Abstract
The present study aimed at determining the histamine production capacity of Gram (+) and Gram (−) bacteria isolated from Octopus maya, along with identifying the presence of amino acid decarboxylase genes. Of the total 80 psychrotrophic microorganisms, 32 strains were identified as histamine-forming bacteria. The recombinant DNA technique was used for genotypic identification of histidine (hdc), ornithine (odc), and lysine decarboxylases (ldc) genes. Thirty-two strains were able to produce 60–100 ppm in trypticase soy broth with 1.0% l-histidine after 6 h at 20 °C. NR6B showed 98% homology with Hafnia alvei. NR73 represented 18.8% of the total isolates and showed 98% homology with Enterobacter xianfengensis and Enterobacter cloacae. NR6A represented 6% of the total isolates, which were identified as Lactococcus sp. The hdc gen from NR6B showed 100% identity with hdc from Morganella morganii; ldc showed 97.7% identity with ldc from Citrobacter freundii. The Odc gene was detected only in NR73 and showed 100% identity with Enterobacter sp. All the isolated were identified as weak histamine–former. The ingestion of a food containing small amounts of histamine has little effect on humans; however, the formation of biogenic amines is often considered as an indicator of hygienic quality; this emphasizes the importance of improving good management practices and storage.
1. Introduction
Mexico ranks among the top 10 exporters of octopus to the United States, representing approximately 13% of the market. US octopus imports from Mexico have increased steadily, with almost 18,000 t imported in 2014. The fishery is mainly based on the red octopus Octopus maya, which is endemic to the Yucatan Peninsula, and a unique species among the 150-known species of octopus worldwide [1]. The extraction is done in a small-scale fleet with vessels between 8 and 10 m in length near the coastal areas (2–5-fathom depth) whereby the organisms have a high microbial load on the surface of the skin at the time of capture [2]. These daily trip-boats are not well equipped for cold storage, which renders the octopus a highly perishable product even in subsequent refrigerated storage.
Histamine (C5H9N3) has been identified as the main causative agent of scombrotoxic fish poisoning (often called “histamine poisoning”) which is caused by ingestion of certain species of marine fish that contain high levels of histamine and possibly other biogenic amines (BAs) [3]. The severity of the symptoms can vary, depending on the amount of histamine and other biogenic amines ingested and the individual’s sensitivity to specific BAs. Since fish handling practices are critical with regard to BAs production, histamine was defined as a chemical hazard regulated by the US Food and Drug Administration (FDA) according to the hazard analysis and critical control point (HACCP) guideline [4]. Failure to comply with these regulations and limitations leads to import rejections and disruptions in fish.
In particular, the presence of microbial populations with decarboxylase activity, the storage temperature and handling practices are the main factors affecting the production of BAs in raw seafood [5,6]. The illness associated with spoiled fish and fishery products is triggered by time/temperature abuse of fish muscle due to improper processing or storage [7,8,9,10]. A hazardous level of histamine (>50 mg/100 g) in muscle is often found with a prolonged storage even at the refrigeration temperatures [11,12]. Cadaverine (C5H14N2) and putrescine (C4H12N2) are known to enhance histamine toxicity by inhibiting histamine metabolizing enzymes such as diamine oxidase and methyl transferase, and they are also involved in nitrosamines formation, such as nitrosopiperidine and nitrosopyrrolidine, respectively [13,14,15].
The association of BAs with seafood safety has promoted empirical research for identifying all types of bacteria-producing decarboxylase enzymes, and such BAs are capable of forming histamine, cadaverine, and putrescine. Despite numerous reports showing that many bacterial species are capable of forming BAs, two groups of bacteria, the enteric and marine bacteria, have been identified as histamine formers in fish [16,17,18]. Both psychrophilic and mesophilic-halophilic histamine forming bacteria have been isolated from marine fish in the Gulf of Mexico [19,20]; however, the main concern related to seafood safety is the growth of psychrotrophic and psychrophilic histamine-forming bacteria (HFB), which can grow even at 0 °C. In particular, psychrotrophic microorganisms show a maximum growth temperature above 20 °C and are widespread in natural environments along with foods, while psychrophiles have a maximum temperature for growth at 20 °C or below and are restricted to permanently cold habitats [21].
Although there is a wealth of information about HFB in fishery products [22,23], they have been seldom studied in cephalopod and very little is known about BA production in octopus. Therefore, the objectives of this study were (a) to identify BA-forming bacteria in red octopus during refrigerated storage (4 ± 2 °C) and (b) to quantify the capacity of these microorganisms to produce histamine in the culture broth.
2. Materials and Methods
2.1. Microorganisms
The microorganisms used in the present study were isolated earlier from the red octopus [24]. Of a total of 80 microorganisms isolated from the edible octopus’ tissues, three bacterial genera coded as NR6A, NR6B, and NR73 were found to be histamine producers.
2.2. Isolation of Histamine Producing Bacteria
The isolated strains were cultured on Niven agar containing 0.5% tryptone, 0.5% yeast extract, 3% l-histidine, 0.5% NaCl, 0.1% CaCO3, 2% agar and 0.012% bromocresol purple; pH = 5.3 [25]. After 72 h incubating at 20 °C, the colonies were identified on the basis of the occurrence of a pH change in the agar adjacent to a growing colony (purple halo). Thus, 32 pure colonies were taken from the plate and sub-cultivated at 20 °C overnight in 5 mL of histidine broth containing 0.10% Bacto Peptone, 0.3% yeast extract, 0.5% glucose, 1.0% l-histidine, and 2% NaCl [26] and cryopreserved for further study. A strain of Klebsiella pneumonia isolated from fish and identified previously as a histamine former, was used as a positive control.
