Some Remarks on Colloid Stability: Selected Examples Taken from the Milk Chain for Food Prepares
Abstract
:1. Introduction
- (a)
- collecting raw matter;
- (b)
- operating with it or mixtures of the like in such a way to get the desired products of a given food chain in substantial amounts; and
- (c)
- obtaining the final products through maturing and storage, if these steps are required.
2. Surface Properties
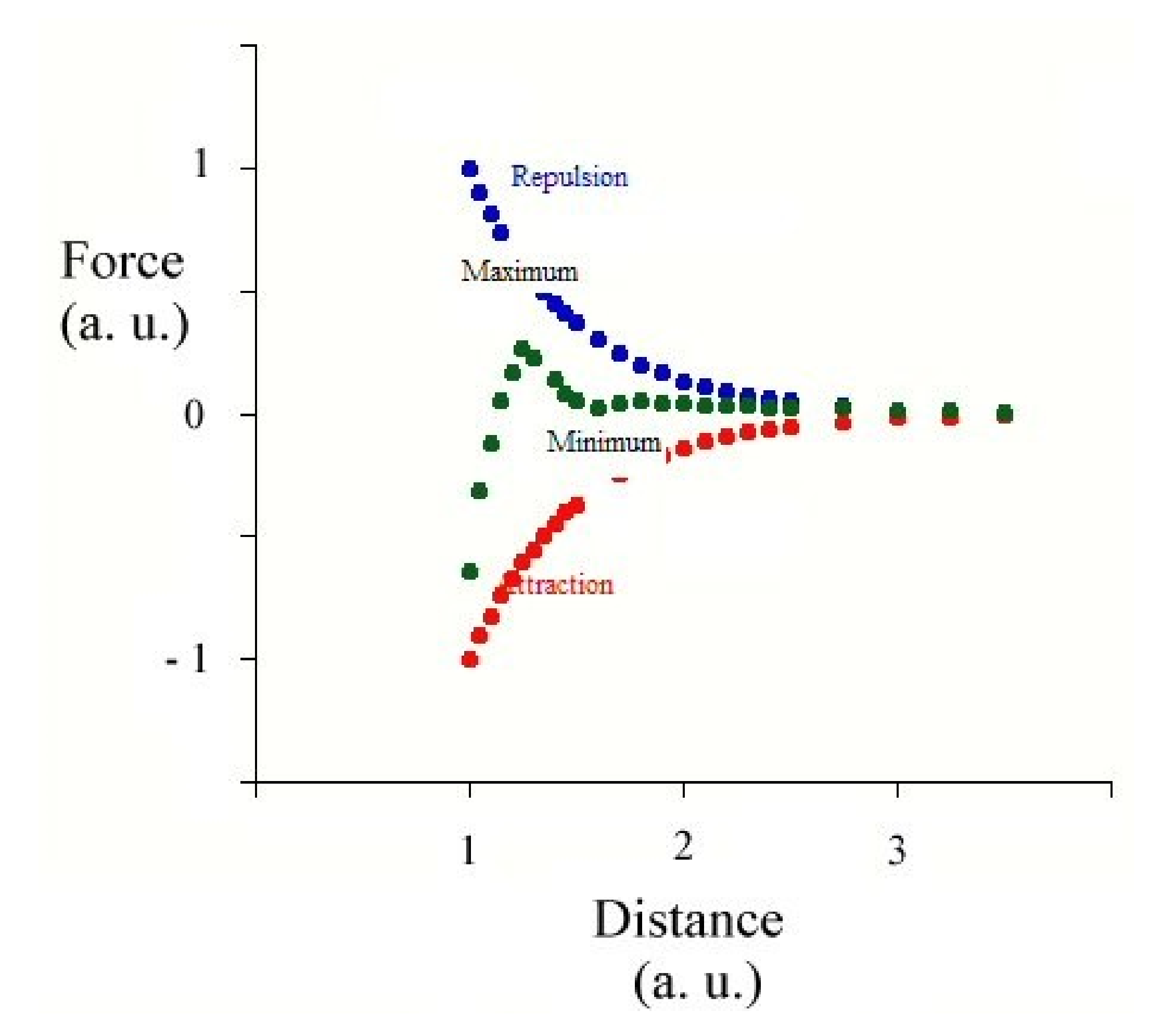
2.1. Forces in Action and Species Responsible for Stabilization
