Theoretical and Experimental Determinations of the Hydrophilic–Lipophilic Balance (HLB) of Representative Oils and Lecithins
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Nanoemulsions Prepared by Ultra-High-Pressure Homogenization
2.3. Analysis
2.4. Mathematical Basis for Calculation of Theoretical HLB
2.5. Stability Studies
3. Results and Discussion
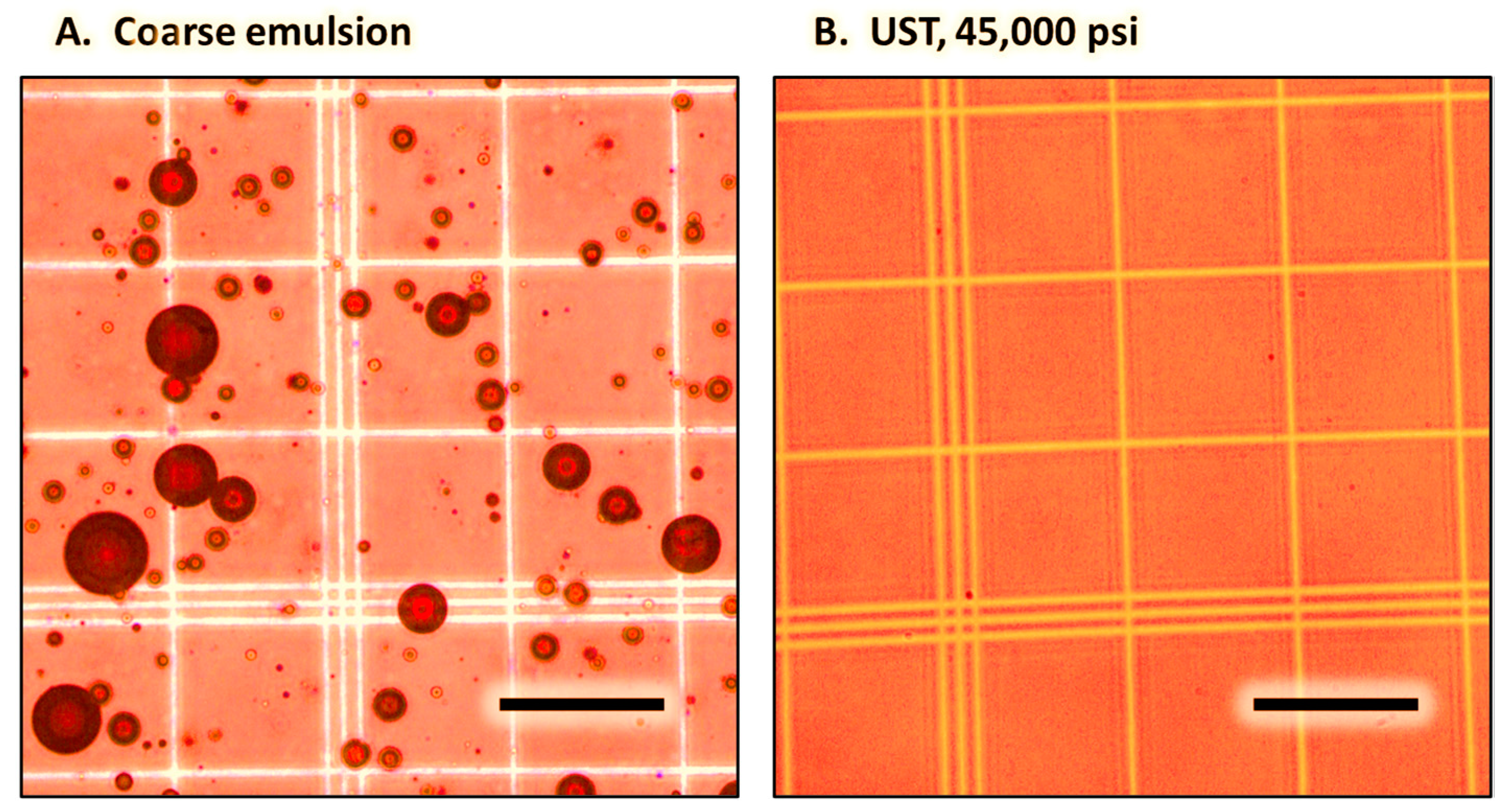
3.1. Coarse Emulsions
3.2. Nanoemulsions Prepared by Ultra-High-Pressure Homogenization
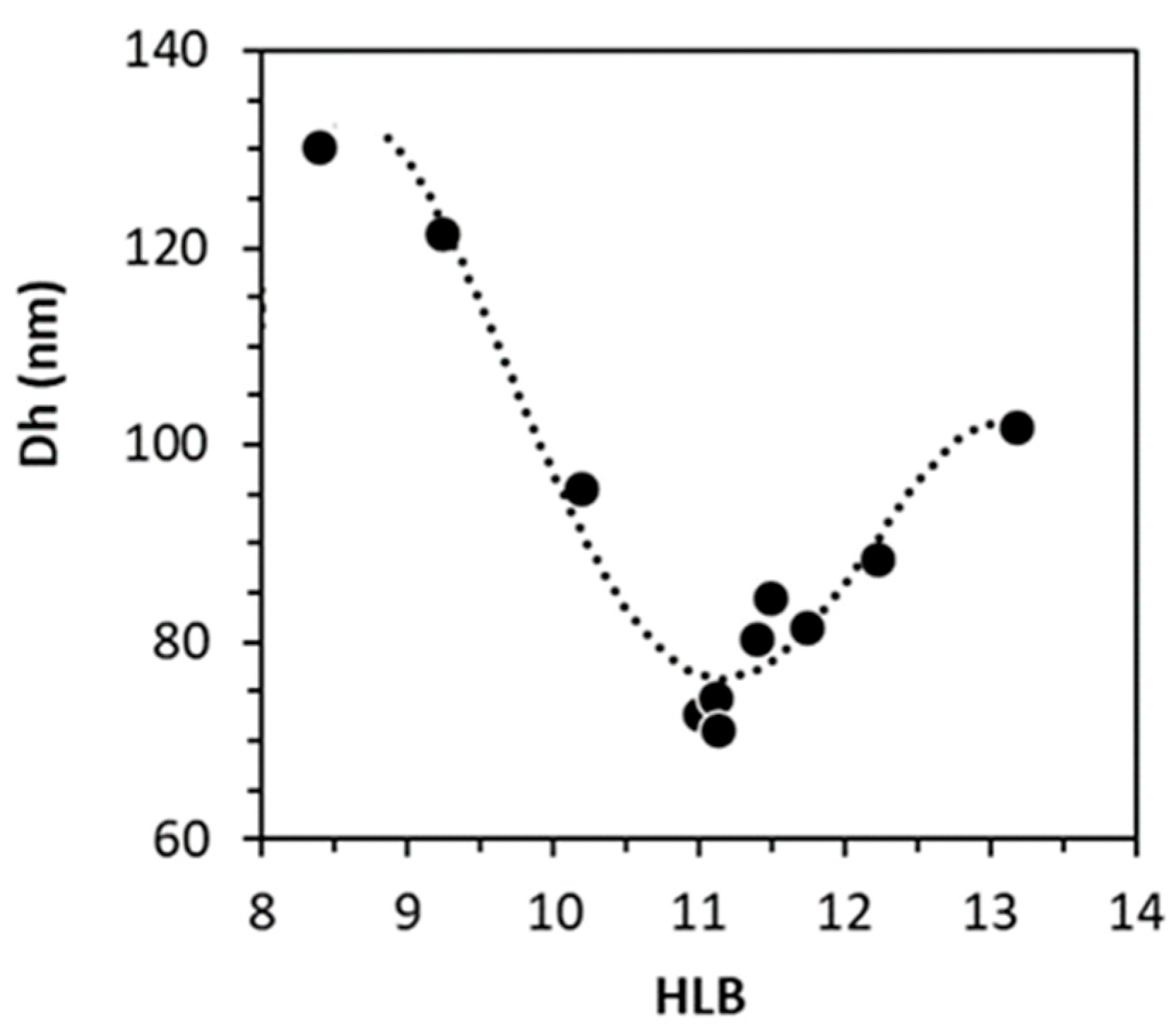
3.3. Experimental and Theoretical Determinations of HLB
3.3.1. Validation of the Method
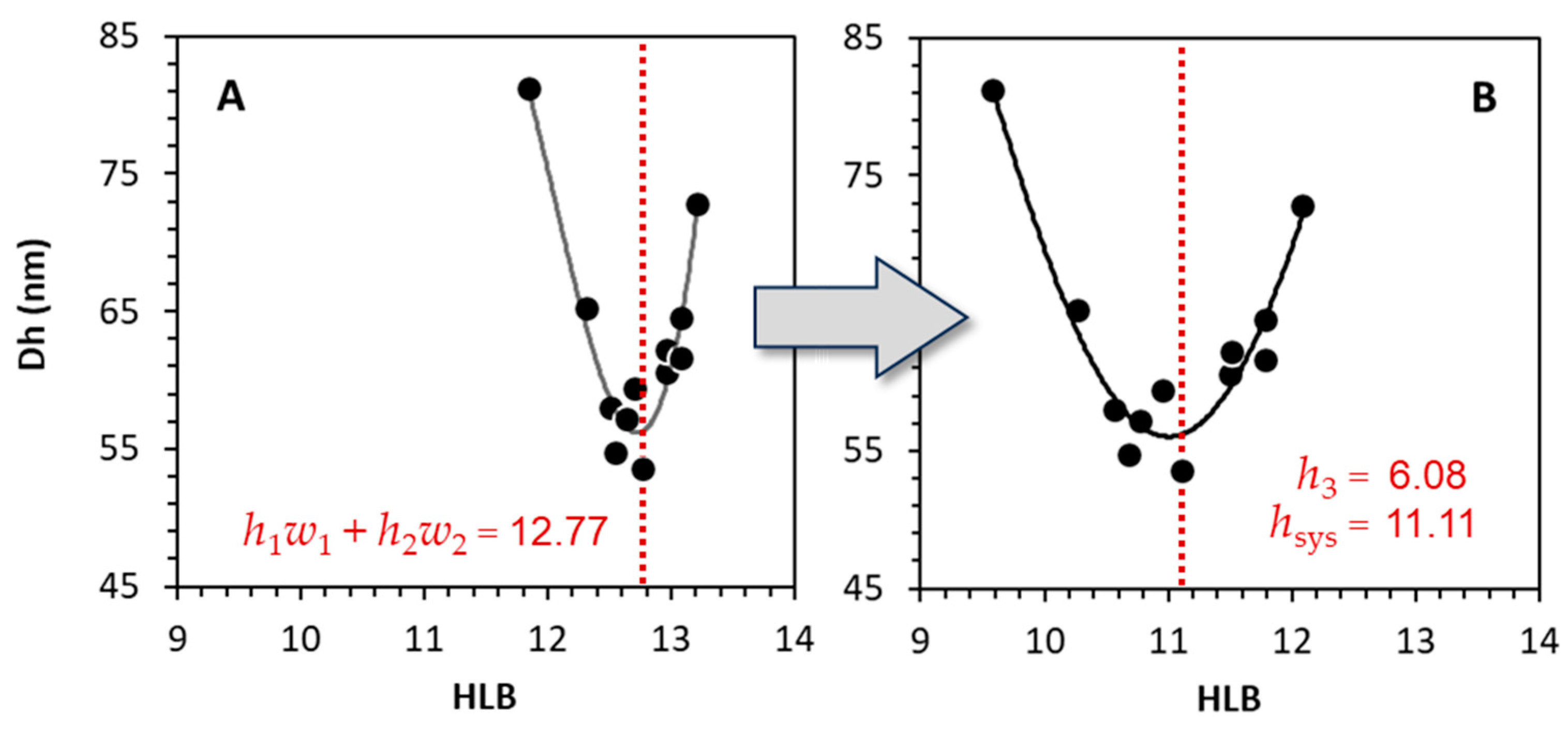
3.3.2. Determination of Alcolec S HLB
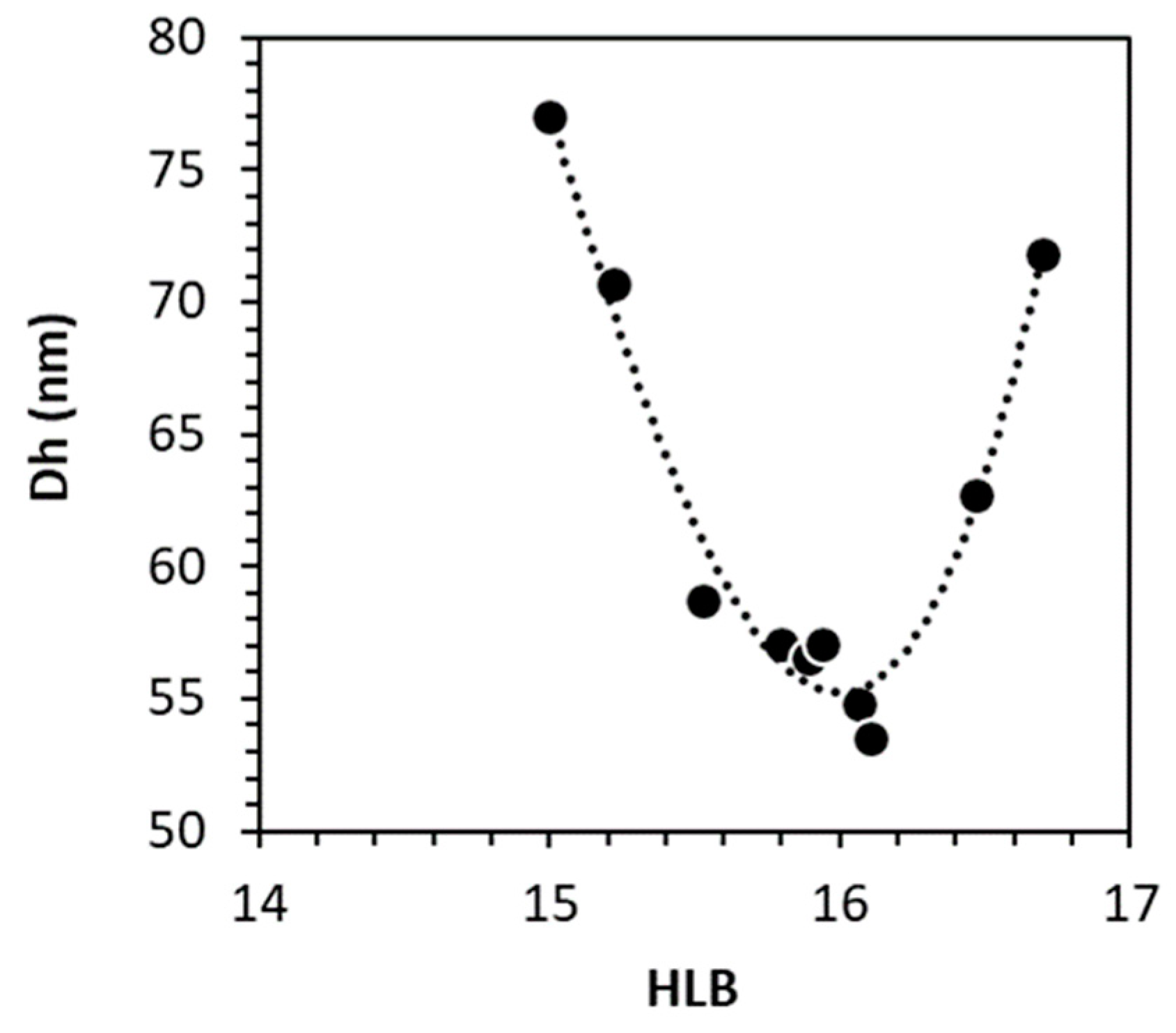
3.3.3. Determination of Black Cumin Seed Oil HLB
3.3.4. Determination of Astaxanthin Oleoresin HLB
3.3.5. Determination of P-LPC-80 HLB
3.3.6. Determination of Kolliphor EL HLB
3.3.7. Estimation of HLB by UV/Vis Spectroscopy
3.3.8. Stability of Nanoemulsions
4. Concluding Remarks
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- McClements, D.J. Nanoemulsions versus microemulsions: Terminology, differences, and similarities. Soft Matter 2012, 8, 1719–1729. [Google Scholar] [CrossRef]
