree pruning. Appl. Therm. Eng. 2017, 113, 1410–1418. [Google Scholar] [CrossRef]
- Damartzis, T.; Vamvuka, D.; Sfakiotakis, S.; Zabaniotou, A. Thermal degradation studies and kinetic modeling of cardoon (Cynara cardunculus) pyrolysis using thermogravimetric analysis (TGA). Bioresour. Technol. 2011, 102, 6230–6238. [Google Scholar] [CrossRef]




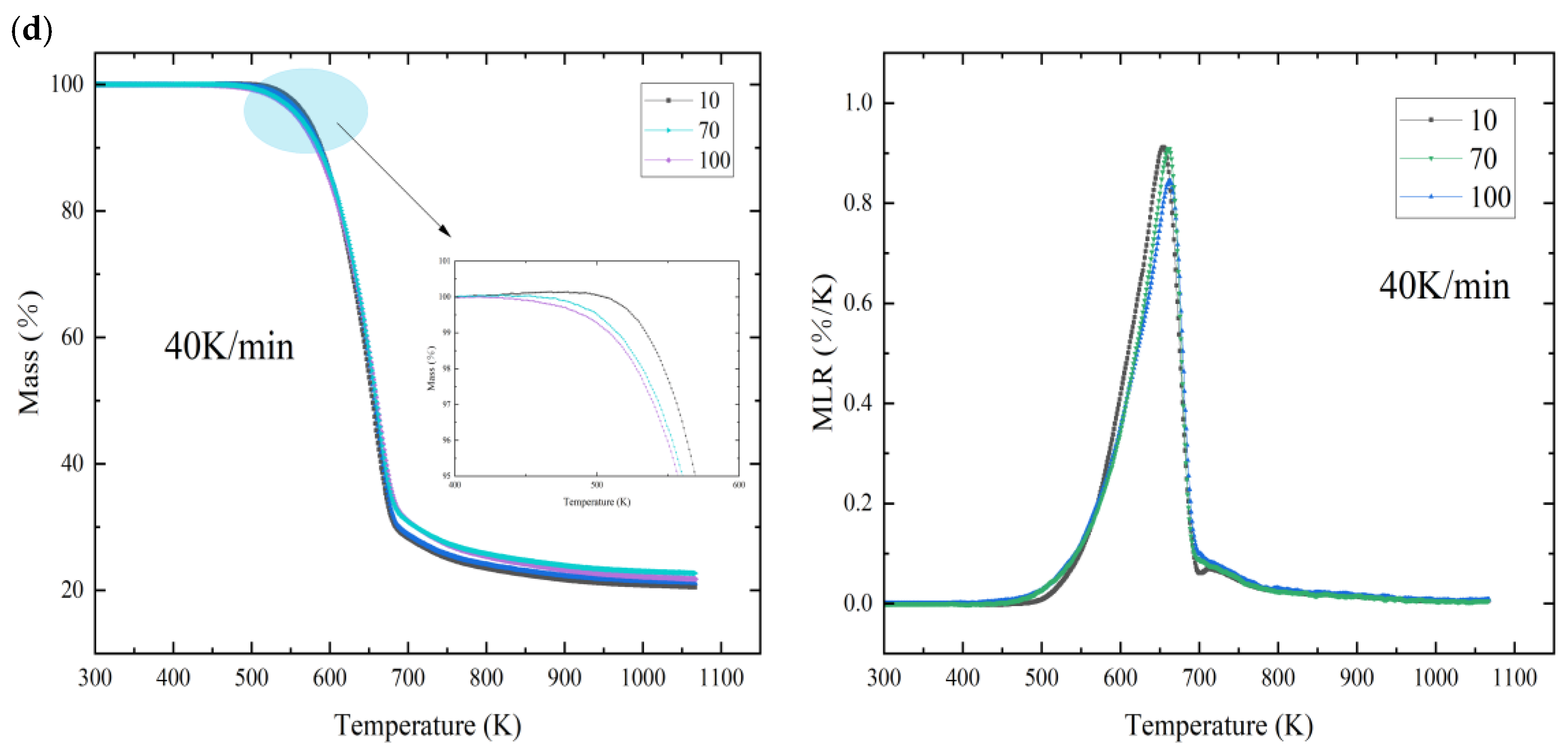
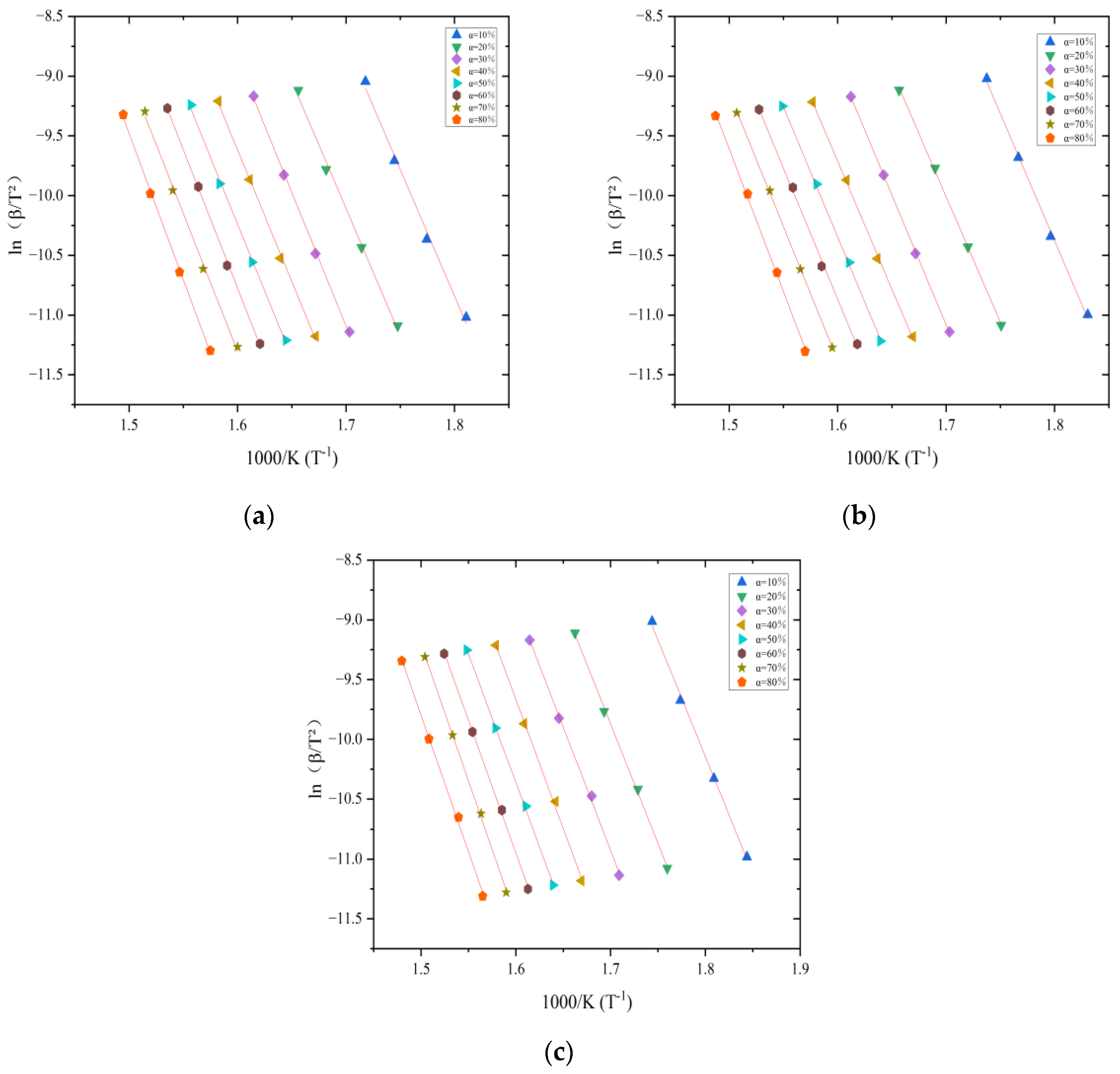
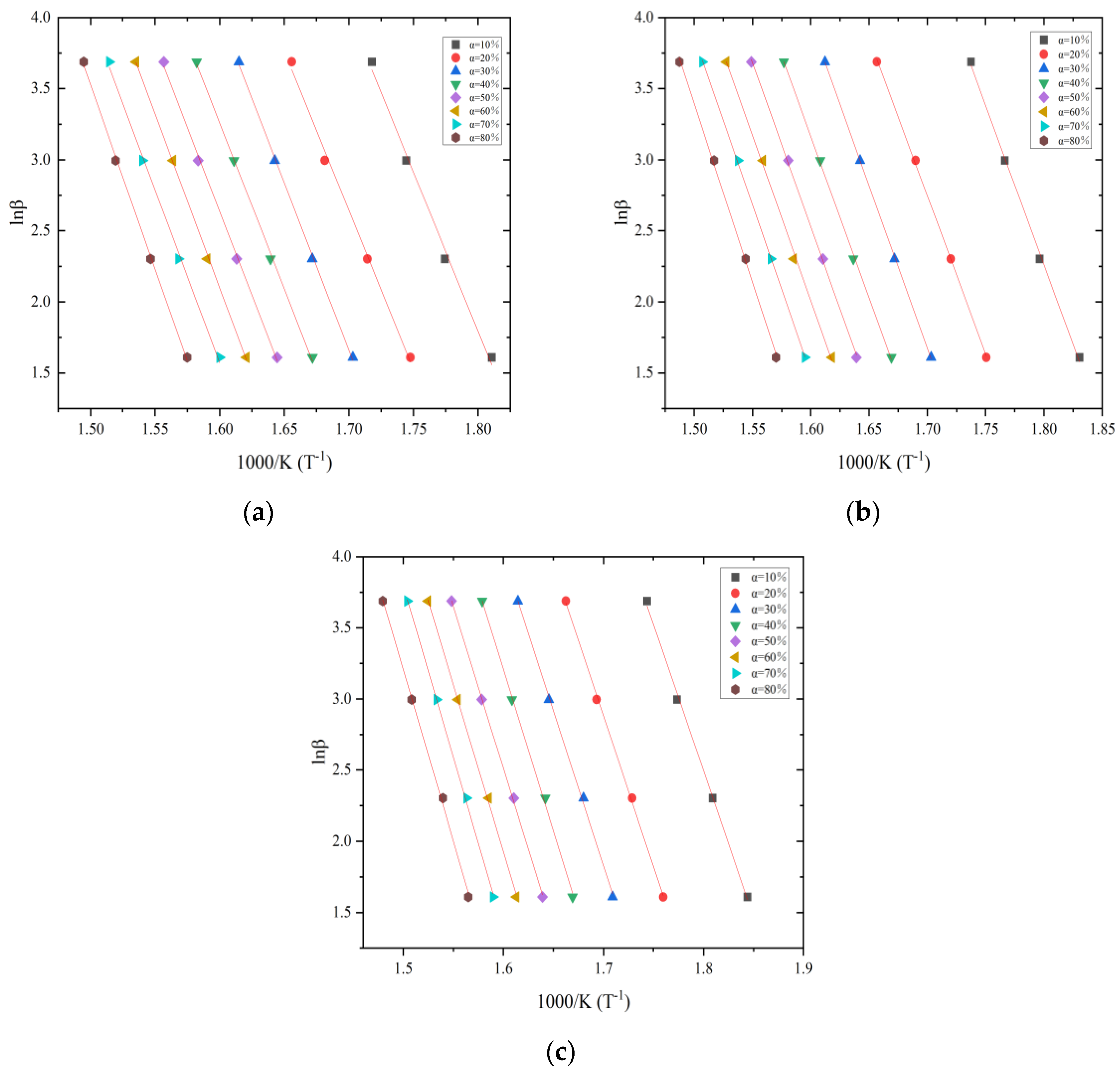

| Heating Rate | Natural Aging—10 | Natural Aging—70 | Natural Aging—100 | ||||||
|---|---|---|---|---|---|---|---|---|---|
| T5% | Tpeak | MLRpeak | T5% | Tpeak | MLRpeak | T5% | Tpeak | MLRpeak | |
| (K) | (%) | (K) | (%) | (K) | (%) | ||||
| 5 | 540 | 620 | 0.896 | 532 | 623 | 0.838 | 527 | 623 | 0.825 |
| 10 | 551 | 632 | 0.856 | 541 | 635 | 0.812 | 538 | 634 | 0.802 |
| 20 | 562 | 647 | 0.854 | 550 | 648 | 0.777 | 549 | 645 | 0.765 |
| 40 | 568 | 650 | 0.884 | 561 | 656 | 0.804 | 557 | 654 | 0.798 |
| α | Natural Aging—10 | Natural Aging—70 | Natural Aging—100 | |||
|---|---|---|---|---|---|---|
| (kJ/mol) | (kJ/mol) | (kJ/mol) | ||||
| FWO | KAS | FWO | KAS | FWO | KAS | |
| 0.1 | 176 | 176 | 176 | 177 | 162 | 162 |
| 0.2 | 176 | 177 | 174 | 175 | 165 | 166 |
| 0.3 | 185 | 186 | 180 | 181 | 171 | 172 |
| 0.4 | 183 | 183 | 178 | 179 | 179 | 180 |
| 0.5 | 186 | 183 | 180 | 181 | 178 | 179 |
