Impact of Polymer Nanoparticles on DPPC Monolayer Properties
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
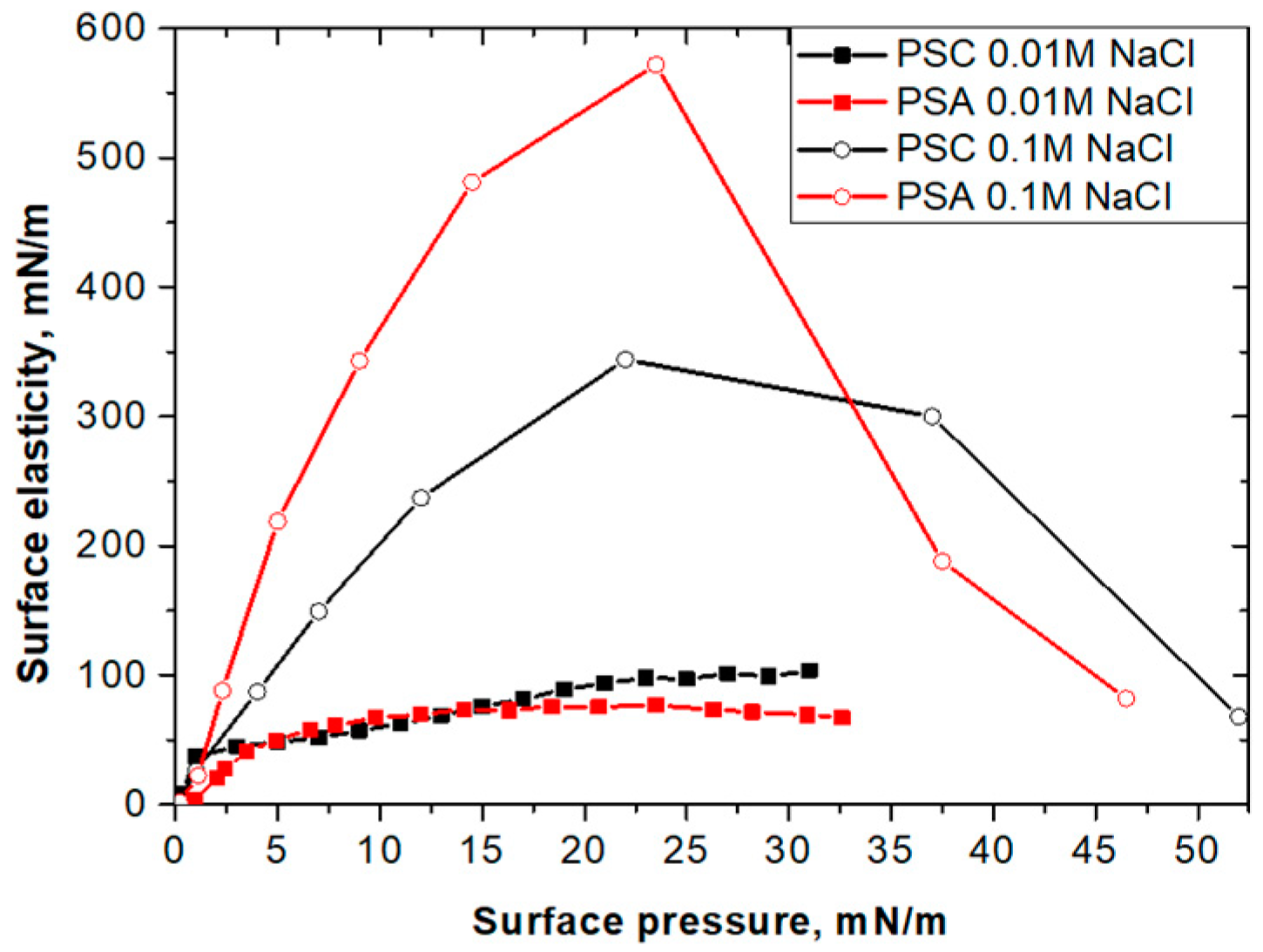
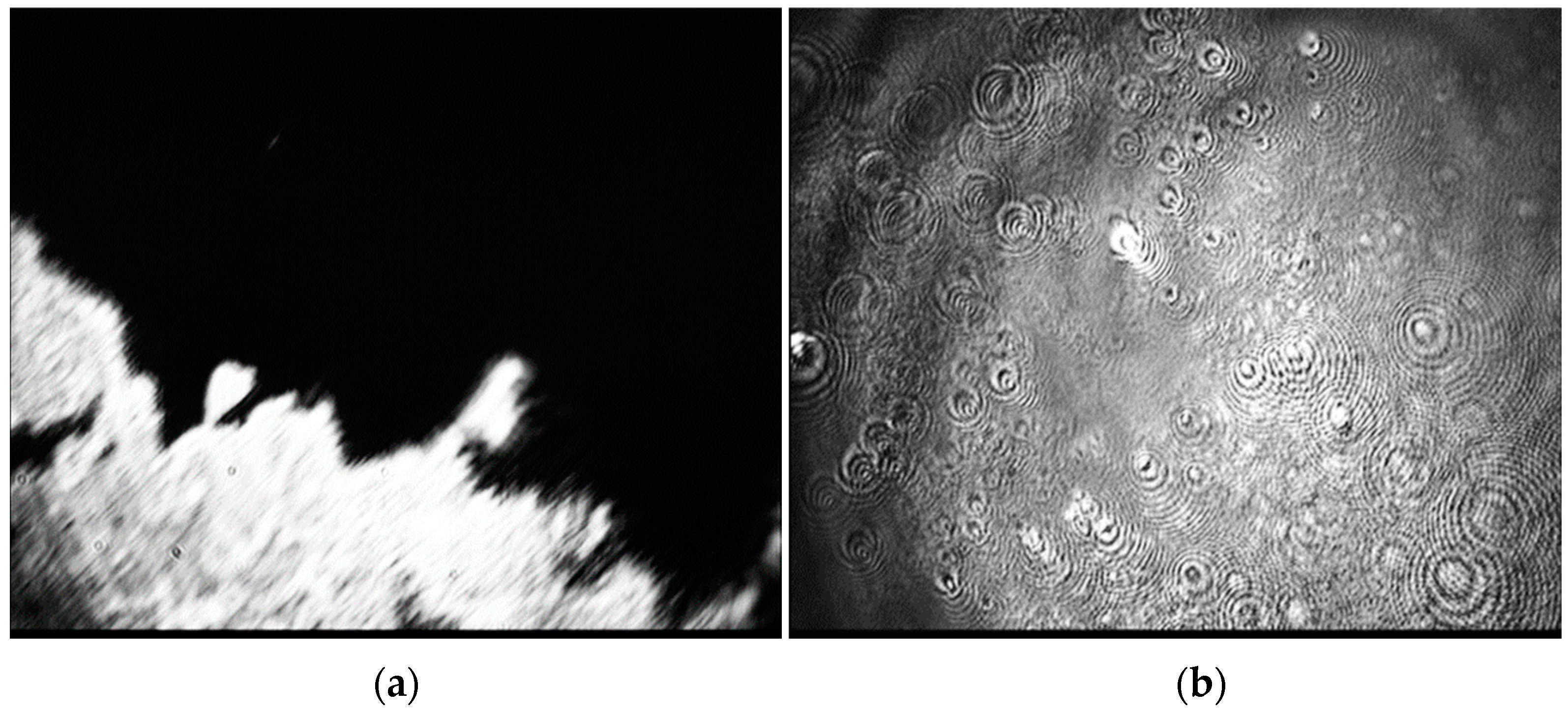
3.1. Nanoparticle Layers
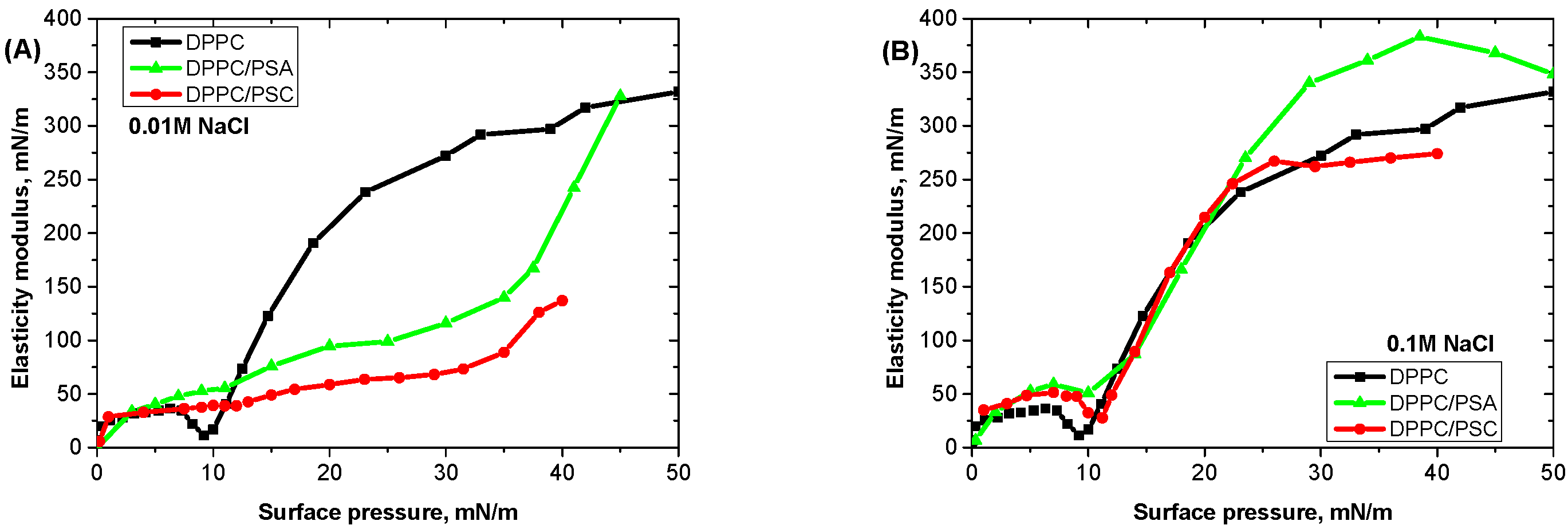
3.2. Mixed Films of Nanoparticles and DPPC at High Surface Tensions
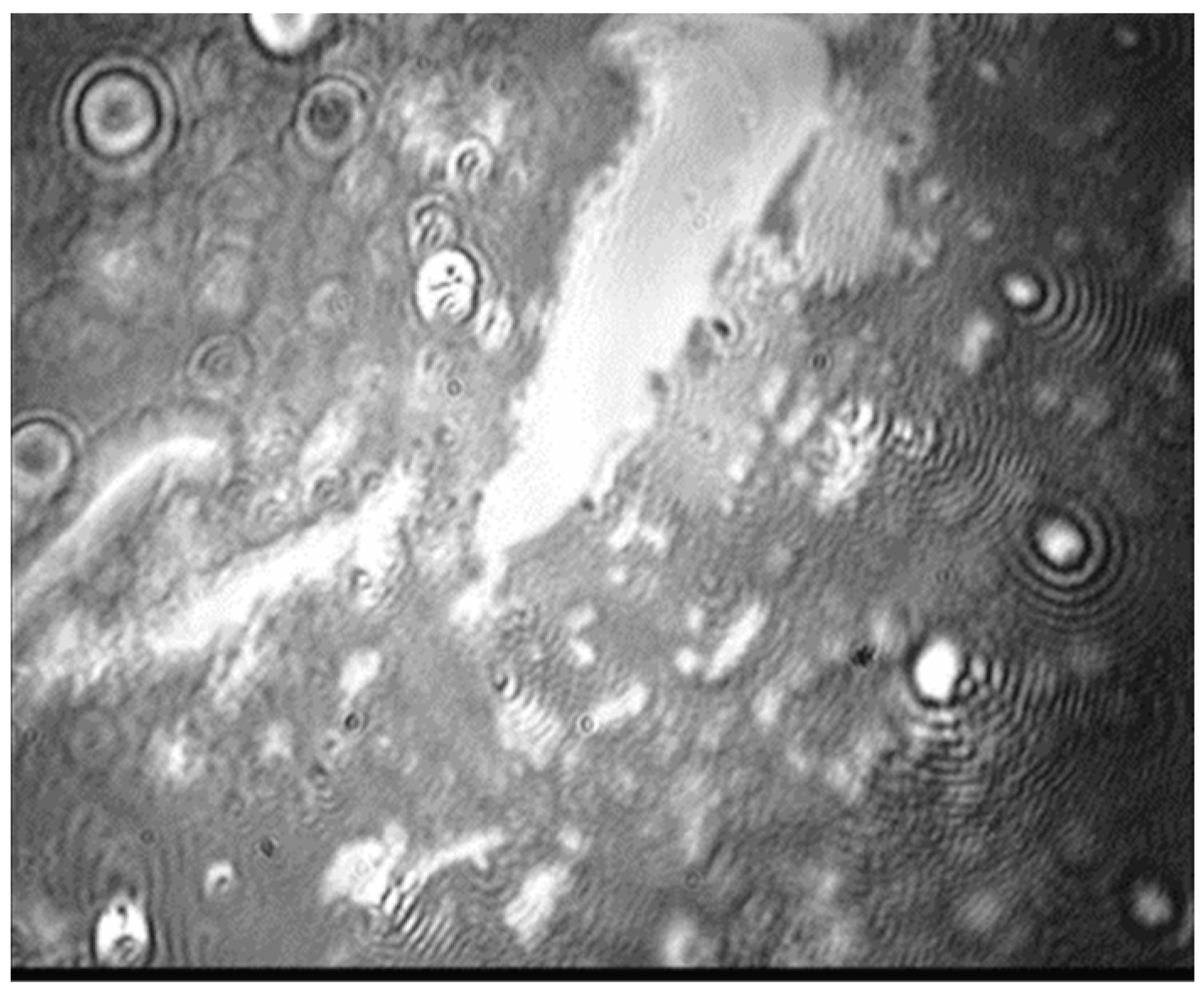
3.3. Mixed Films of Nanoparticles and Lipid at Low Surface Tensions
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Echaide, M.; Autilio, C.; Arroyo, R.; Perez-Gil, J. Restoring pulmonary surfactant membranes and films at the respiratory surface. Biochim. Biophys. Acta Biomembr. 2017, 9, 1725–1739. [Google Scholar] [CrossRef] [PubMed]
- Castillo-Sanchez, J.C.; Cruz, A.; Perez-Gil, J. Structural hallmarks of lung surfactant: Lipid-protein interactions, membrane structure and future challenges. Arch. Biochem. Biophys. 2021, 703, 108850. [Google Scholar] [CrossRef] [PubMed]
- Sosnowski, T.R. Inhaled aerosols: Their role in COVID-19 transmission, including biophysical interactions in the lungs. Curr. Opin. Colloids Interface Sci. 2021, 54, 101451. [Google Scholar] [CrossRef]
- Guzmán, E.; Santini, E. Lung surfactant models and particles at fluid interfaces: Making preliminary toxicity assessments of inhaled particles. Curr. Opin. Colloids Interface Sci. 2019, 39, 24–39. [Google Scholar] [CrossRef]
- Zuo, Y.Y.; Uspal, W.E.; Wei, T. Airborne transmission of COVID-19: Aerosol dispersion, lung deposition, and virus-receptor interactions. ACS Nano 2020, 14, 16502–16524. [Google Scholar] [CrossRef]