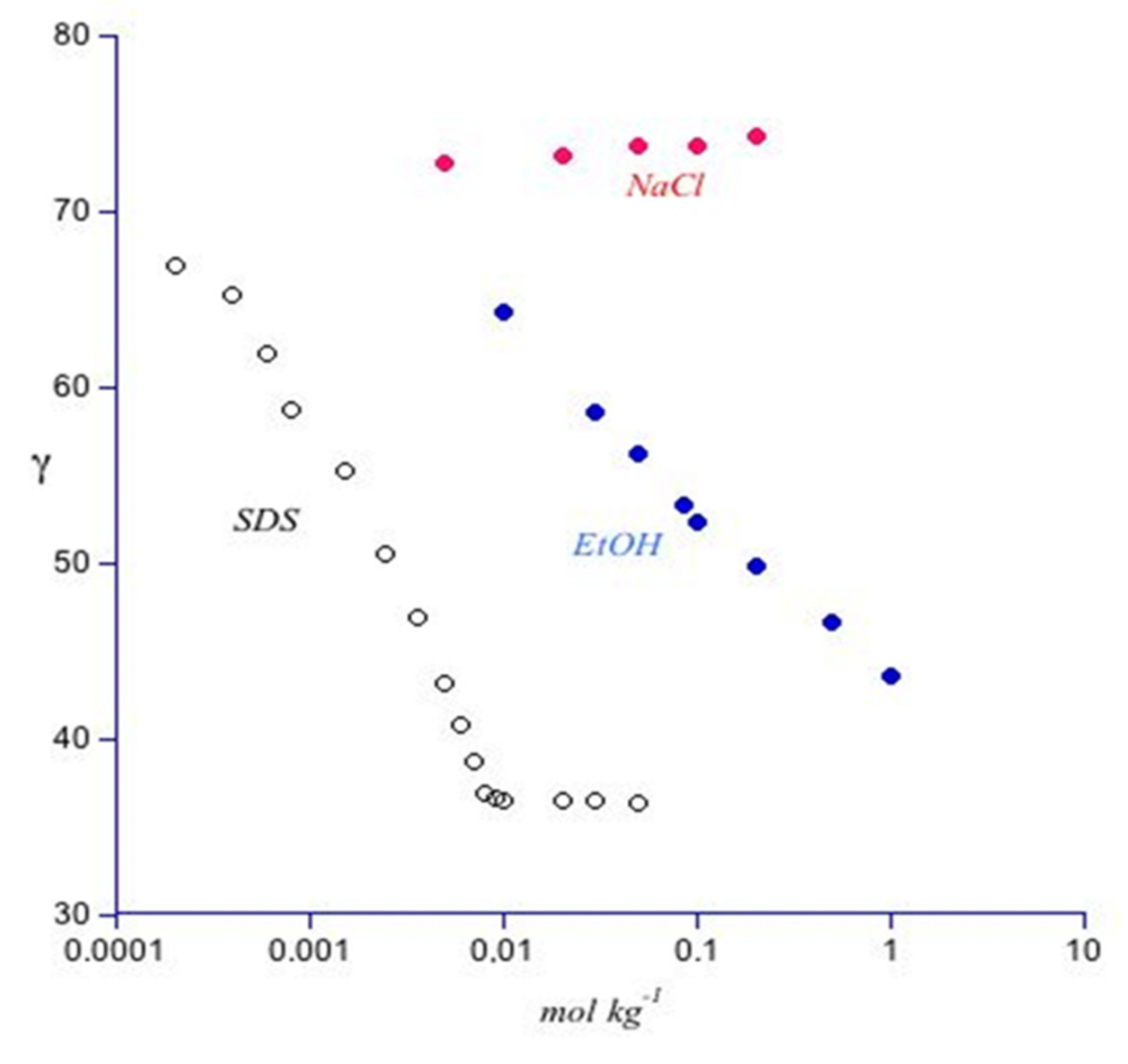
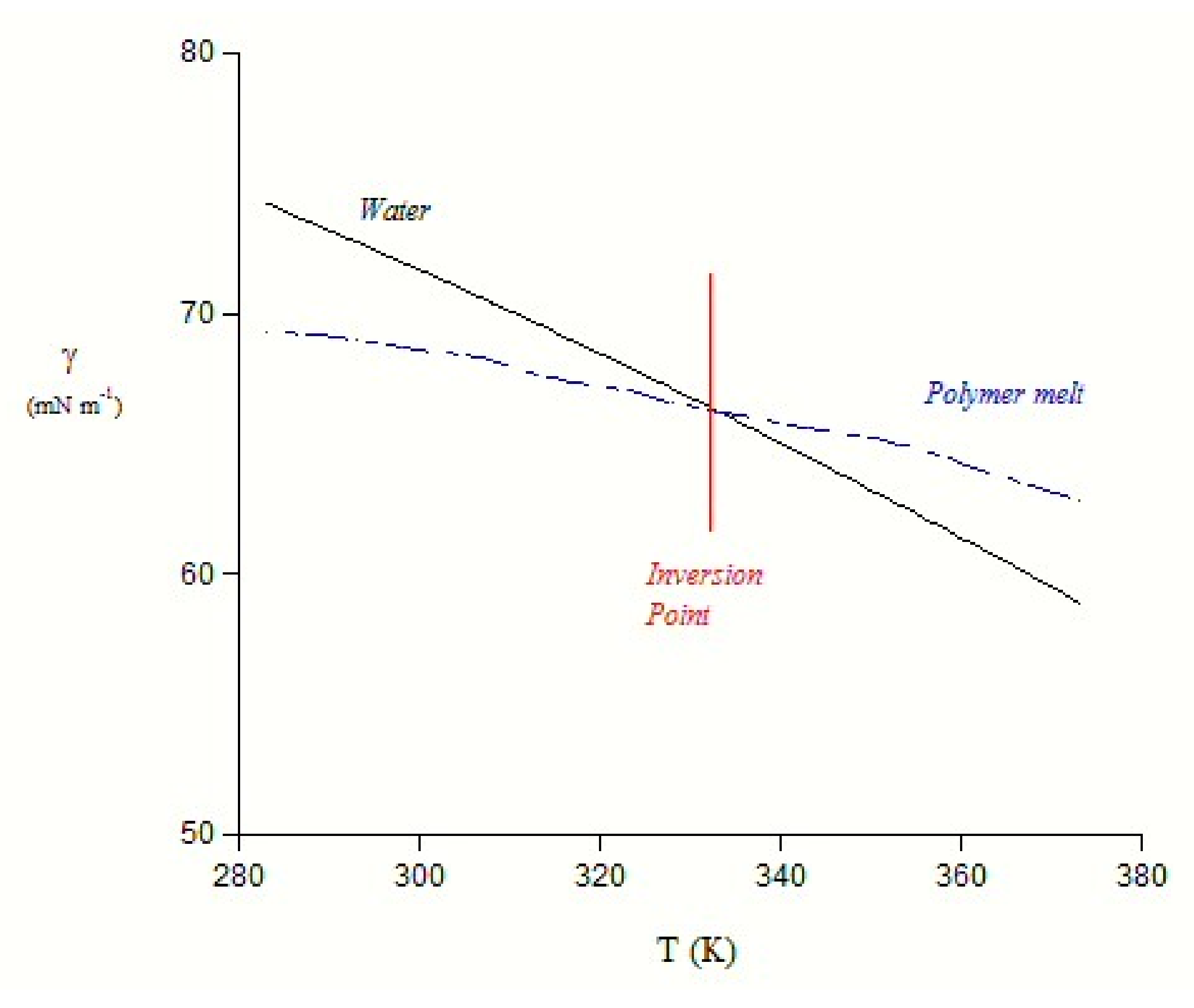
2.2. Surface Properties
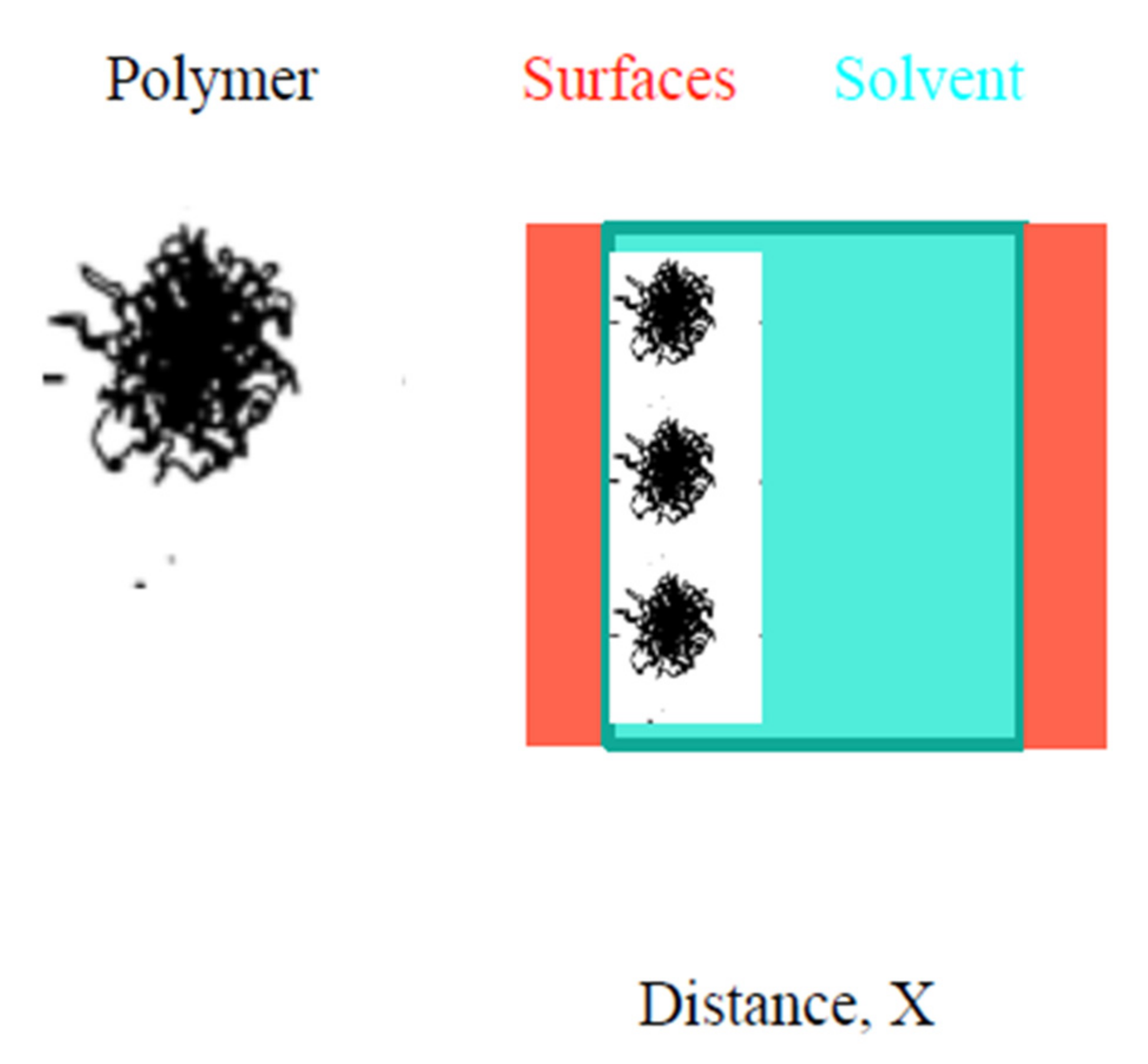
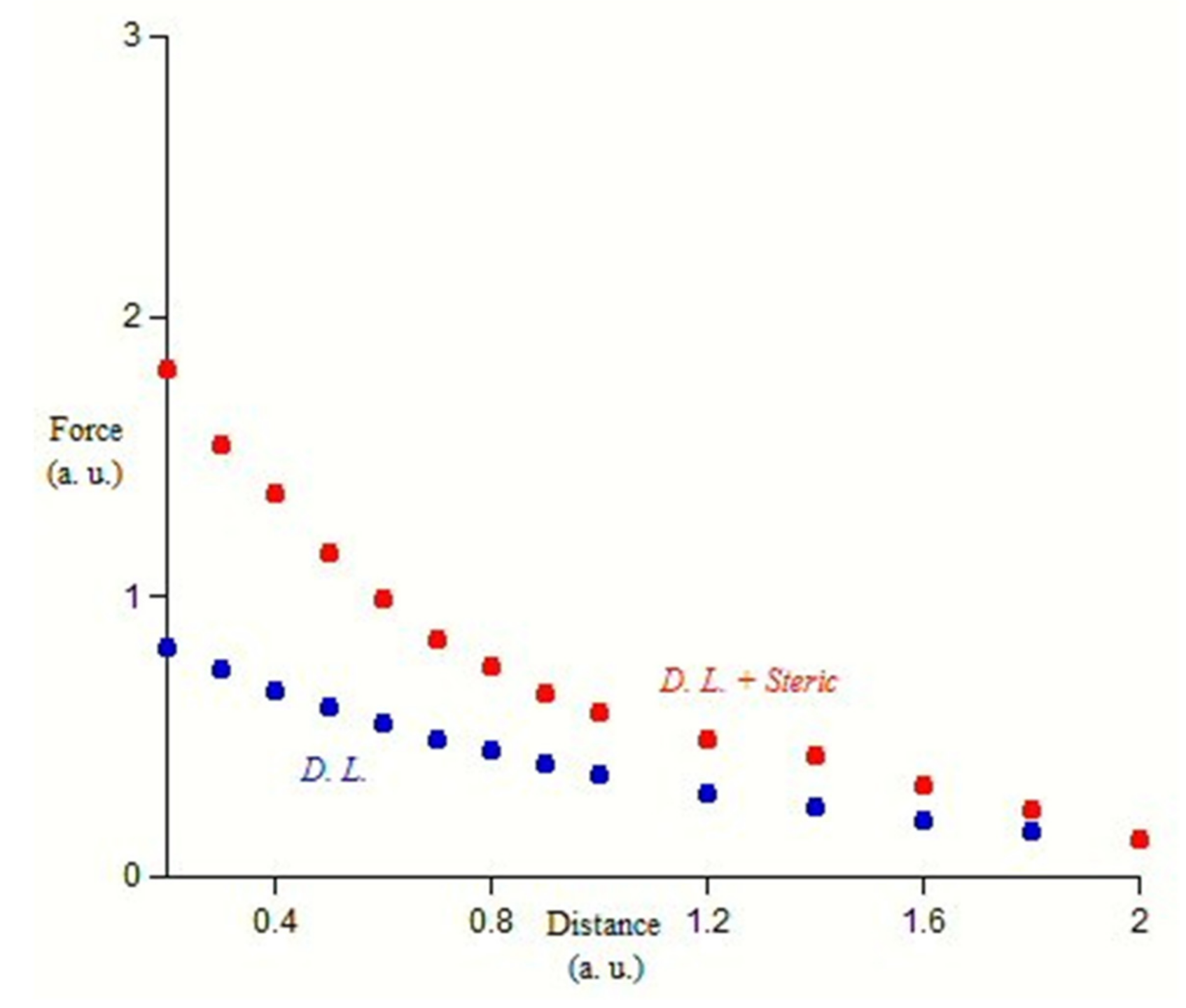
3. Steric Stabilization
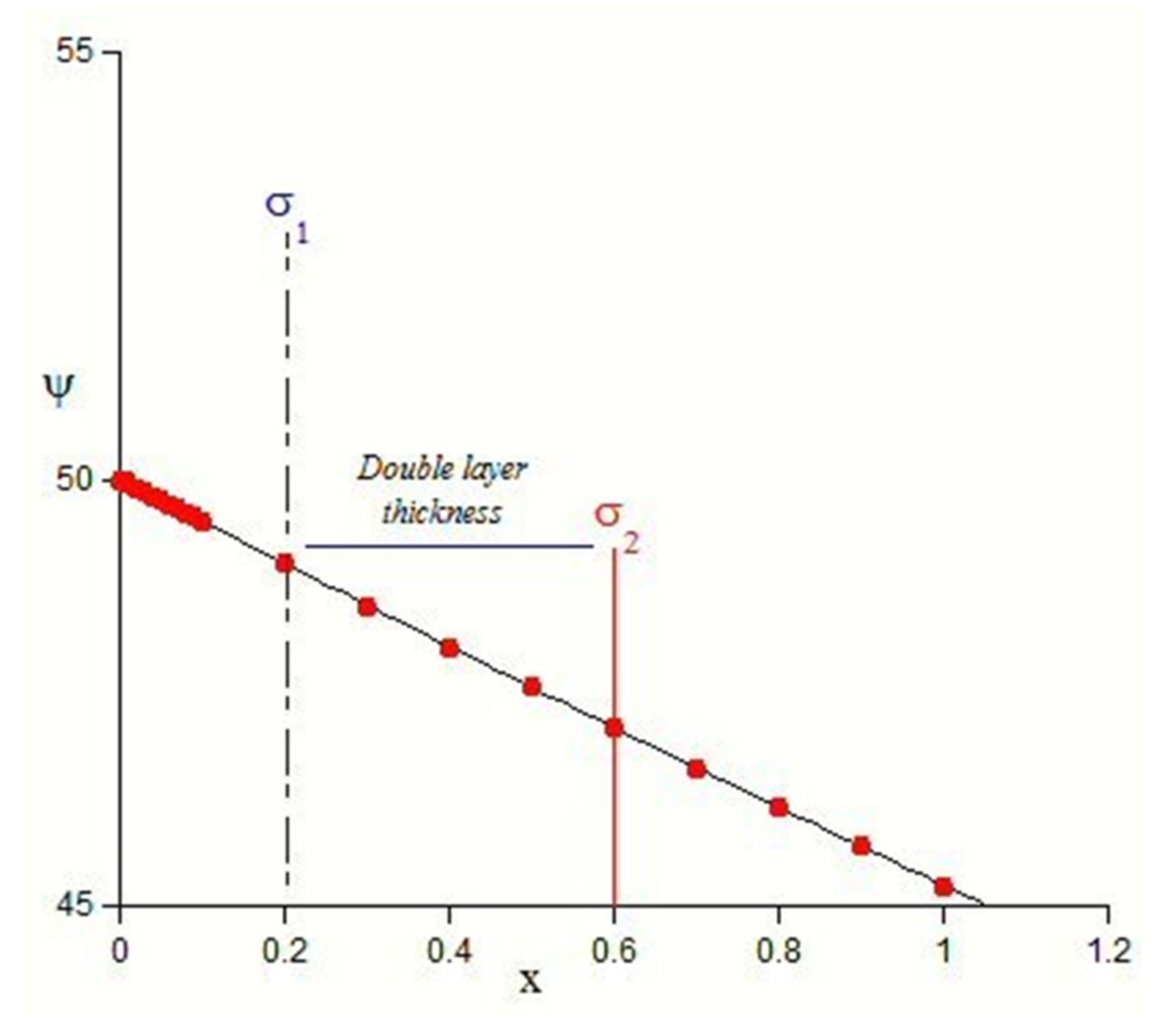