| 0.6 | 193 | 193 | 182 | 183 | 183 | 184 |
| 0.7 | 191 | 192 | 186 | 187 | 189 | 190 |
| 0.8 | 204 | 204 | 198 | 198 | 189 | 190 |
| Avg | 187 | 187 | 182 | 183 | 177 | 178 |
| Model | ||
|---|---|---|
| D1 1D diffusion | ||
| D2 2D diffusion-Valensi | ||
| D3 3D diffusion-Jander | ||
| D4 3D diffusion-Ginstling | ||
| F1 First order | ||
| F2 Second order | ||
| F3 Third order | ||
| R2 Contracting area | ||
| R3 Contracting volume | ||
| P2/3 Power law | ||
| P2 Power law | ||
| P3 Power law | ||
| P4 Power law | ||
| A2 Avrami–Erofeev | ||
| A3 Avrami–Erofeev | ||
| A4 Avrami–Erofeev |
| 5 K/min | 10 K/min | 20 K/min | 40 K/min | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mechanism Function | (kJ/mol) | ln (A) (s−1) | R2 | (kJ/mol) | ln (A) (s−1) | R2 | (kJ/mol) | ln (A) (s−1) | R2 | (kJ/mol) | ln (A) (s−1) | R2 |
| D1 | 137.07 | 20.06 | 0.98 | 140.82 | 20.25 | 0.98 | 143.54 | 20.28 | 0.98 | 143.93 | 19.86 | 0.98 |
| D2 | 149.78 | 22.20 | 0.99 | 153.87 | 22.42 | 0.99 | 156.82 | 22.44 | 0.99 | 157.18 | 21.98 | 0.99 |
| D3 | 164.95 | 24.07 | 0.99 | 169.46 | 24.31 | 0.99 | 172.67 | 24.33 | 0.99 | 172.99 | 23.80 | 0.99 |
| D4 | 154.80 | 21.82 | 0.99 | 159.03 | 22.04 | 0.99 | 162.06 | 22.06 | 0.99 | 162.41 | 21.57 | 0.99 |
| F0 | 63.61 | 5.49 | 0.98 | 65.39 | 5.58 | 0.98 | 66.67 | 5.59 | 0.98 | 66.78 | 5.36 | 0.98 |
