Tailoring Acrylated Soybean Oil-Containing Terpolymers through Emulsion Polymerization
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Modification of Soybean Oil
2.3. Emulsion Polymerization
2.4. Characterization Techniques
3. Results and Discussion
3.1. Modification of Soybean Oil
3.2. Emulsion Polymerization
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Braun, D.; Cherdron, H.; Rehahn, M.; Ritte, H.; Voit, B. Polymer Synthesis: Theory and Practice, 5th ed.; Springer: Berlin/Heidelberg, Germany, 2005; p. 404. ISBN 978-3-642-28979-8. [Google Scholar]
- Ozturk, C.; Mutlu, H.; Meier, M.A.R.; Kusefoglu, S.H. 4-Vinylbenzenesulfonic acid adduct of epoxidized soybean oil: Synthesis, free radical and ADMET polymerizations. Eur. Polym. J. 2011, 47, 1467–1476. [Google Scholar] [CrossRef]
- Meier, M.A.R.; Metzger, J.O.; Schubert, U.S. Plant oil renewable resources as green alternatives in polymer science. Chem. Soc. Rev. 2007, 36, 1788–1802. [Google Scholar] [CrossRef] [PubMed]
- Gandini, A.; Lacerda, T.M. From monomers to polymers from renewable resources: Recent advances. Prog. Polym. Sci. 2015, 48, 1–39. [Google Scholar] [CrossRef]
- Khot, S.N.; Lascala, J.J.; Can, E.; Morye, S.S.; Williams, G.I.; Palmese, G.R.; Kusefoglu, S.H.; Wool, R.P. Development and application of triglyceride-based polymers and composites. J. Appl. Polym. Sci. 2001, 82, 703–723. [Google Scholar] [CrossRef]
- Zhu, Y.Q.; Romain, C.; Williams, C.K. Sustainable polymers from renewable resources. Nature 2016, 540, 354–362. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Espinosa, L.M.; Meier, M.A.R. Plant oils: The perfect renewable resource for polymer science?! Eur. Polym. J. 2011, 47, 837–852. [Google Scholar] [CrossRef]
- Li, F.; Hanson, M.V.; Larock, R.C. Soybean oil-divinylbenzene thermosetting polymers: Synthesis, structure, properties and their relationships. Polymer 2001, 42, 1567–1579. [Google Scholar] [CrossRef]
- Altuna, F.I.; Pettarin, V.; Williams, R.J.J. Self-healable polymer networks based on the cross-linking of epoxidised soybean oil by an aqueous citric acid solution. Green Chem. 2013, 15, 3360–3366. [Google Scholar] [CrossRef]
- Sacristan, M.; Ronda, J.C.; Galia, M.; Cadiz, V. Silicon-Containing Soybean-Oil-Based Copolymers. Synthesis and Properties. Biomacromolecules 2009, 10, 2678–2685. [Google Scholar] [CrossRef] [PubMed]
- Moreno, M.; Lligadas, G.; Ronda, J.C.; Galia, M.; Cadiz, V. Polyketoesters from oleic acid. Synthesis and functionalization. Green Chem. 2014, 16, 1847–1853. [Google Scholar] [CrossRef]
- Lligadas, G.; Ronda, J.C.; Galia, M.; Biermann, U.; Metzger, J.O. Synthesis and characterization of polyurethanes from epoxidized methyl oleate based polyether polyols as renewable resources. J. Polym. Sci. Part A Polym. Chem. 2006, 44, 634–645. [Google Scholar] [CrossRef]
- Ronda, J.C.; Lligadas, G.; Galia, M.; Cadiz, V. A renewable approach to thermosetting resins. React. Funct. Polym. 2013, 73, 381–395. [Google Scholar] [CrossRef]
- Galia, M.; Montero de Espinosa, L.; Carles Ronda, J.; Lligadas, G.; Cadiz, V. Vegetable oil-based thermosetting polymers. Eur. J. Lipid Sci. Technol. 2010, 112, 87–96. [Google Scholar] [CrossRef]