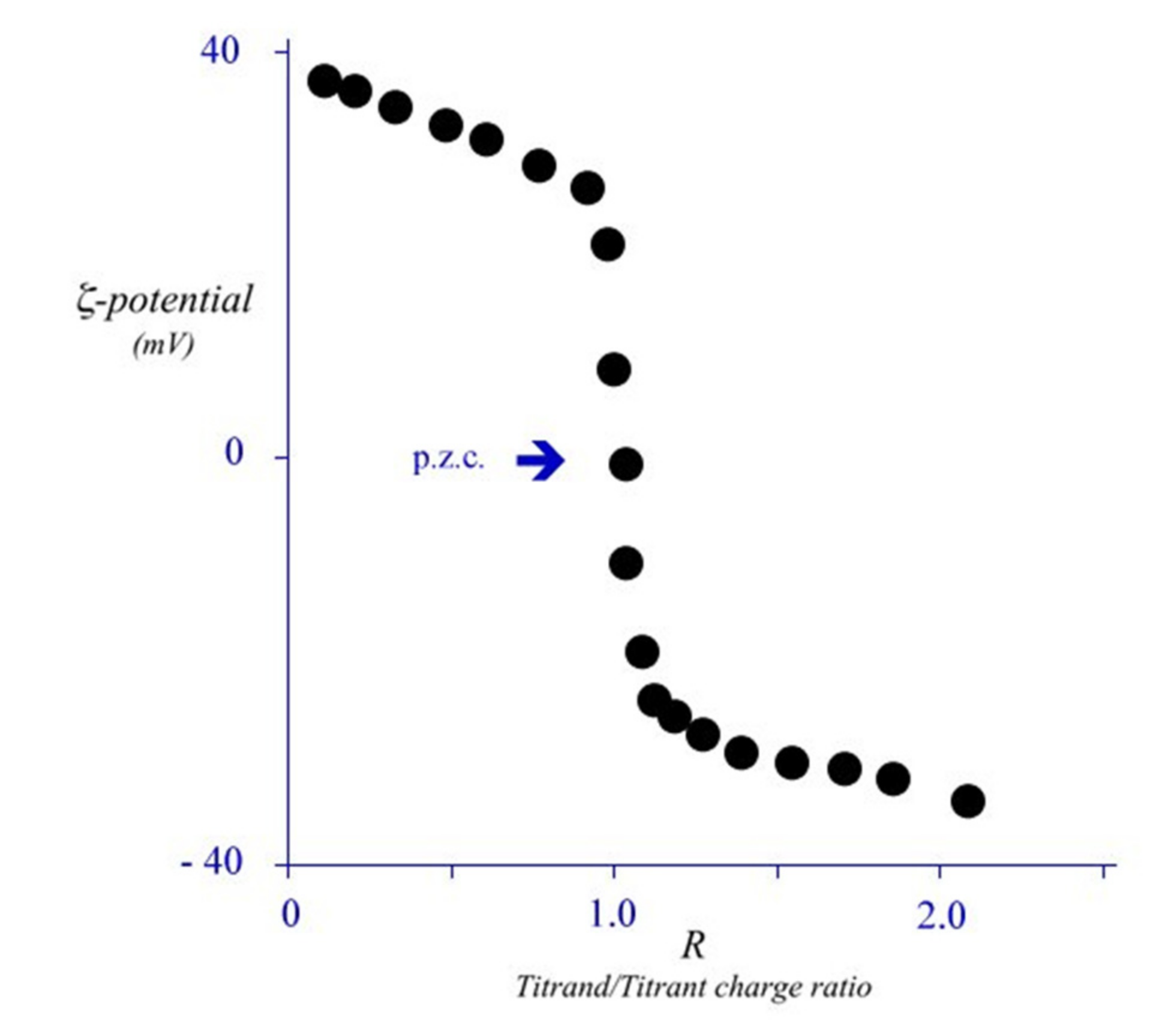
4. Electrostatic Contributions
5. More Fundamentals
5.1. The Fundamentals of Food Colloid Stabilization
5.2. The Case of Food Colloids
6. The Cheese Making Sequence
- Milk coagulation by rennet (sometimes assisted by tiny amounts of citric acid) added to milk kept at mild temperature, 36–39 °C. This step implies optimization of the enzymic activity of rennet and, in consequence of that, favors eliminating steric stabilization by cutting the polypeptide chains protruding outward from casein micelles.