| F1 | 85.54 | 10.52 | 0.99 | 87.92 | 10.65 | 0.99 | 89.58 | 10.65 | 0.99 | 89.64 | 10.33 | 0.99 |
| F2 | 113.70 | 16.85 | 0.98 | 116.83 | 17.02 | 0.98 | 118.97 | 17.01 | 0.98 | 118.94 | 16.58 | 0.98 |
| F3 | 145.25 | 24.33 | 0.96 | 151.54 | 24.56 | 0.96 | 154.28 | 24.55 | 0.96 | 154.10 | 23.98 | 0.96 |
| R1 | 73.82 | 7.15 | 0.99 | 75.87 | 7.26 | 0.99 | 77.32 | 7.26 | 0.99 | 77.42 | 7.00 | 0.99 |
| R2 | 77.55 | 7.60 | 0.99 | 79.71 | 7.72 | 0.99 | 81.22 | 7.72 | 0.99 | 81.31 | 7.44 | 0.99 |
| P2/3 | 46.27 | 0.57 | 0.98 | 40.25 | 0.46 | 0.98 | 41.03 | 0.46 | 0.98 | 41.06 | 0.30 | 0.98 |
| P2 | 65.38 | −1.38 | 0.98 | 27.61 | −2.21 | 0.98 | 28.15 | −2.18 | 0.98 | 28.26 | −2.24 | 0.98 |
| P3 | 84.49 | −3.43 | 0.97 | 15.03 | −5.13 | 0.97 | 15.33 | −5.11 | 0.97 | 15.41 | −5.14 | 0.97 |
| P4 | 8.51 | −6.92 | 0.95 | 8.75 | −6.83 | 0.95 | 8.92 | −6.81 | 0.95 | 8.97 | −6.82 | 0.95 |
| A2 | 37.85 | 0.44 | 0.99 | 38.87 | 0.52 | 0.99 | 39.61 | 0.54 | 0.99 | 39.69 | 0.43 | 0.99 |
| A3 | 21.95 | −3.20 | 0.99 | 22.55 | −3.14 | 0.99 | 22.97 | −3.12 | 0.99 | 23.03 | −3.18 | 0.99 |
| A4 | 14.00 | −5.19 | 0.99 | 14.45 | −5.16 | 0.99 | 14.73 | −5.17 | 0.99 | 14.63 | −5.28 | 0.99 |
| 5 K/min | 10 K/min | 20 K/min | 40 K/min | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mechanism Function | (kJ/mol) | ln (A) (s−1) | R2 | (kJ/mol) | ln (A) (s−1) | R2 | (kJ/mol) | ln (A) (s−1) | R2 | (kJ/mol) | ln (A) (s−1) | R2 |