2.3. Phenotypic and Genotypic Identification of Histamine-Forming Bacteria
The phenotypic identification was carried out using the commercial bacterial identification systems API 20E® (Enterobacteriaceae and Gram-negative bacilli) (Biomerieux, Marcy-l’Étoile, France), API 20NE® (Non-enteric Gram-negative bacilli) (Biomerieux, Marcy-l’Étoile, France), and complementary biochemical tests. Genomic DNA was obtained by lysis and protein digestion according to the method described by Ausubel et al. [27]. Briefly, 1.5 mL of overnight TSBH culture was centrifuged at 2600× g for 15 min at 4 °C (Eppendorf, 5804R, Westbury, NY, USA). Subsequently, the pellet was re-suspended in 600 μL of Tris-EDTA buffer containing 30 μL of 10% SDS and 3 μL of proteinase K (20 mg/mL). After 1 h of incubation at 37 °C, 100 μL of 5 M NaCl and 80 μL of 10% CTAB in 0.7 M NaCl were added and incubated at 65 °C for 10 min. The sample was washed twice, with chloroform/isoamyl alcohol 24:1 and with phenol/chloroform/isoamyl alcohol 25:24:1 and then centrifuged at 13,000× g each time to remove lipid and protein residues. DNA precipitation was achieved with 0.6 volumes of isopropanol. The DNA was washed with 300 μL of 70% ethanol and finally reconstituted in TE buffer (10 mM Tris-HCl, 1 mM Na-EDTA, pH 8.0).
Polymerase Chain Reaction (PCR) and subsequent sequencing of the 1400 bp region of the 16S ribosomal DNA (rDNA) was used for the genotypic identification. The universal primers 27F (5′AGA GTT TGA TCC TGG CTC AG 3′) and 1492R (5′GGT CT CTT GTT ACG ACT T 3′) were used for this purpose [26]. The PCR mixture reaction (25 μL) consisted of 10 mM Tris–HCL (pH 9.0), 50 mM KCl, 0.1% Triton X-100, 2.1 mM MgCl2, 0.2 μM of each deoxynucleoside triphosphate (dNTP), 0.4-μM primer concentration, 50 ng of bacterial DNA, and 1 U of Taq DNA polymerase (Promega Corp., Madison, WI, USA). The amplification profile was as follows: 1 cycle of 240 s at 94 °C, 40 cycles of 5 s at 94 °C, 45 s at 46 °C, and 90 s at 72 °C; and a final cycle of 10 min at 72 °C. PCR products were electrophoresed at 85 V in a 2% agarose gel. Amplicons were extracted from the gel and then purified and sequenced in an automated DNA sequencing instrument by the Sanger method (Applied Biosystems 3730xl; Thermo Fisher Scientific, Waltham, MA, USA).
The phylogenetic relationship of the strains was established considering on the statistical significance of matches of the representative 16S rDNA sequences retrieved from by the National Center for Biotechnology Information (NCBI) database (Bacteria and Archaea). The sequences were aligned with ClustalW using MEGA 7.0 software [28,29]. A phylogenetic tree was inferred using the Maximum Parsimony (MP) method with gaps deletion and bootstrapping of 500 replicates. MP tree was obtained by the random addition of sequences using the subtree pruning-regrafting algorithm [30].
2.4. Amplification of Histidine (Hdc) Ornithine (Odc) and Lysine Decarboxylase (Ldc) Genes
The amplification of the target genes was carried out using oligonucleotides designed by De las Rivas et al. [31]. The hdc gene was amplified using the primer set 106 (5′AAY TCNTTYGAYTTYGARAARGARG3′) and 107 (5′ATN GGN GAN CCD ATC ATY TTR TGN CC 3′) that amplifies a region of 534 bp. The odc gene was amplified using PUT1-F (5′TWYMAYGCNGAYAARACNTAYYYTGT3′) and PUT1-R (5′ACRCANAGNACNCCNGGNGGRTANGG3′) amplifying a region of 1440 bp. Finally, the ldc gene was amplified with CAD1-F (5′TTYGAYWCNGCNTGGGTNCCNTAYAC3′) and CAD1-R (5′CCRTGDATRTCNGTYTCRAANCCNGG3′) with an amplification region of 1098 bp. The primer set 106/107 failed to amplify the hdc gene from the NR6A strain, which was then amplified by JV16HC (5′ AGA TGG TAT TGT TTC TTA TG 3′) and JV17HC (5′ AGA CCA TAC ACC ATA ACC TT 3′) primer set, specific for Gram-positive bacteria. This primer set generated a PCR product of 367-bp [32].
Amplification was performed in a 50 μL reaction mixture that contained 25 μL of DreamTaq Green PCR Master Mix (2X) (Thermo Scientific, Waltham, MA, USA) 75 pmol of each primer and 2 μg of DNA. The amplification was carried out for 35 cycles (95 °C for 30 s, 58 °C for 30 s and 72 °C for 1 min) in a Thermal Cycler (Major Science, Sea Gull Way Saratoga, CA, USA) with an initial denaturation of 94 °C for 4 min and a final extension at 72 °C for 7 min. The PCR products were separated on a 1.5% agarose gel at 80 V in 1X TAE buffer (400 mM Tris-acetate, 10 mM EDTA, pH = 8.2–8.4). The amplified fragments containing ethidium bromide (0.5 μg/mL) were visualized in a 3UV-Benchtop transilluminator (UVP, Upland, CA, USA). The product size was confirmed by comparison with a 100-bp molecular marker (Invitrogen, Carlsbad, CA, USA). The amplified products were purified using the Wizard SV Gel and PCR Clean-Up System (Promega Corp., Madison, WI, USA). Finally, the purified products were eluted and solubilized in nuclease-free water, quantified by fluorescence (Quantus Flurorometer E6150, Promega Corp., Madison, WI, USA) and stored at −20 °C for subsequent cloning and sequencing.