- Aswathanarayan, J.B.; Vittal, R.R. Nanoemulsions and Their Potential Applications in Food Industry. Front. Sustain. Food Syst. 2019, 3, 95. [Google Scholar] [CrossRef]
- Wang, Q.; Zhang, H.; Han, Y.; Cui, Y.; Han, X. Study on the relationships between the oil HLB value and emulsion stabilization. RSC Adv. 2023, 13, 24692–24698. [Google Scholar] [CrossRef] [PubMed]
- Silva, H.D.; Cerqueira, M.A.; Vicente, A.A. Influence of surfactant and processing conditions in the stability of oil-in-water nanoemulsions. J. Food Eng. 2015, 167, 89–98. [Google Scholar] [CrossRef]
- Hadnadev, T.D.; Dokic, P.; Krstonosic, V.; Hadnadev, M. Influence of oil phase concentration on droplet size distribution and stability of oil-in-water emulsions. Tamara Eur. J. Lipid Sci. Technol. 2013, 115, 313–321. [Google Scholar] [CrossRef]
- Nejadmansouri, M.; Hosseini, S.M.H.; Niakosari, M.; Yousefi, G.H.; Golmakani, M.T. Physicochemical properties and oxidative stability of fish oil nanoemulsions as affected by hydrophilic lipophilic balance, surfactant to oil ratio and storage temperature. Colloids Surf. A Physicochem. Eng. Asp. 2016, 506, 821–832. [Google Scholar] [CrossRef]