- Curd breaking and serum discharge jointly imply a phase separation of colloids. A heating stage at 50 °C is required to completely discharge and favor the onset of cheese lumps.
- Curd is left maturing in acid serum for some hours. In this stage, solubilization of calcium salts and de-mineralization of cheese paste occur. The paste becomes plastic (is a reverse phase separation), giving rise to “pasta filata” (also termed cooked pasta), and is reduced in fibers by the action of gravitation or by simple stretching, as happens for mozzarella and provolone. In the absence of shear forces, which favor the formation of fibers, cheese grains collapse.
- Salting is made in dry or wet conditions, depending on the needs. Ion valence and content are relevant in this step. Salting implies water loss due to the onset of osmotic gradients outside the cheese peel, which contracts, deforms, and progressively becomes harder.
- Cheese maturing is usually performed at 3–8 °C and 85–90% humidity. It helps to give cheese due consistency and appearance. It implies further water loss. Maturing depends on the cheese kind obtained, and the same holds for working conditions. In niche products, mildewing, coverage with straw, or chestnut leaves (which are rich in tannins) are sometimes used. Sometimes, mildewed conditions are induced on the cheese peel or in its interior. In the latter eventuality, a wide variety of “blue cheese” types may occur. These can be stiff (Stilton) or creamy (Gorgonzola).
7. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Chandrasekkhar, S. Stochastic Problems in Physics and Astronomy. Rev. Mod. Phys. 1943, 15, 1. [Google Scholar] [CrossRef]
- Arbasino, A. Un Paese Senza; Garzanti: Milano, Italy, 1980; p. 40. [Google Scholar]
- Mukhopadhyay, S. Nano Drugs: A Critical Review of Their Patents and Market. In Characterization and Biology of Nanomaterials for Drug Delivery; Elsevier: Amsterdam, The Netherlands, 2019; Chapter 18; pp. 523–551. [Google Scholar]
- Van Boekel, M.A.J.S.; Jongen, W.M.F. Product quality and food processing: How to quantify the healthiness of a product. Cancer Lett. 1997, 114, 65–69. [Google Scholar] [CrossRef]
- Toussaint-Samat, M. A History of Food; Wiley: New York, NY, USA, 2009. [Google Scholar]
- Post, D.M. The long and short of food-chain length. Trends Ecol. Evol. 2002, 117, 269–277. [Google Scholar] [CrossRef]
- Martínez-Guido, S.I.; González-Campos, J.B.; El-Halwagi, M.M.; Ponce-Ortega, J.M. Sustainable Optimization of Food Networks in Disenfranchised Communities. ACS Sustain. Chem. Eng. 2017, 5, 8895–8907. [Google Scholar] [CrossRef]
- Rea, S.; Storani, G.; Mascaro, N.; Stocchi, R.; Loschi, A.R. Species identification in anchovy pastes from the market by PCR-RFLP technique. Food Control 2009, 20, 515–520. [Google Scholar] [CrossRef]
- Guinee, T.P.; O’Kennedy, B.T.; Kelly, P.M. Effect of Milk Protein Standardization Using Different Methods on the Composition and Yields of Cheddar Cheese. J. Dairy Sci. 2006, 89, 468–482. [Google Scholar] [CrossRef]
- Zhong, Y.; Cavender, G.; Zhao, Y. Investigation of different coating application methods on the performance of edible coatings on Mozzarella cheese. LWT Food Sci. Technol. 2014, 56, 1–8. [Google Scholar] [CrossRef]
- Longinotti, M.P.; Mazzobre, M.F.; Buera, M.P.; Corti, H.R. Effect of salts on the properties of aqueous sugar systems in relation to biomaterial stabilization. Part 2: Sugar crystallization rate and electrical conductivity behavior. PCCP 2002, 4, 533–540. [Google Scholar] [CrossRef]
- Leser, M.E.; Sagalowicz, L.; Michel, M.; Watzke, H.J. Self-assembly of polar food lipids. Adv. Colloid Interface Sci. 2006, 123, 125–136. [Google Scholar] [CrossRef]
- Murray, B.S.; Durga, K.; Yusoff, A.; Stoyanov, S.D. Stabilization of foams and emulsions by mixtures of surface active food-grade particles and proteins. Food Hydrocoll. 2011, 25, 627–638. [Google Scholar] [CrossRef]
- Schmitt, C.; Turgeon, S.L. Protein/polysaccharide complexes and coacervates in food systems. Adv. Colloid Interface Sci. 2011, 167, 63–70. [Google Scholar] [CrossRef] [PubMed]
- Eftimov, T.; Korošec, P.; Seljak, B.K. StandFood: Standardization of Foods Using a Semi-Automatic System for Classifying and Describing Foods According to FoodEx2. Nutrients 2017, 9, 542. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Milk Proteins from Expression to Food, 3rd ed.; Boland, M.; Singh, H. (Eds.) Academic Press: Cambridge, MA, USA, 2019. [Google Scholar]
- Getaneh, G.; Mebrat, A.; Wubie, A.; Kendie, H. Review on Goat Milk Composition and its Nutritive Value. J. Nutr. Health Sci. 2016, 3, 1–10. [Google Scholar]
- Schott, H. Saturation adsorption at interfaces of surfactant solutions. J. Pharm. Sci. 1980, 69, 852–854. [Google Scholar] [CrossRef] [PubMed]