- Schleh, C.; Hohlfeld, J.M. Interaction of nanoparticles with the pulmonary surfactant system. Inhal. Toxicol. 2009, 21, 97–103. [Google Scholar] [CrossRef]
- Guzmán, E.; Santini, E.; Ferrari, M.; Liggieri, L.; Ravera, F. Evaluating the impact of hydrophobic silicon dioxide in the interfacial properties of lung surfactant films. Environ. Sci. Technol. 2022; in press. [Google Scholar] [CrossRef]
- Gregory, T.J.; Steinberg, K.P.; Spragg, R.; Gadek, J.E.; Hyers, T.M.; Longmore, W.J.; Moxley, M.A.; Cai, G.Z.; Hite, R.D.; Smith, R.M.; et al. Bovine surfactant therapy for patients with acute respiratory distress syndrome. Am. J. Respir. Crit. Care Med. 1997, 155, 1309–1315. [Google Scholar] [CrossRef]
- Veldhuizen, R.A.W.; Zuo, Y.Y.; Petersen, N.O.; Lewis, J.F.; Possmayer, F. The COVID-19 pandemic: A target for surfactant therapy? Expert Rev. Respir. Med. 2021, 15, 597–608. [Google Scholar] [CrossRef] [PubMed]
- Baer, B.; Souza, L.M.P.; Pimentel, A.S.; Veldhuizen, R.A.W. New insights into exogenous surfactant as a carrier of pulmonary therapeutics. Biochem. Pharmacol. 2019, 164, 64–73. [Google Scholar] [CrossRef]
- Hidalgo, A.; Cruz, A.; Pérez-Gil, J. Barrier or carrier? Pulmonary surfactant and drug delivery. Eur. J. Pharm. Biopharm. 2015, 95, 117–127. [Google Scholar] [CrossRef]
- Kim, H.C.; Suresh, M.V.; Singh, V.V.; Arick, D.Q.; Machado-Aranda, D.A.; Raghavendran, K.; Won, Y.Y. Polymer Lung Surfactants. ACS Appl. Bio Mater. 2018, 1, 581–592. [Google Scholar] [CrossRef]
- Sosnowski, T.R. Particles on the lung surface—Physicochemical and hydrodynamic effects. Curr. Opin. Colloids Interface Sci. 2018, 36, 1–9. [Google Scholar] [CrossRef]