- Badruddoza, A.Z.M.; Gupta, A.; Myersonm, A.S.; Trout, B.L.; Doyle, P.S. Low Energy Nanoemulsions as Templates for the Formulation of Hydrophobic Drugs. Adv. Therap. 2017, 1, 10. [Google Scholar] [CrossRef]
- Hait, S.K.; Moulik, S.P. Determination of critical micelle concentration (CMC) of nonionic surfactants by donor-acceptor interaction with lodine and correlation of CMC with hydrophile-lipophile balance and other parameters of the surfactants. J. Surfact Deterg. 2001, 4, 303–309. [Google Scholar] [CrossRef]
- Griffin, W.C. Calculation of HLB Values of Non-Ionic Surfactants. J. Soc. Cosmet. Chem. 1954, 5, 249–256. [Google Scholar]
- Pasquali, R.C.; Sacco, N.; Bregni, C. The Studies on Hydrophilic-Lipophilic Balance (HLB): Sixty Years after William C. Griffin’s Pioneer Work (1949–2009). Lat. Am. J. Pharm. 2009, 28, 313–317. [Google Scholar]
- Davies, J.T. A quantitative kinetic theory of emulsion type. Physical chemistry of the emulsifying agent. Proc. Int. Congr. Surf. Act. 1957, 1, 426–438. [Google Scholar]
- Nihon Emulsion Company. The Effective Formulation Development Using the Organic Conceptual Diagram (IOB System). Available online: http://Nihon-emulsion.co.jp/shared/pdf/tech/emulsification_prescriptionE.pdf (accessed on 30 January 2023).