- Accordino, S.R.; Morini, M.A.; Sierra, M.B.; Fris, J.A.R.; Appignanesi, G.A.; Fernández, A. Wrapping mimicking in drug-like small molecules disruptive of protein–protein interfaces. Proteins 2012, 80, 1755–1765. [Google Scholar] [CrossRef]
- Mohrhusen, L.; Osmić, M. Steric ligand stabilization of nanocrystals versus electrostatic shielding by ionic compounds: A principle model study with TEM and XPS. RSC Adv. 2017, 7, 12897–12907. [Google Scholar] [CrossRef] [Green Version]
- Lekkerkerker, H.N.W. Osmotic Equilibrium Treatment of the Phase Separation in Colloidal Dispersions Containing Non-Adsorbing Polymer Molecules. Colloids Surf. 1990, 51, 419–426. [Google Scholar] [CrossRef] [Green Version]
- Schneider, C.; Hanisch, M.; Wedel, B.; Jusufi, A.; Ballauff, M. Experimental study of electrostatically stabilized colloidal particles: Colloidal stability and charge reversal. J. Colloid Interface Sci. 2011, 358, 62–67. [Google Scholar] [CrossRef]
- Tadros, T.F. Interfacial Phenomena and Colloid Stability: Industrial Applications; Wiley: Hoboken, NJ, USA, 2015. [Google Scholar]
- Usov, A.I. Polysaccharides of the red algae. Adv. Carbohydr. Chem. Biochem. 2011, 65, 115–217. [Google Scholar]
- Marangoni, A.G.; van Duynhoven, J.P.M.; Acevedo, N.C.; Nicholson, R.A.; Patel, A.R. Advances in our understanding of the structure and functionality of edible fats and fat mimetics. Soft Matter 2020, 16, 289–306. [Google Scholar] [CrossRef]
- Dickinson, E. Stability and rheological implications of electrostatic milk protein–polysaccharide interactions. Trends Food Sci. Technol. 1998, 9, 347–354. [Google Scholar] [CrossRef]
- Yaranossian, N.; Lynch, M.G. Confectionery Containing Low Viscosity Iota Carrageenan. European Patent EP3197287A2, 27 February 2019. [Google Scholar]
- Finney, D.J.; Dea, I.C.M. Stabilization of Ice-Cream Compositions. U.S. Patent 4145454, 20 March 1979. [Google Scholar]
- Everett, D.H. Basic Principles of Colloid Science; Royal Society of Chemistry: London, UK, 1994; Chapter V; pp. 63–75. [Google Scholar]
- Ottewill, R.H.; Rochester, C.H.; Smith, A.L. (Eds.) Adsorption from Solution; Academic Press: London, UK, 1983. [Google Scholar]
- Lunkenheimer, K.; Wantke, K.-D. Determination of the surface tension of surfactant solutions applying the method of Lecomte du Noüy (ring tensiometer). Colloid Polym. Sci. 1981, 259, 354–366. [Google Scholar] [CrossRef]
- Alahverdjieva, V.S.; Grigoriev, D.O.; Ferri, J.K.; Fainerman, V.B.; Aksenenko, E.V.; Leser, M.E.; Michel, M.; Miller, R. Adsorption behaviour of hen egg-white lysozyme at the air/water interface. Colloids Surf. A Physicochem. Engin. Aspects 2008, 323, 167–174. [Google Scholar] [CrossRef]
- Hua, X.Y.; Rosen, M.J. Dynamic surface tension of aqueous surfactant solutions: I. Basic parameters. J. Colloid Interface Sci. 1988, 124, 652–659. [Google Scholar]
- Bonfillon, A.; Sicoli, F.; Langevin, D. Dynamic Surface Tension of Ionic Surfactant Solutions. J. Colloid Interface Sci. 1994, 168, 497–504. [Google Scholar] [CrossRef]
- Rouquerol, F.; Rouquerol, J.; Sing, K.S.W.; Llewellyn, P.; Maurin, G. Adsorption by Powders and Porous Solids. Principles, Methodology and Applications; Academic Press: New York, NY, USA, 2013. [Google Scholar]
- De Gennes, P.G. Scaling theory of polymer adsorption. J. Phys. 1976, 37, 1445–1452. [Google Scholar] [CrossRef]
- Hone, D.; Ji, H.; Pincus, P.A. Polymer Adsorption on Rough Surfaces.1. Ideal Long Chain. Macromolecules 1987, 20, 2543–2549. [Google Scholar] [CrossRef]
- Evans, R.; Napper, D.H. Steric stabilization I. Comparison of theories with experiment. Koll.-Z. u. Z. Polym. 1973, 251, 409–414. [Google Scholar] [CrossRef]
- Israelachvili, J.; Min, Y.; Akbulut, M.; Alig, A.; Carver, G.; Greene, W.; Kristiansen, K.; Meyer, E.; Pesika, N.; Rosenberg, K.; et al. Recent advances in the surface forces apparatus (SFA) technique. Rep. Prog. Phys. 2010, 73, 036601. [Google Scholar] [CrossRef]
- Zhulina, E.B.; Borisov, O.V.; Priamitsyn, V.A. Theory of steric stabilization of colloid dispersions by grafted polymers. J. Colloid Interface Sci. 1990, 137, 495–511. [Google Scholar] [CrossRef]
- Landau, L.D. Collected Works; Nauka: Moscow, Russia, 1969; Volume I, pp. 386–411. [Google Scholar]
- Verwey, E.J.W.; Overbeek, J.T.G. Theory of the Stability of Lyophobic Colloids; Dover Books on Chemistry: New York, NY, USA, 1999. [Google Scholar]
- Deriaguin, R.V. Theory of the Stability of Colloids and Thin Films; Consultants Bureau: New York, NY, USA; London, UK, 1989. [Google Scholar]
- Ninham, B.W. On progress in forces since DLVO theory. Adv. Colloid Interface Sci. 1999, 83, 1–17. [Google Scholar] [CrossRef]
- Moskin, J. Cookie Master, (Column). The New York Times. Available online: https://www.nytimes.com/2004/11/07/magazine/cookie-master.html (accessed on 7 November 2004).