| D1 | 124.94 | 17.51 | 0.98 | 127.68 | 17.58 | 0.98 | 129.19 | 17.41 | 0.98 | 128.91 | 16.83 | 0.98 |
| D2 | 136.39 | 19.41 | 0.99 | 139.34 | 19.47 | 0.99 | 140.97 | 19.28 | 0.99 | 140.64 | 18.66 | 0.99 |
| D3 | 150.05 | 20.97 | 0.99 | 153.23 | 21.02 | 0.99 | 155.00 | 20.81 | 0.99 | 154.59 | 20.12 | 0.99 |
| D4 | 140.91 | 18.92 | 0.99 | 143.93 | 18.98 | 0.99 | 145.61 | 18.79 | 0.99 | 145.26 | 18.14 | 0.99 |
| F0 | 57.57 | 4.17 | 0.98 | 58.85 | 4.19 | 0.98 | 59.52 | 4.10 | 0.98 | 59.28 | 3.79 | 0.98 |
| F1 | 77.32 | 8.76 | 0.99 | 78.93 | 8.77 | 0.99 | 79.81 | 8.65 | 0.99 | 79.47 | 8.24 | 0.99 |
| F2 | 102.61 | 14.51 | 0.98 | 104.63 | 14.51 | 0.98 | 105.75 | 14.34 | 0.98 | 105.27 | 13.81 | 0.98 |
| F3 | 132.95 | 21.30 | 0.96 | 135.42 | 21.28 | 0.96 | 136.85 | 21.07 | 0.96 | 136.18 | 20.39 | 0.96 |
| R1 | 66.76 | 5.62 | 0.99 | 68.20 | 5.65 | 0.99 | 68.96 | 5.54 | 0.99 | 68.69 | 5.18 | 0.99 |
| R2 | 70.12 | 6.00 | 0.99 | 71.62 | 6.02 | 0.99 | 72.42 | 5.90 | 0.99 | 72.12 | 5.54 | 0.99 |
| P2/3 | 35.11 | −0.52 | 0.99 | 35.90 | −0.50 | 1.00 | 36.29 | −0.58 | 1.00 | 36.07 | −0.80 | 1.00 |
| P2 | 24.71 | −2.80 | 0.99 | 25.24 | −2.76 | 0.99 | 25.56 | −2.77 | 0.99 | 25.56 | −2.87 | 1.00 |
| P3 | 13.48 | −5.50 | 0.98 | 13.78 | −5.47 | 0.99 | 13.95 | −5.47 | 0.99 | 13.95 | −5.52 | 0.99 |
| P4 | 7.86 | −7.09 | 0.97 | 8.04 | −7.06 | 0.97 | 8.14 | −7.05 | 0.97 | 8.15 | −7.08 | 0.98 |