- Salager, J.L.; Antón, R.; Bullón, J.; Forgiarini, A.; Marquez, R. How to Use the Normalized Hydrophilic-Lipophilic Deviation (HLDN) Concept for the Formulation of Equilibrated and Emulsified Surfactant-Oil-Water Systems for Cosmetics and Pharmaceutical Products. Cosmetics 2020, 7, 57. [Google Scholar] [CrossRef]
- Aubry, J.M.; Ontiveros, J.F.; Salager, J.L.; Nardello-Rataj, V. Use of the normalized hydrophilic-lipophilic-deviation (HLDN) equation for determining the equivalent alkane carbon number (EACN) of oils and the preferred alkane carbon number (PACN) of nonionic surfactants by the fish-tail method (FTM). Adv. Colloid Interface Sci. 2020, 276, 102099. [Google Scholar] [CrossRef]
- Salager, J.L.; Marquez, N.; Graciaa, A. Lachaise Partitioning of Ethoxylated Octylphenol Surfactants in Microemulsion−Oil−Water Systems: Influence of Temperature and Relation between Partitioning Coefficient and Physicochemical Formulation. Langmuir 2000, 16, 5534–5539. [Google Scholar] [CrossRef]
- Abbott, S.R. Surfactant Science: Principles and Practice. Available online: https://www.stevenabbott.co.uk/practical-surfactants/hld.php#:~:text=At%20the%20point%20of%20perfect,of%20the%20HLD%20%3D%200%20point (accessed on 30 January 2023).