- Sosnowski, T.R.; Kubski, P.; Wojciechowski, K. New experimental model of pulmonary surfactant for biophysical studies. Colloids Surf. A 2017, 519, 27–33. [Google Scholar] [CrossRef]
- Schüer, J.J.; Arndt, A.; Wölk, C.; Pinnapireddy, S.R.; Bakowsky, U. Establishment of a Synthetic In Vitro Lung Surfactant Model for Particle Interaction Studies on a Langmuir Film Balance. Langmuir 2020, 36, 4808–4819. [Google Scholar] [CrossRef]
- Farnoud, A.M.; Fiegel, J. Calf lung surfactant recovers surface functionality after exposure to aerosols containing polymeric particles. J. Aerosol Med. Pulm. Drug Deliv. 2015, 29, 10–23. [Google Scholar] [CrossRef] [PubMed]
- Guzman, E.; Ferrari, M.; Santini, E.; Liggieri, L.; Ravera, F. Effect of silica nanoparticles on the interfacial properties of a canonical lipid mixture. Colloids Surf. B 2015, 136, 971–980. [Google Scholar] [CrossRef]
- Farnoud, A.M.; Fiegel, J. Low concentrations of negatively charged sub-micronparticles alter the microstructure of DPPC at the air–water interface. Colloids Surf. A 2012, 415, 320–327. [Google Scholar] [CrossRef]
- Farnoud, A.M.; Fiegel, J. Interaction of dipalmitoyl phosphatidylcholine monolayers with a particle-laden subphase. J. Phys. Chem. B 2013, 117, 12124–12134. [Google Scholar] [CrossRef]
- Guzman, E.; Santini, E.; Ferrari, M.; Liggieri, L.; Ravera, F. Interfacial properties of mixed DPPC-hydrophobic fumed silica nanoparticle layers. J. Phys. Chem. C 2015, 119, 21024–21034. [Google Scholar] [CrossRef]
- Ravera, F.; Miller, R.; Zuo, Y.Y.; Noskov, B.A.; Bykov, A.G.; Kovalchuk, V.I.; Loglio, G.; Javadi, A.; Liggieri, L. Methods and models to investigate the physico-chemical functionality of pulmonary surfactant. Curr. Opin. Colloids Interface Sci. 2021, 55, 101467. [Google Scholar] [CrossRef]