| A2 | 34.58 | −0.32 | 1.00 | 35.29 | −0.28 | 1.00 | 35.71 | −0.31 | 1.00 | 35.65 | −0.46 | 0.99 |
| A3 | 20.06 | −3.67 | 1.00 | 20.47 | −3.64 | 1.00 | 20.72 | −3.65 | 1.00 | 20.68 | −3.74 | 0.99 |
| A4 | 11.98 | −5.76 | 0.99 | 12.25 | −5.76 | 0.99 | 12.33 | −5.82 | 0.99 | 12.11 | −5.96 | 0.98 |
| 5 K/min | 10 K/min | 20 K/min | 40 K/min | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mechanism Function | (kJ/mol) | ln (A) (s−1) | R2 | (kJ/mol) | ln (A) (s−1) | R2 | (kJ/mol) | ln (A) (s−1) | R2 | (kJ/mol) | ln (A) (s−1) | R2 |
| D1 | 114.93 | 15.44 | 0.98 | 118.60 | 15.77 | 0.98 | 121.31 | 15.81 | 0.98 | 121.71 | 15.41 | 0.98 |
| D2 | 125.53 | 17.17 | 0.99 | 129.55 | 17.53 | 0.99 | 132.49 | 17.56 | 0.99 | 132.92 | 17.13 | 0.99 |
| D3 | 138.16 | 18.52 | 0.99 | 142.60 | 18.92 | 0.99 | 145.81 | 18.96 | 0.99 | 146.26 | 18.48 | 0.99 |
| D4 | 129.71 | 16.61 | 0.99 | 133.87 | 16.98 | 0.99 | 136.90 | 17.02 | 0.99 | 137.33 | 16.58 | 0.99 |
| F1 | 70.84 | 7.38 | 0.99 | 73.19 | 7.60 | 0.99 | 74.84 | 7.61 | 0.99 | 74.99 | 7.33 | 0.99 |
| F2 | 94.22 | 12.75 | 0.98 | 97.35 | 13.04 | 0.98 | 99.50 | 13.06 | 0.98 | 99.68 | 12.70 | 0.98 |
| F3 | 122.25 | 19.09 | 0.96 | 126.31 | 19.47 | 0.96 | 129.07 | 19.49 | 0.96 | 129.30 | 19.03 | 0.96 |
| R1 | 61.07 | 4.41 | 0.99 | 63.10 | 4.59 | 0.99 | 64.54 | 4.61 | 0.99 | 64.67 | 4.36 | 0.99 |