2.5. Cloning and Sequencing of Decarboxylase Genes
The amplicons from decarboxylase genes were treated with polyethylene glycol, cooled on the ice for 1 h, and pelleted by centrifugation at 15,000× g for 20 min. The pellet was washed with 70% ethanol, dried, and dissolved in Tris-EDTA buffer. The DNA fragments were ligated into a pGEM® vector using the pGEM®-T Easy Vector System I ligation kit (Promega Corp., Madison, WI, USA) and transformed into Escherichia coli JM109 competent cells [33]. The ligation reaction was performed at 26 °C for 1 h and 4 °C for 48 h using 150 μL of competent cells. Heat shock was given by incubating the cells at 0 °C for 20 min, 42 °C for 45 s, and at 0 °C for 2 min. Subsequently, 1 mL of Luria-Bertani (LB) broth was added to each reaction and incubated at 37 °C with shaking. After 1 h, the cells were centrifuged at 5000× g for 15 min and re-suspended in 150 μL of LB broth. The transformed colonies were identified on LB agar plates containing ampicillin (100 μg/mL), 0.5 mM IPTG (isopropyl-β-d-thiogalactoside), and X-Gal (5-bromo-4-chloro-3-indolyl-β-d-galactopyranoside) (80 μg/mL) at 37 °C. After 18 h, the transformed cells with the insert (white colonies) and without the insert (blue colonies) were selected and cultured on LB agar plates with ampicillin (100 μg/mL) to extract the plasmid DNA.
2.6. Identification of Decarboxylase Genes in Transformed Cells
The identification of the cloned decarboxylase gene segments was carried out from the extraction of the plasmid DNA from the transformed cells by the alkaline lysis method [33]. Briefly, 1.5 mL of overnight TSBH culture of transformed cells was centrifuged at 2600× g for 3 min at 4 °C (Eppendorf, 5804R, Westbury, NY, USA). The pellet was then re-suspended in 100 μL of a cold solution containing 50 mM glucose, 25 mM Tris-Cl, 10 mM sodium EDTA at pH 8.0 and 200 μL of a solution consisting of 0.2 N NaOH and 1% SDS. After 3–5 min of cold rest, 150 μL of a cold solution containing 60 mL of 5 M potassium acetate, 11.5 mL of glacial acetic acid, and 28.5 mL of H2O were added. The sample was centrifuged at 2600× g for 10 min, and the pellet was washed with phenol/chloroform 1:1 (v/v). After centrifugation, the final pellet was washed with 100% and 70% ethanol, subsequently, and reconstituted with TE buffer containing 20 μg/mL of pancreatic RNAase.
The presence of the insert was verified using universal primers pUC/M13F (5′GTT TTC CCA GTC ACG AC 3′) and pUC/M13R (5′CAG GAA ACA GCT ATG AC 3′). The PCR amplification was performed in a 50 μL reaction mixture using 25 μL of the Dream Taq™ Green PCR Master Mix kit (2X), 1 μL of 2 pmol of each primer, and 2 μL of 1 μg of plasmid DNA sample. The amplification was carried out in 30 cycles (94 °C for 60 s, 55 °C for 60 s, and 72 °C for 2 min) with an initial denaturation at 94 °C for 5 min and a final extension at 72 °C for 10 min. The cloned DNA segments were sequenced by an automated DNA sequencer based on Sanger’s method (Applied Biosystems 3730xl; Thermo Fisher Scientific, Waltham, MA, USA). The deduced protein sequences were compared with UniProtKB database sequences (Swiss-Prot and TrEMBL). The sequences were aligned with ClustalW using MEGA 7.0 software [28,29]. The evolutionary relationship among the amino acid sequences was inferred by the Neighbor-Joining method [34]. The bootstrap consensus tree inferred from 500 replicates was taken to represent the relationships of the sequences analyzed [35]. The distances were computed using the Poisson’s correction method [36].
2.7. Determining of the Concentration of Histamine Produced by Bacteria
The concentration of histamine produced by the strains was determined by direct enzyme-linked immunosorbent assays (Multiskan EX, Thermo Scientific, Rockford, IL, USA) using the Veratox® commercial kit. One colony was suspended in 50 mL of trypticase soy broth supplemented with 1.0% l-histidine (TSBH) and incubated at 20 °C in a shaking incubator at 150 rpm (Thermo Fisher Scientific, Waltham, MA, USA). At every hour, an aliquot of 3 mL was removed from the culture and used to estimate the total concentration of bacteria in soy trypcasein agar (TSA) and cell transmittance using a UV-visible spectrophotometer at 540 nm (GENESYSTM 10S, Thermo Fisher Scientific, Waltham, MA, USA). The remnant sample was centrifuged at 2600× g 15 min and the supernatant was used to quantify the histamine concentration.
3. Results
3.1. Molecular Identification of Histamine Producing Strains
Among the total 80 bacterial isolates from the muscle tissues, 32 strains were identified as histamine-producing by PCR. Table 1 shows the identity of the HFB determined by comparison of the sequences with NCBI-16S rDNA database. NR6B strain, which represented 15% of the total isolates, showed 98% homology with Hafnia alvei (NR_112985.1). NR73 represented 18.8% of the total isolates and 15 of them had 98% homology with Enterobacter xiangfangensis (NR_126208.1) and Enterobacter cloacae (NR_118568.1). Finally, the strain NR6A which represented 6% of the total isolates was identifying as Lactococcus sp.

Table 1.
Identification of histamine-forming bacteria (HFB) from red octopus (Octopus maya) based on 16S rDNA sequences of pure isolates.
3.2. Phenotypic Characterization of Histamine Producing Strains
Of the 80 strains tested, 38 were identified as histamine forming by the Niven’s agar method but only 31 strains were positive by the molecular PCR-identification. With the exception of a Lactococcus sp. most of the histamine producing strains were Gram-negative. Two strains, viz., NR6B and NR73, from the Enterobacteriacea group, were characterized by catalyzing the decomposition of hydrogen peroxide to oxygen as well as the lack of cytochrome oxidase activity. Both strains were negative for the production of sodium thiosulfate, sodium pyruvate, and indole, and positive for glucose fermentation and nitrate reduction.
On the other hand, an immobile strain NR6A strain was catalase and oxidase negative and homofermentative, degrading glucose via glycolysis to produce lactic acid. Table 2 shows the complete phenotypic characterization of the HFB from red octopus.