- Acosta, E.J.; Yuan, J.S.; Bhakta, A.S. The Characteristic Curvature of Ionic Surfactants. J. Surfact Deterg. 2008, 11, 145–158. [Google Scholar] [CrossRef]
- Kampa, J.; Frazier, R.; Rodriguez-Garcia, J. Physical and Chemical Characterisation of Conventional and Nano/Emulsions: Influence of Vegetable Oils from Different Origins. Foods 2022, 11, 681. [Google Scholar] [CrossRef]
- Ga, S.; Jovanovi, B.; Jovanovi, S. The stability of emulsions in the presence of additives. J. Serb. Chem. Soc. 2002, 67, 31–39. [Google Scholar]
- Orafidiya, L.O.; Oladimeji, F.A. Determination of the Required HLB Values of Some Essential Oils. Int. J. Pharm. 2002, 237, 241–249. [Google Scholar] [CrossRef]
- Niczinger, N.A.; Kállai-Szabó, N.; Dredán, J.; Budai, L.; Hajdú, M.; Antal, I. Application of Droplet Size Analysis for the Determination of the Required HLB of Lemon Oil in O/W Emulsion. Curr. Pharm. Anal. 2015, 11, 11–15. [Google Scholar] [CrossRef]
- Van Hoogevest, P. An update on the use of oral phospholipid excipients. Eur. J. Pharm. Sci. 2017, 108, 1–12. [Google Scholar] [CrossRef]
- Otto, F.; Van Hoogevest, P.; Syrowatka, F.; Heinl, V.; Neubert, R.H.H. Assessment of the applicability of HLB values for natural phospholipid emulsifiers for preparation of stable emulsions. Pharmazie 2020, 75, 365–370. [Google Scholar]
- Smejkal, G.B.; Ting, E.Y.; Arul Nambi, K.; Schumacher, R.T.; Lazarev, A.V. Characterization of astaxanthin nanoemulsions produced by intense fluid shear through a self-throttling nanometer range annular orifice valve-based high pressure homogenizer. Molecules 2021, 26, 2856. [Google Scholar] [CrossRef]
- Janahar, J.J.; Marciniak, A.; Balasubramaniam, V.M.; Jimenez-Flores, R.; Ting, E. Effects of pressure, shear, temperature, and their interactions on selected milk quality attributes. J. Dairy Sci. 2021, 104, 1531–1547. [Google Scholar] [CrossRef]
- Rasband, W.S. ImageJ, U.S. National Institutes of Health, Bethesda, Maryland, USA. 1997–2018. Available online: https://imagej.nih.gov/ij/ (accessed on 30 January 2023).
- Yaghmur, A.; Aserin, A.; Mizrahi, Y.; Nerd, A.; Garti, N. Argan oil-in-water emulsions: Preparation and stabilization. J. Am. Oil Chem. Soc. 1999, 76, 15–18. [Google Scholar] [CrossRef]
- American Lecithin Company. Lipids and Phospholipids: A Simple Guide to Use and Selection. Product Brochure. 2020, pp. 1–23. Available online: https://americanlecithin.us/wp-content/uploads/2022/08/ALC-Lecithin-and-Phopholipids-Brochure.pdf (accessed on 30 January 2023).
- Mohd Nor, N.H.; Mohd Shafri, M.A.; Mohamed, F. Preparation and characterization of nigella sativa microemulsions. Int. J. Pharm. Pharm. Sci. 2014, 6, 485–489. [Google Scholar]
- Khader, M.; Eckl, P.M. Thymoquinone: An emerging natural drug with a wide range of medical applications. Iran. J. Basic Med. Sci. 2014, 17, 950–957. [Google Scholar]
- Akram Khan, M.; Afzal, M. Chemical composition of Nigella sativa Linn: Part 2 Recent advances. Inflammopharmacol 2016, 24, 67–79. [Google Scholar] [CrossRef]
- Legen, I.; Peternel, L.; Novak, S.; Homar, M.; Rozman, P.; Klancar, U. Self-Microemulsifying Drug Delivery System of Abiraterone or Abiraterone Acetate. Patent WO2014009434, 10 July 2013. [Google Scholar]
- Smail, S.S.; Ghareeb, M.M.; Omer, H.K.; Al-Kinani, A.A.; Alany, R.G. Studies on Surfactants, Cosurfactants, and Oils for Prospective Use in Formulation of Ketorolac Tromethamine Ophthalmic Nanoemulsions. Pharmaceutics 2021, 13, 467. [Google Scholar] [CrossRef]
- Pharma, B.A.S.F.; Kolliphor, E.L. Technical Bulletin. 2019, pp. 1–5. Available online: https://pharma.basf.com/products/kolliphor-el (accessed on 30 January 2023).