- Kondej, D.; Sosnowski, T.R. Interfacial rheology for the assessment of potential health effects of inhaled carbon nanomaterials at variable breathing conditions. Sci. Rep. 2020, 10, 14044. [Google Scholar] [CrossRef]
- Bykov, A.G.; Gochev, G.; Loglio, G.; Miller, R.; Panda, A.K.; Noskov, B.A. Dynamic surface properties of mixed monolayers of polystyrene micro- and nanoparticles with DPPC. Colloids Surf. A Physicochem. Eng. Asp. 2017, 521, 239–246. [Google Scholar] [CrossRef]
- Xu, L.; Yang, Y.; Zuo, Y.Y. Atomic force microscopy imaging of adsorbed pulmonary surfactant films. Biophys. J. 2020, 119, 756–766. [Google Scholar] [CrossRef] [PubMed]
- Guzman, E.; Santini, E.; Zabiegaj, D.; Ferrari, M.; Liggieri, L.; Ravera, F. Interaction of carbon black particles and dipalmitoylphosphatidylcholine at the water/air interface: Thermodynamics and rheology. J. Phys. Chem. C 2015, 119, 26937–26947. [Google Scholar] [CrossRef]
- Finot, E.; Markey, L.; Hane, F.; Amrein, M.; Leonenko, Z. Combined atomic force microscopy and spectroscopic ellipsometry applied to the analysis of lipid-protein thin films. Colloids Surf. B Biointerfaces 2013, 104, 289–293. [Google Scholar] [CrossRef]
- Przybyla, R.J.; Wright, J.; Parthiban, R.; Nazemidashtarjandi, S.; Kaya, S.; Farnoud, A.M. Electronic cigarette vapor alters the lateral structure but not tensiometric properties of calf lung surfactant. Respir. Res. 2017, 18, 193. [Google Scholar] [CrossRef] [PubMed]