Table 2.
Phenotypic identification of histamine-forming bacteria (HFB) isolated from the red octopus (Octopus maya).
3.3. Genotypic Identification of Histidine, Ornithine, and Lysine Decarboxylase Genes
The detection of the decarboxylating genes by PCR confirmed the ability of the isolates to decarboxylate BAs and produce histamine, cadaverine, and putrescine. The 106/107 primer set (hdc gene) generated a typical PCR product of 534-bp for H. alvei NR6B and for Enterobacter NR73 (Figure 1a). This primer set failed to amplify the hdc in Lactococcus NR6A, which was later amplified by the JV16HC/JV17HC primers resulting in a 367-bp PCR product (Figure 1b). The odc gene was only detected in Enterobacter NR73 and generated a 1400-bp product (Figure 1c). The ldc gene was detected in all strains giving an amplicon of 1098 bp (Figure 1d). The results of the sequencing of decarboxylase enzymes are presented in Table 3.
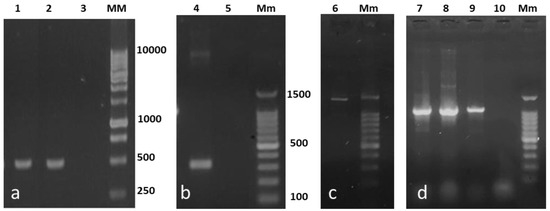
Figure 1.
Identification of the histidine (hdc), ornithine (odc), and lysine (ldc) decarboxylase genes in the strains isolated from red octopus (Octopus maya). The hdc amplification of Hafnia alvei NR6B (1a) and Enterobacter NR73 (2a) was performed using the specific primer set 106/107 (534 bp); the primers JV16HC/JV17HC (367 bp) for Lactococcus NR6A (4b). Odc from Enterobacter NR73 (6c) was performed using the PUT1 primer set (1400 bp). Ldc from H. alvei NR6B (7d), Enterobacter NR73 (8d), and Lactococcus NR6A (9d) was amplified with the CAD1 primer set (1098 bp). Negative control = 3a, 5b, 10d; MM = 1 Kb DNA Ladder; Mm = DNA Ladder 100–1500 bp.

Table 3.
Amino Acids Sequences of Decarboxylase Enzymes Isolated from Red Octopus (Octopus maya) Bacteria.
The hdc gene from H. alvei NR6B and Lactococcus NR6A presented 100% identity with the hdc sequence of Morganella morganii; Enterobacter NR74 showed 99.4% identity. For the ldc gene, Enterobacter NR74 showed 100% identity with Enterobacter cloacae; H. alvei NR6B presented 87% identity with the ldc gene from H. alvei, and Lactococcus NR6A had a 97.7% identity with Citrobacter freundii. The Odc gene was detected only in the Enterobacter NR74 showing 100% identity with the genus Enterobacter. The e-value, match length, and identity percentage for the referred alignment are shown in Table 4. The evolutionary relationship of the taxa based on the amino acid sequences of decarboxylase enzymes and inferred by the Neighbor-Joining method is shown in Figure 2.

Table 4.
Closest relative amino acids sequences of decarboxylase enzymes from strains isolated from red octopus (Octopus maya).

Figure 2.
Evolutionary relationships of taxa of histidine (Hdc), ornithine (Odc), and lysine (Ldc) decarboxylase enzymes isolated from red octopus (Octopus maya) bacteria.
The evolutionary history was inferred using the Neighbor-Joining method. The bootstrap consensus tree inferred from 500 replicates was taken to represent the evolutionary history of the taxa analyzed. The percentage of replicate trees in which the associated taxa clustered together in the bootstrap test (500 replicates) are shown next to the branches.
3.4. Histamine Production
The concentration of histamine produced of the isolated strains in TSBH medium is shown in Figure 3. The histamine concentration was increased as a function of growth time. In the first six hours, the concentration of H. alvei NR6B, Lactococcus NR6A, and Enterobacter NR74 attained up to 9.0–10.5 log CFU/mL, but the production of histamine was variable. The highest histamine concentration was produced by Lactococcus NR6A reaching 100 ppm at 6 h of growth. H. alvei NR6B produced 60 ppm in the same time and Enterobacter NR74 reached less than 40 ppm in 6 h of growth.
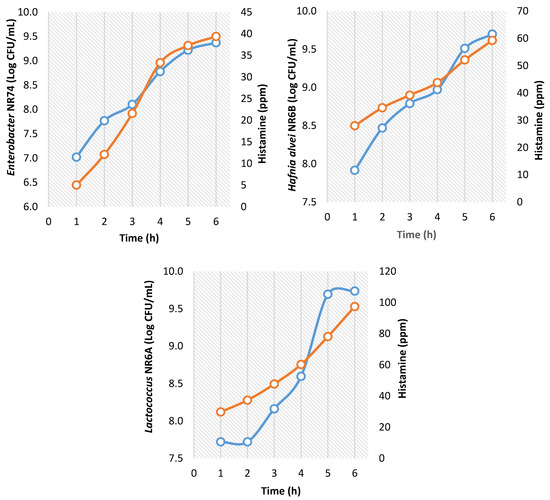
Figure 3.
Bacterial concentration (blue) and TSBH-histamine production (orange) of the strains isolated from red octopus (Octopus maya); (The points correspond to an average of 3 replicates).
4. Discussion
Three bacterial strains isolated from the refrigerated octopus’ muscle were identified as BA-forming and were identified as Hafnia, Enterobacter sp. and Lactococcus sp. Hafnia was identified up to species level as Hafnia alvei (DNA group 1).