- The cheese that melts and stretches. Italian Food Tech. 11 October 2012. Editorial.
- Manufacturing Method for Fibrous Cheese Products. Japan Patent JP2017510252A, 19 February 2014.
- Beumer, C.; Wulferink, M.; Raaben, W.; Fiechter, D.; Brands, R.; Seinen, W. Calf Intestinal Alkaline Phosphatase, a Novel Therapeutic Drug for Lipopolysaccharide (LPS)-Mediated Diseases, Attenuates LPS Toxicity in Mice and Piglets. J. Pharmacol. Exp. Ther. 2003, 307, 737–744. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Manning, G.S. Counterion Condensation on Charged Spheres, Cylinders and Planes. J. Phys. Chem. B 2007, 111, 8554–8559. [Google Scholar] [CrossRef] [PubMed]
- Nájera, A.I.; de Renobales, M.; Barrona, L.J.R. Effects of pH, temperature, CaCl2 and enzyme concentrations on the rennet-clotting properties of milk: A multifactorial study. Food Chem. 2003, 80, 345–352. [Google Scholar] [CrossRef]
- Carles, C.; Dumas, B.R. Kinetics of the action of chymosin (rennin) on a peptide bond of bovine αs1-casein: Comparison of the behavior of this substrate with that of β- and κo-caseins. FEBS Lett. 1985, 185, 282–286. [Google Scholar] [CrossRef] [Green Version]
- Montilla, A.; Calvo, M.M. Goat’s Milk Stability during Heat Treatment: Effect of pH and Phosphates. J. Agric. Food Chem. 1997, 45, 931934. [Google Scholar] [CrossRef]
- Israelachvili, J.N. Intermolecular and Surface Forces, 3rd ed.; Elsevier: Amsterdam, The Netherlands, 2011. [Google Scholar]
- Adamson, A.V. Physical Chemistry of Surfaces, 5th ed.; Wiley: Hoboken, NJ, USA, 1991; Chapter V. [Google Scholar]
- Ohshima, H. Electrophoretic mobility of soft particles. Colloids Surf. A Physicochem. Eng. Asp. 1995, 103, 249–255. [Google Scholar] [CrossRef]
- Dalgleish, D.G. Measurement of electrophoretic mobilities and zeta-potentials of particles from milk using laser Doppler electrophoresis. J. Dairy Res. 1984, 51, 425–438. [Google Scholar] [CrossRef]
- Kantardjieff, K.A.; Rupp, B. Protein isoelectric point as a predictor for increased crystallization screening efficiency. Bioinformatics 2004, 20, 2162–2168. [Google Scholar] [CrossRef] [Green Version]

| State of Matter 1st Component (Dispersant) | The 2nd Component is a Solid (Dispersed) | The 2nd Component is a Liquid (Dispersed) | The 2nd Component is a Gas (Dispersed) |
|---|---|---|---|
| Solid | opals | bitumens | foams |
| Liquid | muds | Emulsions * | bubbles |
| Gas | smokes | aerosols | no |
| Mammal Ruminant | Country | Fat % in Fresh Milk |
|---|---|---|
| Jersey cow | United Kingdom | 5.2% |
| Zebu | India, Madagascar, Sri Lanka | 4.7% |
| Brown Swiss cow | Switzerland | 4.0% |
| Holstein Friesian cow | Netherlands, FRG, Denmark | 3.6% |
| Neapolitan Buffalo | Italy | 8.3% |
| Spanish sheep | Spain, Italy, Greece | 5.6% |
| Merinos-modified sheep | Spain, Australia | 5.3% |
| Appenzell goat | Switzerland | 4.6% |
| Manchega goat | Spain, France | 4.8% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
La Mesa, C.; Risuleo, G. Some Remarks on Colloid Stability: Selected Examples Taken from the Milk Chain for Food Prepares. Colloids Interfaces 2020, 4, 58. https://doi.org/10.3390/colloids4040058
La Mesa C, Risuleo G. Some Remarks on Colloid Stability: Selected Examples Taken from the Milk Chain for Food Prepares. Colloids and Interfaces. 2020; 4(4):58. https://doi.org/10.3390/colloids4040058
Chicago/Turabian StyleLa Mesa, Camillo, and Gianfranco Risuleo. 2020. "Some Remarks on Colloid Stability: Selected Examples Taken from the Milk Chain for Food Prepares" Colloids and Interfaces 4, no. 4: 58. https://doi.org/10.3390/colloids4040058