- Arora, S.; Kappl, M.; Haghi, M.; Young, P.M.; Traini, D.; Jain, S. An investigation of surface properties, local elastic modulus and interaction with simulated pulmonary surfactant of surface modified inhalable voriconazole dry powders using atomic force microscopy. RSC Adv. 2016, 6, 25789–25798. [Google Scholar] [CrossRef] [Green Version]
- Valle, R.P.; Huang, C.L.; Loo, J.S.C.; Zuo, Y.Y. Increasing hydrophobicity of nanoparticles intensifies lung surfactant film inhibition and particle retention. ACS Sustain. Chem. Eng. 2014, 2, 1574–1580. [Google Scholar] [CrossRef]
- Yang, Y.; Xu, L.; Dekkers, S.; Zhang, L.G.; Cassee, F.R.; Zuo, Y.Y. Aggregation state of metal-based nanomaterials at the pulmonary surfactant film determines biophysical inhibition. Environ. Sci. Technol. 2018, 52, 8920–8929. [Google Scholar] [CrossRef]
- Bykov, A.G.; Liggieri, L.; Noskov, B.A.; Pandolfini, P.; Ravera, F.; Loglio, G. Surface dilational rheological properties in the nonlinear domain. Adv. Colloids Interface Sci. 2015, 222, 110–118. [Google Scholar] [CrossRef]
- Bykov, A.G.; Loglio, G.; Ravera, F.; Liggieri, L.; Miller, R.; Noskov, B.A. Dilational surface elasticity of spread monolayers of pulmonary lipids in a broad range of surface pressure. Colloids Surf. A 2018, 541, 137–144. [Google Scholar] [CrossRef]
- Bykov, A.G.; Loglio, G.; Miller, R.; Milyaeva, O.Y.; Michailov, A.V.; Noskov, B.A. Dynamic properties and relaxation processes in surface layer of pulmonary surfactant solutions. Colloids Surf. A Physicochem. Eng. Asp. 2019, 573, 14–21. [Google Scholar] [CrossRef]