There are only a few studies on the bacteria related to the production of BAs in octopus. Lougovois et al. [37] studied the spoilage potential of fresh musky octopus (Eledone moschata) by evaluating the changes in the biochemical properties, microbial growth, and sensory quality of the mantle and tentacles over a period of 18 days, and reported that the members of the Enterobacteriaceae were present only in small numbers. Other authors reported Pseudomonas spp., Alteromonas putrefaciens, Flavobacterium spp., Shewanella spp., Acinetobacter spp., and Photobacerium as prevalent bacterial species found in fish during cold storage; however, these genera have rarely been confirmed as HFB [26,38]. It is true that mesophilic Enterobacteriaceae exist as a minor bacterial flora in seafood stored at 0 °C, but psychrotolerant Enterobacteriaceae are capable of growing at refrigeration temperature and associate with the presence of BAs [26]. In the present study, 15 Enterobacter strains codified as NR73 were isolated from the muscle of red octopus and identified as HFB. Other Enterobacter species, e.g., Enterobacter intermedium, have previously been isolated from Bluefin tuna (Thunnus thynnus) at 8 °C confirming not only their adaptability to low temperatures but also the ability to produce histamine [39]. Identification at the species level in the genus Enterobacter is difficult on the basis of 16S rDNA, as the genus is polyphyletic [40]. The strain Enterobacter NR73 showed 98% identity with Enterobacter xiangfangensis sp. nov, and Enterobacter cloacae indicating a close relationship; however, the concatenated partial analysis based on the sequence of rpoB, atpD, gyrB, and infB genes should be analyzed in future studies to confirm a species [41].
Hafnia alvei was isolated from fresh and temperature-abused albacore (Thunnus alalunga) and found to be HFB by Kim et al. [16]. The genus Hafnia originally contained a single species, H. alvei, which was later described to include another species H. paralvei and some of the strains previously designated as the now obsolete Obesumbacterium proteus. H. alvei is genetically heterogeneous and consists of at least two DNA hybridization groups [42,43]. Recently, hybridization studies have distinguished the original H. alvei into H. alvei sensu stricto (DNA group 1) and H. paralvei, formerly known as H. alvei hybridization group 2 [44]. Based on the results of the biochemical tests carried out by Abbot et al. [45] for the identification of Hafnia species, the NC6B strain isolated in the present study was identified as Hafnia alvei (DNA group 1). H. alvei sensu stricto is typically malonate, salicin, and β-glucosidase positive, and d-arabinose negative, while H. paralvei has an opposite pattern. In our study, salicine, esculine, malonate, and β-glucosidase were typically positive and d-arabinose was negative.
Even knowing that decarboxylase is considered as species-specific or sometimes even strain specific activity, in the present study, Hafnia alvei NR6B and Lactococcus NR6A were identified as potential histamine and cadaverine producers, and Enterobacter NR73 as histamine, cadaverine, and putrescine producer. In fact, several studies have reported that the genera isolated from different foodstuffs show lysine and ornithine decarboxylase activity [46]. The ldc gene was found in the Enterbocateriaceae strains (Hafnia NR6B and Enterobacter NR73) amplifying a specific region of 1098-bp, but this region was also found in Streptococcaceae (Lactococcus NR6A). The primer set, CAD1-F, and CAD1-R, used in this study, were previously designed for some representative species of Enterobacteriaceae such as Shigella flexneri, Shigella sonnei, and Escherichia coli [31]. In the present study, this oligonucleotide set also amplified the ldc region from Gram-positive Lactococcus NR6A, which showed 97.7% identity with ldc from Citrobacter freundii (A0A0J1MZ87). The non-amplication of the hdc gene from Lactococcus NR6A with the primer set 106/107 was expected since the oligonucleotides were previously developed considering the two distinct classes of histidine decarboxylase enzymes; the homometric pyridoxal 5-phosphate (PLP)-dependent enzyme from Gram-negative bacteria [47], and the heterometric enzyme that contains an essential pyruvoyl group from Gram-positive bacteria [32,48].
It is difficult to predict the presence of cadaverine and putrescine in the octopus on the basis of gene expression. De Filippis et al. [49] mentioned that the expression of the decarboxylase genes, and the consequent production of cadaverine and putrescine in some microorganisms of the family Enterobacteriaceae, is influenced by the temperature, where a low temperature is associated with gene downregulation. In fact, the strains isolated from octopus, in particular, H. alvei NC6B and Enterobacter NC73 were found to be weak HFB compared with those reported by previous authors. For example, Raoultella planticola and M. morganii isolated from fish were identified as prolific histamine former with an ability to produce 1000 to 4000 ppm in 24 h at 37 °C in a TSBH medium [16,50,51].
The time and temperature of storage play a significant role in histamine accumulation. Torrido et al. [26] measured the histamine production of mesophilic and psychrotrophic isolates and concluded that, unlike the mesophilic, more than half of the 118 psychrophilic isolates produced no more than 500 ppm histamine. H. alvei, in particular, produced >88.7 ppm in fish infusion broth at 30 °C [52] but was identified as weak histamine former (42.1 ppm) in TSBH at 15 °C [53]. Kim et al. [38] also mentioned that only a few isolates of H. alvei and E. cloacae can produce >1000 ppm histamine in culture broth and the most of them are weak histamine formers. In our study, the histamine concentrations produced by the psychrotrophic Enterobacter NC73 and H. alvei NC6B after 6 h of incubation in TSBH were 40 and 60 ppm, respectively. This production classifies them as weak histamine forming strains.
Very little is known about the Gram-positive bacteria isolated from seafood and their role as HFB. The genus Tetragenococcus, created after reclassification of the halophilic lactic acid bacterium Pediococcus halophilus as T. halophilus is a Gram-positive microorganism isolated from salted and fermented fish products and was identified as an HFB [23,54]. A study reported the histamine production of 10.67 ppm from several strains of the Lactobacillus genus after 10 days at 25 °C [55]. This is relatively low compared to the 100 ppm produced for Lactococcus NC6A after 6 h in TSBH. The presence of the genus Lactoccoccus in a refrigerated octopus is unusual and possibly its presence is related to cross-contamination.