- Einstein, A. Über einen die Erzeugung und Verwandlung des Lichtes betreffenden heuristischen Gesichtspunkt. Ann. Der. Phys. 1905, 322, 132–148. [Google Scholar] [CrossRef]
- Stetefeld, J.; McKenna, S.A.; Patel, T.R. Dynamic light scattering: A practical guide and applications in biomedical sciences. Biophys. Rev. 2016, 8, 409–427. [Google Scholar] [CrossRef]
- Mikulcová, V.; Kašpárková, V.; Humpolíček, P.; Buňková, L. Formulation, Characterization and Properties of Hemp Seed Oil and Its Emulsions. Molecules 2017, 22, 700. [Google Scholar] [CrossRef]
- Meor Mohd Affandi, M.M.R.; Julianto, T.; Majeed, A.B.A. Enhanced Oral Bioavailability of Astaxanthin with Droplet Size Reduction. Food Sci. Technol. Res. 2012, 18, 549–554. [Google Scholar] [CrossRef]
- Onodera, T.; Kuriyama, I.; Andoh, T.; Ichikawa, H.; Sakamoto, Y.; Lee-Hiraiwa, E.; Mizushina, Y. Influence of particle size on the in vitro and in vivo anti-inflammatory and anti-allergic activities of a curcumin lipid nanoemulsion. Int. J. Mol. Med. 2015, 35, 1720–1728. [Google Scholar] [CrossRef]
- Khattab, A.; Hassanin, L.; Zaki, N. Self-Nanoemulsifying Drug Delivery System of Coenzyme (Q10) with Improved Dissolution, Bioavailability, and Protective Efficiency on Liver Fibrosis. AAPS PharmSciTech 2017, 18, 1657–1672. [Google Scholar] [CrossRef]
- Nakano, Y.; Tajima, M.; Sigiyama, E.; Sato, V.H.; Sato, H. Development of a Novel Nanoemulsion Formulation to Improve Intestinal Absorption of Cannabidiol. Med. Cannabis Cannabinoids 2019, 2019, 2. [Google Scholar]