| R2 | 64.19 | 4.73 | 0.99 | 66.32 | 7.97 | 0.99 | 67.82 | 4.94 | 0.99 | 67.96 | 4.69 | 0.99 |
| P2/3 | 38.48 | 0.26 | 0.98 | 32.89 | −1.17 | 0.99 | 33.66 | −1.17 | 0.99 | 33.67 | −1.32 | 1.00 |
| P2 | 27.66 | −2.09 | 0.98 | 23.57 | −3.15 | 0.99 | 24.11 | −3.12 | 0.99 | 24.22 | −3.18 | 0.99 |
| P3 | 15.06 | −5.07 | 0.97 | 12.85 | −5.71 | 0.98 | 13.15 | −5.68 | 0.98 | 13.21 | −5.71 | 0.99 |
| P4 | 8.76 | −6.79 | 0.95 | 7.50 | −7.23 | 0.97 | 7.67 | −7.20 | 0.97 | 7.71 | −7.22 | 0.97 |
| A2 | 38.98 | 0.70 | 0.99 | 33.00 | −0.79 | 1.00 | 33.74 | −0.75 | 1.00 | 33.87 | −0.85 | 1.00 |
| A3 | 22.61 | −3.03 | 1.00 | 19.15 | −3.96 | 1.00 | 19.57 | −3.93 | 1.00 | 19.65 | −3.99 | 1.00 |
| A4 | 12.65 | −5.56 | 1.00 | 10.82 | −6.16 | 0.99 | 11.09 | −6.17 | 0.99 | 10.98 | −6.27 | 0.99 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yan, H.; Zhu, G.; Zhao, Y. Study on Pyrolysis Characteristics of Chinese Fir under Different Natural Aging Times. Fire 2022, 5, 161. https://doi.org/10.3390/fire5050161
Yan H, Zhu G, Zhao Y. Study on Pyrolysis Characteristics of Chinese Fir under Different Natural Aging Times. Fire. 2022; 5(5):161. https://doi.org/10.3390/fire5050161
Chicago/Turabian StyleYan, Huailin, Guoqing Zhu, and Yongchang Zhao. 2022. "Study on Pyrolysis Characteristics of Chinese Fir under Different Natural Aging Times" Fire 5, no. 5: 161. https://doi.org/10.3390/fire5050161