5. Conclusions
Although it is generally believed that histamine fish poisoning is caused by mesophilic histamine-producing bacteria that are active when temperature abuse occurs, this study showed that psychrophilic HFB are also prevalent in refrigerated octopus. To the best of our knowledge, this is the first report to demonstrate the occurrence of HFB in octopus; this emphasizes the importance of improving good management practices and storage. Although some of the enteric bacteria are naturally present in octopus’s tissues, most seem to arise from handling during harvesting and storage [24].
The isolated strains were found to produce a low level of histamine in the culture broth. The ingestion of food containing small amounts of histamine has little effect on humans; however, the formation of BAs is important not only from the standpoint of their toxicity, their presence is often being used as an indicator of hygienic quality and possible fecal contamination. Rapid chilling and refrigeration are mandatory to prevent microbial growth and consequent histamine formation. These are the main suggested control measures for seafood. In this sense, this study could assist in identifying better critical control points to minimize contamination of BAs-forming microorganisms in red octopus.
Author Contributions
M.G.K. conceived and designed the experiment; M.D.D. and M.J.S.S. performed the experiments; M.G.K. analyzed the data and wrote the paper. All authors approved the content of the submitted manuscript.
Funding
This research was funded by the Research Department of the University Marist of Mérida (UMM).
Acknowledgments
The authors are grateful with F.J. Espinosa Faller from UMM for the support given to this research. We like to acknowledge Seelyna Ramos Narváez from the UMM and Betsabe Pérez Hernández from the UMAR, Puerto Ángel, Oaxaca for their lab assistance.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Solís-Ramírez, M.J. Octopus maya: Biology and fishery in Mexico. In The Fishery and Market Potential of Octopus in California; Lang, M., Hochberg, F.G., Ambrose, R.A., Engle, J.M., Eds.; Smithsonian Institution: Washington, DC, USA; University of Southern California: Los Angeles, CA, USA, 1997; pp. 105–113. [Google Scholar]
- Gullian-Klanian, M.; Terrats-Preciat, M.; Pech-Jiménez, E.C.; Cutz De Ocampo, J. Effect of frozen storage on protein denaturation and fatty acids profile of the red octopus (Octopus maya). J. Food Process. Preserv. 2017, 41, e13072. [Google Scholar] [CrossRef]
- Morrow, J.D.; Margolies, G.R.; Rowland, J.; Roberts, L.J. Evidence that histamine is the causative toxin of scombroid-fish poisoning. N. Engl. J. Med. 1991, 324, 716–720. [Google Scholar] [CrossRef] [PubMed]
- Food and Drug Administration. FDA’s Evaluation of the Seafood HACCP Program for Fiscal Years 2000/2001; Food and Drug Administration: Washington, DC, USA, 2002.
- Visciano, P.; Schirone, M.; Tofalo, R.; Suzzi, G. Biogenic amines in raw and processed seafood. Front. Microbiol. 2012, 3, 188. [Google Scholar] [CrossRef] [PubMed]
- Costa, M.P.; Rodrigues, B.L.; Frasao, B.S.; Conte-Junior, C.A. Biogenic Amines as Food Quality Index and Chemical Risk for Human Consumption. In Food Quality: Balancing Health and Disease; Handbook of Food Bioengineering; Holban, A.M., Grumezescu, A.M., Eds.; Academic Press: London, UK, 2018; pp. 75–108. ISBN 9780128114421. [Google Scholar]
- Kim, S.H.; Price, R.J.; Morrissey, M.T.; Field, K.G.; Wei, C.I.; An, H. Occurrence of histamine-forming bacteria in albacore and histamine accumulation in muscle at ambient temperature. J. Food Sci. 2002, 67, 1515–1521. [Google Scholar] [CrossRef]
- Duflos, G. Histamine risk in fishery products. Bull. Acad. Vet. France 2009, 162, 241–246. [Google Scholar] [CrossRef]
- Hungerford, J.M. Scombroid poisoning: A review. Toxicon 2010, 56, 231–243. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Satomi, M.; Furushita, M.; Oikawa, H.; Yano, Y. Diversity of plasmids encoding histidine decarboxylase gene in Tetragenococcus spp. isolated from Japanese fish sauce. Int. J. Food Microbiol. 2011, 148, 60–65. [Google Scholar] [CrossRef] [PubMed]
- Marrakchi, A.E.; Bennour, M.; Bouchriti, N.; Hamama, A.; Tagafait, H. Sensory, chemical, and microbiological assessments of Moroccan sardines (Sardina pilchardus) stored in ice. J. Food Prot. 1990, 53, 600–605. [Google Scholar] [CrossRef]
- Bennour, M.; Marrakchp, A.E.; Bouchritf, N.; Hamama, A.; Ouadaa, M.E. Chemical and microbiological assessments of mackerel (Scomber scombrus) stored in ice. J. Food Prot. 1991, 54, 784–792. [Google Scholar] [CrossRef]
- Bjeldanes, L.F.; Schutz, D.E.; Morris, M.M. On the aetiology of scombroid poisoning: Cadaverine potentiation of histamine toxicity in the guinea-pig. Food Cosmet. Toxicol. 1978, 16, 157–159. [Google Scholar] [CrossRef]
- Lehane, L.; Olley, J. Histamine fish poisoning revisited. Int. J. Food Microbiol. 2000, 58, 1–37. [Google Scholar] [CrossRef]