- Elsohly, M.A.; Shahzadi, I.; Waseem Gul, W. Absorption and Bioavailability of Novel UltraShear Nanoemulsion of Cannabidiol in Rats. Med. Cannabis Cannabinoids 2023, 6, 148–159. [Google Scholar] [CrossRef]
- Yen, C.C.; Chen, Y.C.; Wu, M.T.; Wang, C.C.; Wu, Y.T. Nanoemulsion as a strategy for improving the oral bioavailability and anti-inflammatory activity of andrographolide. Int. J. Nanomed. 2018, 13, 669–680. [Google Scholar] [CrossRef]
- Murphy, D.J.; Goggin, K.; Paterson, R.R.M. Oil palm in the 2020s and beyond: Challenges and solutions. CABI Agric. Biosci. 2021, 2, 39. [Google Scholar] [CrossRef]
- Guerin, C.; Joët, T.; Serret, J.; Lashermes, P.; Vaissayre, V.; Agbessi, M.D.; Beule, T.; Severac, D.; Amblard, P.; Tregear, J.; et al. Gene coexpression network analysis of oil biosynthesis in an interspecific backcross of oil palm. Plant J. 2016, 87, 423–441. [Google Scholar] [CrossRef]
- Yi, Y.; Jin, Y.; Menon, R.; Yeung, B. Polysorbate, the Good, the Bad, and the Ugly. American Pharmaceutical Review 2020. Available online: https://www.americanpharmaceuticalreview.com/Featured-Articles/569560-Polysorbate-the-Good-the-Bad-and-the-Ugly/ (accessed on 30 January 2023).
- Lv, G.; Wang, F.; Cai, W.; Zhang, X. Characterization of the addition of lipophilic Span 80 to the hydrophilic Tween 80-stabilized emulsions. Colloids Surf. A Physicochem. Eng. Asp. 2014, 447, 8–13. [Google Scholar] [CrossRef]
- Ben Hmida, R.; Gargouri, B.; Chtourou, F.; Abichou, M.; Sevim, D.; Bouaziz, M. Study on the Effect of Climate Changes on the Composition and Quality Parameters of Virgin Olive Oil “Zalmati” Harvested at Three Consecutive Crop Seasons: Chemometric Discrimination. ACS Omega 2022, 7, 40078–40090. [Google Scholar] [CrossRef] [PubMed]