- Bykov, A.G.; Milyaeva, O.Y.; Isakov, N.A.; Michailov, A.V.; Loglio, G.; Miller, R.; Noskov, B.A. Dynamic properties of adsorption layers of pulmonary surfactants. Influence of matter exchange with bulk phase. Colloids Surf. A 2021, 611, 125851. [Google Scholar] [CrossRef]
- Bykov, A.G.; Noskov, B.A.; Loglio, G.; Lyadinskaya, V.V.; Miller, R. Dilational surface elasticity of spread monolayers of polystyrene microparticles. Soft Matter 2014, 10, 6499–6505. [Google Scholar] [CrossRef] [PubMed]
- Bykov, A.G.; Loglio, G.; Miller, R.; Noskov, B.A. Dilational surface elasticity of monolayers of charged polystyrene nano- and microparticles at liquid/fluid interfaces. Colloids Surf. A Physicochem. Eng. Asp. 2015, 485, 42–48. [Google Scholar] [CrossRef]
- Noskov, B.A.; Bykov, A.G. Dilational rheology of monolayers of nano- and microparticles at the liquid-fluid interfaces. Curr. Opin. Colloids Interface Sci. 2018, 37, 1–12. [Google Scholar] [CrossRef]
- Wüstneck, R.; Perez-Gil, J.; Wüstneck, N.; Cruz, A.; Fainerman, V.B.; Pison, U. Interfacial properties of pulmonary surfactant layers. Adv. Colloids Interface Sci. 2005, 117, 33–58. [Google Scholar] [CrossRef]
- Guzman, E.; Orsi, D.; Cristofolini, L.; Liggieri, L.; Ravera, F. Two-dimensional DPPC based emulsion-like structures stabilized by silica nanoparticles. Langmuir 2014, 30, 11504–11512. [Google Scholar] [CrossRef]
- Orsi, D.; Guzmán, E.; Liggieri, L.; Ravera, F.; Ruta, B.; Chushkin, Y.; Rimoldi, T.; Cristofolini, L. 2D dynamical arrest transition in a mixed nanoparticle-phospholipid layer studied in real and momentum spaces. Sci. Rep. 2015, 5, 17930. [Google Scholar] [CrossRef] [Green Version]