- Bulushi, I.A.; Poole, S.; Deeth, H.C.; Dykes, G.A. Biogenic amines in fish: Roles in intoxication, spoilage, and nitrosamine formation—A review. Crit. Rev. Food Sci. Nutr. 2009, 49, 369–377. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.H.; Field, K.G.; Morrissey, M.T.; Price, R.J.; Wei, C.I.; An, H. Source and identification of histamine-producing bacteria from fresh and temperature-abused albacore. J. Food Prot. 2001, 64, 1035–1044. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, H.; Ogai, M.; Miya, S.; Kuda, T.; Kimura, B. Effects of environmental factors on histamine production in the psychrophilic histamine-producing bacterium Photobacterium iliopiscarium. Food Control 2015, 52, 39–42. [Google Scholar] [CrossRef]
- Zou, Y.; Hou, X. Histamine production by Enterobacter aerogenes in chub mackerel (Scomber japonicus) at various storage temperatures. Food Sci. Tecnol. 2017, 37, 76–79. [Google Scholar] [CrossRef]
- Bjornsdottir-Butler, K.; Bowers, J.C.; Benner, R.A., Jr. Prevalence and characterization of high histamine–producing bacteria in Gulf of Mexico fish species. J. Food Prot. 2015, 78, 1335–1342. [Google Scholar] [CrossRef] [PubMed]
- Bjornsdottir-Butler, K.; McCarthy, S.A.; Dunlap, P.V.; Benner, R.A. Photobacterium angustum and Photobacterium kishitanii, psychrotrophic high-level histamine-producing bacteria indigenous to tuna. Appl. Environ. Microbiol. 2016, 82, 2167–2176. [Google Scholar] [CrossRef] [PubMed]
- Gounot, A.M. Psychrophilic and psychrotrophic microorganisms. Experientia 1986, 42, 1192–1197. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.H.; Barros-Velázquez, J.; Ben-Gigirey, B.; Eun, J.B.; Jun, S.H.; Wei, C.I.; An, H. Identification of the main bacteria contributing to histamine formation in seafood to ensure product safety. Food Sci. Biotechnol. 2003, 12, 451–460. [Google Scholar]
- Satomi, M. Effect of histamine-producing bacteria on fermented fishery products. Food Sci. Technol. Res. 2016, 22, 1–21. [Google Scholar] [CrossRef]
- Gullian-Klanian, M.; Sánchez-Solís, M.J.; Terrats-Preciat, M.; Delgadillo-Diaz, M.; Aranda, J. Quality indicators and shelf life of red octopus (Octopus maya) in chilling storage. Food Sci. Tecnol. 2016, 36, 304–312. [Google Scholar] [CrossRef]
- Niven, C.F.; Jeffrey, M.B.; Corlett, D.A. Differential plating medium for quantitative detection of histamine-producing bacteria. Appl. Environ. Microbiol. 1981, 41, 321–322. [Google Scholar] [PubMed]
- Torido, Y.; Ohshima, C.; Takahashi, H.; Miya, S.; Iwakawa, A.; Kuda, T.; Kimura, B. Distribution of psychrophilic and mesophilic histamine-producing bacteria in retailed fish in Japan. Food Control 2014, 46, 338–342. [Google Scholar] [CrossRef]
- Ausubel, F.M.; Brent, R.; Kingston, R.E.; Moore, D.D.; Seidman, J.G.; Smith, J.A.; Struhl, K. Short Protocols in Molecular Biology; John Wiley & Sons: New York, NY, USA, 1992; 836p, ISBN 0-471-13781-2. [Google Scholar]
- Chenna, R.; Sugawara, H.; Koike, T.; Lopez, R.; Gibson, T.J.; Higgins, D.G.; Thompson, J.D. Multiple sequence alignment with the Clustal series of programs. Nucleic Acids Res. 2003, 31, 3497–3500. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kumar, S.; Stecher, G.; Tamura, K. MEGA7: Molecular Evolutionary Genetics Analysis version 7.0 for bigger datasets. Mol. Biol. Evol. 2016, 33, 1870–1874. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nei, M.; Kumar, S. Molecular Evolution and Phylogenetics; Oxford University Press: Oxford, UK, 2000; 333p, ISBN 9780195135855. [Google Scholar]
- De las Rivas, B.; Marcobal, A.; Carrascosa, A.V.; Munoz, R. PCR detection of foodborne bacteria producing the biogenic amines histamine, tyramine, putrescine, and cadaverine. J. Food Prot. 2006, 69, 2509–2514. [Google Scholar] [CrossRef] [PubMed]
- Jeune, C.; Lonvaud-Funel, A.; Brink, B.T.; Hofstra, H.; Vossen, J.M.B.M. Development of a detection system for histidine decarboxylating lactic acid bacteria based on DNA probes, PCR and activity test. J. Appl. Microbiol. 1995, 78, 316–326. [Google Scholar] [CrossRef]
- Green, M.R.; Sambrook, J. Molecular Cloning: A Laboratory Manual, 4th ed.; Cold Spring Harbor Laboratory Press: New York, NY, USA, 2012; 1882p. [Google Scholar]
- Saitou, N.; Nei, M. The neighbor-joining method: A new method for reconstructing phylogenetic trees. Mol. Biol. Evol. 1987, 4, 406–425. [Google Scholar] [CrossRef] [PubMed]
- Felsenstein, J. Confidence limits on phylogenies: An approach using the bootstrap. Evolution 1985, 39, 783–791. [Google Scholar] [CrossRef] [PubMed]
- Zuckerkandl, E.; Pauling, L. Evolutionary divergence and convergence in proteins. In Evolving Genes and Proteins; Bryson, V., Vogel, H.J., Eds.; Academic Press: New York, NY, USA, 1965; pp. 97–166. [Google Scholar]
- Lougovois, V.P.; Kolovou, M.K.; Savvaidis, I.N.; Kontominas, M.G. Spoilage potential of ice-stored whole musky octopus (Eledone moschata). Int. J. Food Sci. Technol. 2008, 43, 1286–1294. [Google Scholar] [CrossRef]