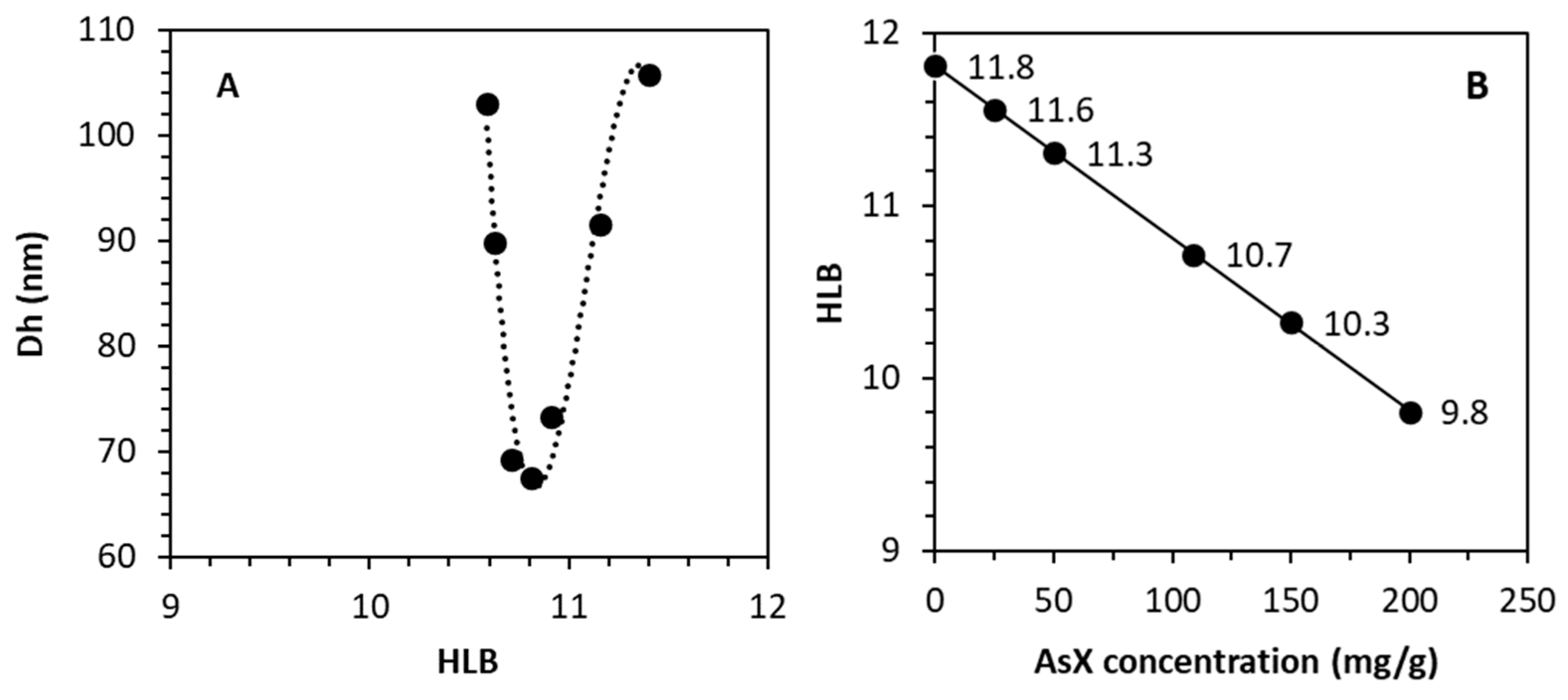
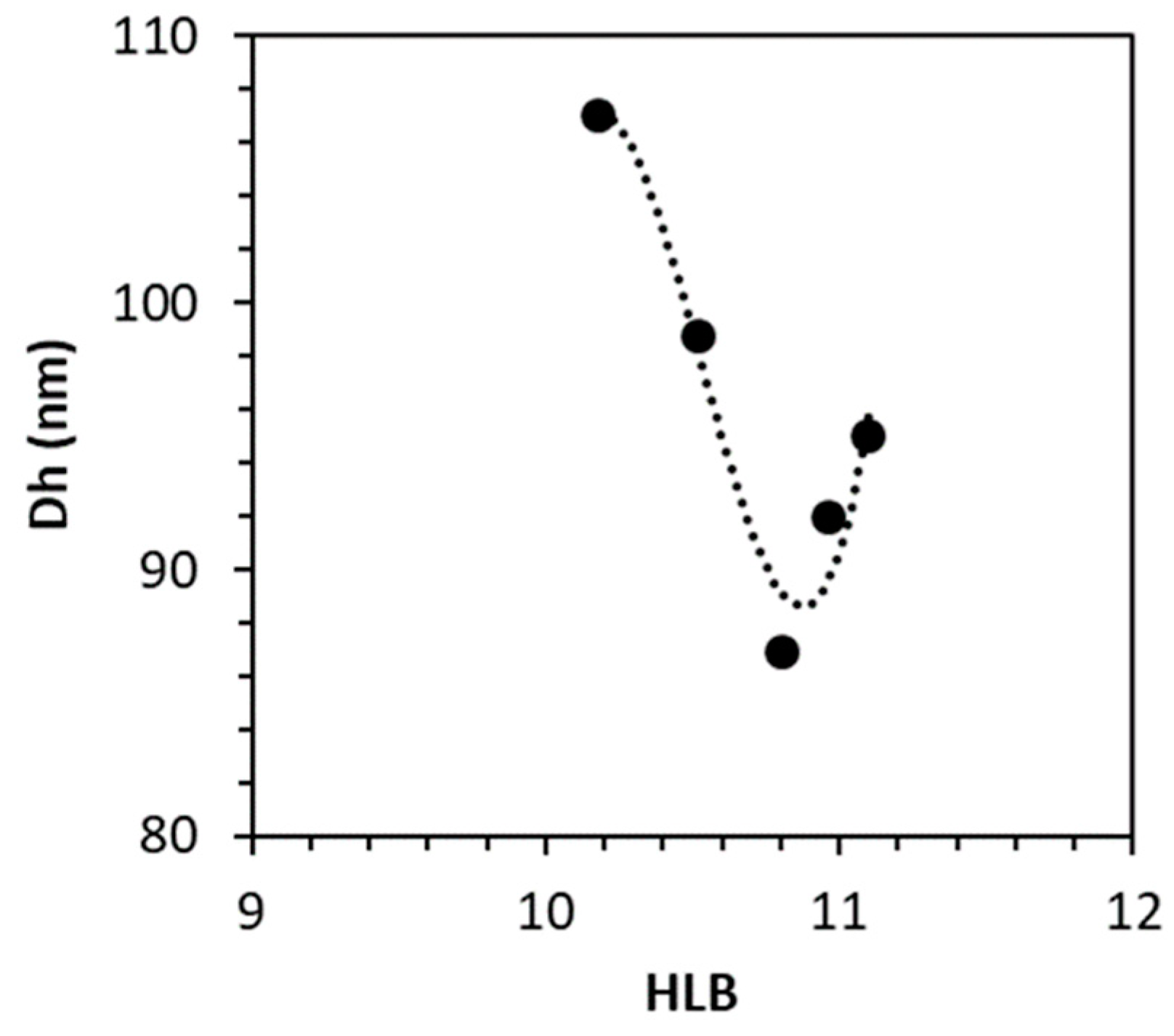

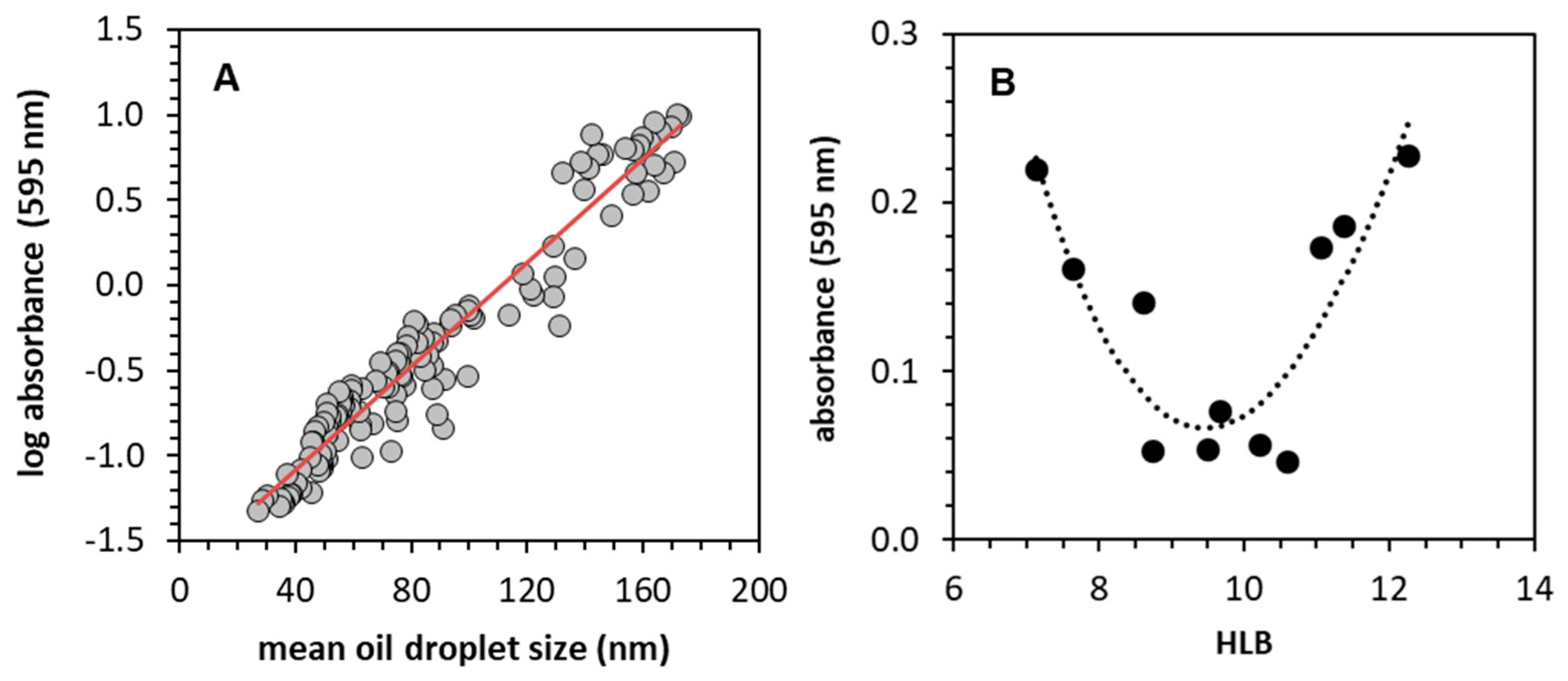
| Observed * | Interpolated ** | Published rHLB | Reference | |
|---|---|---|---|---|
| oils | ||||
| argan oil | 11.12 | 11.19 | 11.0 | [27] |
| black cumin seed oil | 16.1 | 16.0 | 16.0 | [30] |
| astaxanthin oleoresin | 10.81 | 10.84 | - | - |
| hemp seed oil | 9.5 | 9.38 | 9.0 | [37] |
| lecithins | ||||
| Alcolec S | 6.08 | 5.72 | 4.0 | [28] |
| P-LPC-80 | 12.23 | 12.38 | 9–12 | [14] |
| synthetic surfactants | ||||
| Kolliphor EL | 16.12 | 16.15 | 13–14 | [32,33] |
| PS20 | - | - | 16.7 | [46] |
| PS80 | - | - | 15.0 | [46] |
| Span 80 | - | - | 4.3 | [47] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Smejkal, G.; Gross, V.; Lazarev, A. Theoretical and Experimental Determinations of the Hydrophilic–Lipophilic Balance (HLB) of Representative Oils and Lecithins. Colloids Interfaces 2024, 8, 21. https://doi.org/10.3390/colloids8020021
Smejkal G, Gross V, Lazarev A. Theoretical and Experimental Determinations of the Hydrophilic–Lipophilic Balance (HLB) of Representative Oils and Lecithins. Colloids and Interfaces. 2024; 8(2):21. https://doi.org/10.3390/colloids8020021
Chicago/Turabian StyleSmejkal, Gary, Vera Gross, and Alexander Lazarev. 2024. "Theoretical and Experimental Determinations of the Hydrophilic–Lipophilic Balance (HLB) of Representative Oils and Lecithins" Colloids and Interfaces 8, no. 2: 21. https://doi.org/10.3390/colloids8020021