- Bykov, A.G.; Guzmán, E.; Rubio, R.G.; Krycki, M.M.; Milyaeva, O.Y.; Noskov, B.A. Influence of temperature on dynamic surface properties of spread DPPC monolayers in a broad range of surface pressures. Chem. Phys. Lipids 2019, 225, 104812. [Google Scholar] [CrossRef] [PubMed]
- Zuo, Y.Y.; Veldhuizen, R.A.; Neumann, A.W.; Petersen, N.O.; Possmayer, F. Current perspectives in pulmonary surfactant-inhibition, enhancement and evaluation. Biochim. Biophys. Acta 2008, 1778, 1947–1977. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liekkinen, J.; Enkavi, G.; Javanainen, M.; Olmeda, B.; Perez-Gil, J.; Vattulainen, I. Pulmonary surfactant lipid reorganization induced by the adsorption of the oligomeric surfactant protein B complex. J. Mol. Biol. 2020, 432, 3251–3268. [Google Scholar] [CrossRef] [PubMed]
- Binks, B.P. Particles as surfactants similarities and differences. Curr. Opin. Colloids Interface Sci. 2002, 7, 21–41. [Google Scholar] [CrossRef]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bykov, A.; Milyaeva, O.; Akentiev, A.; Panaeva, M.; Isakov, N.; Miller, R.; Noskov, B. Impact of Polymer Nanoparticles on DPPC Monolayer Properties. Colloids Interfaces 2022, 6, 28. https://doi.org/10.3390/colloids6020028
Bykov A, Milyaeva O, Akentiev A, Panaeva M, Isakov N, Miller R, Noskov B. Impact of Polymer Nanoparticles on DPPC Monolayer Properties. Colloids and Interfaces. 2022; 6(2):28. https://doi.org/10.3390/colloids6020028
Chicago/Turabian StyleBykov, Alexey, Olga Milyaeva, Alexander Akentiev, Maria Panaeva, Nikolaj Isakov, Reinhard Miller, and Boris Noskov. 2022. "Impact of Polymer Nanoparticles on DPPC Monolayer Properties" Colloids and Interfaces 6, no. 2: 28. https://doi.org/10.3390/colloids6020028