- Kim, S.H.; An, H.; Field, K.G.; Wei, C.I.; Velazquez, J.B.; Ben-Gigirey, B.; Morrissey, M.T.; Price, R.J.; Pitta, T.P. Detection of Morganella morganii, a prolific histamine former, by the polymerase chain reaction assay with 16S rDNA–targeted primers. J. Food Prot. 2003, 66, 1385–1392. [Google Scholar] [CrossRef] [PubMed]
- López-Sabater, E.I.; Rodriguez-Jerez, J.J.; Roig-Sagues, A.X.; Mora-Ventura, M.A. Bacteriological quality of tuna fish (Thunnus thynnus) destined for canning: Effect of tuna handling on presence of histidine decarboxylase bacteria and histamine level. J. Food Prot. 1994, 57, 318–323. [Google Scholar] [CrossRef]
- Stephan, R.; Van Trappen, S.; Cleenwerck, I.; Vancanneyt, M.; De Vos, P.; Lehner, A. Enterobacter turicensis sp. nov. and Enterobacter helveticus sp. nov., isolated from fruit powder. Int. J. Syst. Evol. Microbiol. 2007, 57, 820–826. [Google Scholar] [CrossRef] [PubMed]
- Brady, C.; Cleenwerck, I.; Venter, S.; Coutinho, T.; De Vos, P. Taxonomic evaluation of the genus Enterobacter based on multilocus sequence analysis (MLSA): Proposal to reclassify E. nimipressuralis and E. amnigenus into Lelliottia gen. nov. as Lelliottia nimipressuralis comb. nov. and Lelliottia amnigena comb. nov., respectively, E. gergoviae and E. pyrinus into Pluralibacter gen. nov. as Pluralibacter gergoviae comb. nov. and Pluralibacter pyrinus comb. nov., respectively, E. cowanii, E. radicincitans, E. oryzae and E. arachidis into Kosakonia gen. nov. as Kosakonia cowanii comb. nov., Kosakonia radicincitans comb. nov., Kosakonia oryzae comb. nov. and Kosakonia arachidis comb. nov., respectively, and E. turicensis, E. helveticus and E. pulveris into Cronobacter as Cronobacter zurichensis nom. nov., Cronobacter helveticus comb. nov. and Cronobacter pulveris comb. nov., respectively, and emended description of the genera Enterobacter and Cronobacter. Syst. Appl. Microl. 2013, 36, 309–319. [Google Scholar] [CrossRef]
- Brenner, D.J. Characterization and clinical identification of Enterobacteriaceae by DNA hybridization. Prog. Clin. Pathol. 1978, 7, 71–117. [Google Scholar] [PubMed]
- Steigerwalt, A.G.; Fanning, G.R.; Fife-Asbury, M.A.; Brenner, D.J. DNA relatedness among species of Enterobacter and Serratia. Can. J. Microbiol. 1976, 22, 121–137. [Google Scholar] [CrossRef] [PubMed]
- Huys, G.; Cnockaert, M.; Abbott, S.L.; Janda, J.M.; Vandamme, P. Hafnia paralvei sp. nov., formerly known as Hafnia alvei hybridization group 2. Int. J. Syst. Evol. Microbiol. 2010, 60, 1725–1728. [Google Scholar] [CrossRef] [PubMed]
- Abbott, S.L.; Moler, S.; Green, N.; Tran, R.; Wainwright, K.; Janda, J.M. Clinical and laboratory diagnostic characteristics and cytotoxigenic potential of Hafnia alvei and Hafnia paralvei strains. J. Clin. Microbiol. 2011, JCM-00866. [Google Scholar] [CrossRef] [PubMed]
- Kuley, E.; Balikci, E.; Özogul, İ.; Cengiz, D. Interaction between lactic acid bacteria and food-borne pathogens on putrescine production in ornithine-enriched broth. Int. J. Food Sci. Technol. 2013, 48, 394–404. [Google Scholar] [CrossRef]
- Kamath, A.V.; Vaaler, G.L.; Snell, E.E. Pyridoxal phosphate-dependent histidine decarboxylases. Cloning, sequencing, and expression of genes from Klebsiella planticola and Enterobacter aerogenes and properties of the overexpressed enzymes. J. Biol. Chem. 1991, 266, 9432–9437. [Google Scholar] [PubMed]
- Van Poelje, P.D.; Snell, E.E. Pyruvoyl-dependent enzymes. Annu. Rev. Biochem. 1990, 59, 29–59. [Google Scholar] [CrossRef] [PubMed]
- De Filippis, F.; Pennacchia, C.; Di Pasqua, R.; Fiore, A.; Fogliano, V.; Villani, F.; Ercolini, D. Decarboxylase gene expression and cadaverine and putrescine production by Serratia proteamaculans in vitro and in beef. Int. J. Food Microbiol. 2013, 165, 332–338. [Google Scholar] [CrossRef] [PubMed]
- Ababouch, L.; Afila, M.E.; Rhafiri, S.; Busta, F.F. Identification of histamine-producing bacteria isolated from sardine (Sardina pilchardus) stored in ice and at ambient temperature (25 °C). Food Microbiol. 1991, 8, 127–136. [Google Scholar] [CrossRef]
- Tsai, W.C.; Chang, L.K. Morganella morganii causing solitary liver abscess complicated by pyopericardium and left pleural effusion in a nondiabetic patient. J. Microbiol. Immunol. Infect. (Wei Mian Yu Gan Ran Za Zhi) 2002, 35, 191–194. [Google Scholar] [PubMed]
- Behling, A.R.; Taylor, S.L. Bacterial histamine production as a function of temperature and time of incubation. J. Food Sci. 1982, 47, 1311–1314. [Google Scholar] [CrossRef]
- Kung, H.F.; Lee, Y.H.; Chang, S.C.; Wei, C.I.; Tsai, Y.H. Histamine contents and histamine-forming bacteria in sufu products in Taiwan. Food Control 2007, 18, 381–386. [Google Scholar] [CrossRef]
- Kuda, T.; Izawa, Y.; Ishii, S.; Takahashi, H.; Torido, Y.; Kimura, B. Suppressive effect of Tetragenococcus halophilus, isolated from fish-nukazuke, on histamine accumulation in salted and fermented fish. Food Chem. 2012, 130, 569–574. [Google Scholar] [CrossRef]
- Long Nguyen, P.T.; Tu Nguyen, H.K. Histamine Production by Lactobacillus rhamnosus. Glob. J. Biol. Agric. Health Sci. 2015, 3, 70–74. [Google Scholar]
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).