- Sinadinovic-Fiser, S.; Jankovic, M.; Petrovic, Z.S. Kinetics of in situ epoxidation of soybean oil in bulk catalyzed by ion exchange resin. J. Am. Oil Chem. Soc. 2001, 78, 725–731. [Google Scholar] [CrossRef]
- Findley, T.W.; Swern, D.; Scanlan, J.T. Epoxidation of unsaturated fatty materials with peracetic acid in glacial acetic acid solution. J. Am. Chem. Soc. 1945, 67, 412–414. [Google Scholar] [CrossRef]
- Leveneur, S.; Ledoux, A.; Estel, L.; Taouk, B.; Salmi, T. Epoxidation of vegetable oils under microwave irradiation. Chem. Eng. Res. Des. 2014, 92, 1495–1502. [Google Scholar] [CrossRef]
- Jensen, A.T.; Sayer, C.; Araujo, P.H.H.; Machado, F. Emulsion copolymerization of styrene and acrylated methyl oleate. Eur. J. Lipid Sci. Technol. 2014, 116, 37–43. [Google Scholar] [CrossRef]
- Ferreira, G.R.; Braquehais, J.R.; da Silva, W.N.; Machado, F. Synthesis of soybean oil-based polymer lattices via emulsion polymerization process. Ind. Crops Prod. 2015, 65, 14–20. [Google Scholar] [CrossRef]
- Sinadinovic-Fiser, S.; Jankovic, M.; Borota, O. Epoxidation of castor oil with peracetic acid formed in situ in the presence of an ion exchange resin. Chem. Eng. Process. 2012, 62, 106–113. [Google Scholar] [CrossRef]
- Jankovic, M.R.; Sinadinovic-Fiser, S.V.; Govedarica, O.M. Kinetics of the Epoxidation of Castor Oil with Peracetic Acid Formed in Situ in the Presence of an Ion-Exchange Resin. Ind. Eng. Chem. Res. 2014, 53, 9357–9364. [Google Scholar] [CrossRef]
- Park, S.J.; Jin, F.L.; Lee, J.R. Synthesis and thermal properties of epoxidized vegetable oil. Macromol. Rapid Commun. 2004, 25, 724–727. [Google Scholar] [CrossRef]
- Saithai, P.; Lecomte, J.; Dubreucq, E.; Tanrattanakul, V. Effects of different epoxidation methods of soybean oil on the characteristics of acrylated epoxidized soybean oil-co-poly(methyl methacrylate) copolymer. Express Polym. Lett. 2013, 7, 910–924. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.H.; Wang, D.H.; Sun, X.S. Copolymers from epoxidized soybean oil and lactic acid oligomers for pressure-sensitive adhesives. RSC Adv. 2015, 5, 27256–27265. [Google Scholar] [CrossRef]
- Gobin, M.; Loulergue, P.; Audic, J.L.; Lemiegre, L. Synthesis and characterisation of bio-based polyester materials from vegetable oil and short to long chain dicarboxylic acids. Ind. Crops Prod. 2015, 70, 213–220. [Google Scholar] [CrossRef] [Green Version]
- Mazzon, E.; Habas-Ulloa, A.; Habas, J.P. Lightweight rigid foams from highly reactive epoxy resins derived from vegetable oil for automotive applications. Eur. Polym. J. 2015, 68, 546–557. [Google Scholar] [CrossRef]
- Jensen, A.T.; de Oliveira, A.C.C.; Goncalves, S.B.; Gambetta, R.; Machado, F. Evaluation of the emulsion copolymerization of vinyl pivalate and methacrylated methyl oleate. J. Appl. Polym. Sci. 2016, 133, 44129. [Google Scholar] [CrossRef]
- Costa, C.; Wagner, M.; Musyanovych, A.; Landfester, K.; Sayer, C.; Araujo, P.H.H. Decrease of methyl methacrylate miniemulsion polymerization rate with incorporation of plant oils. Eur. J. Lipid Sci. Technol. 2016, 118, 93–103. [Google Scholar] [CrossRef]
- Erdemir, U.; Sancakli, H.S.; Yaman, B.C.; Ozel, S.; Yucel, T.; Yıldız, E. Clinical comparison of a flowable composite and fissure sealant: A 24-month split-mouth, randomized, and controlled study. J. Dent. 2014, 42, 149–157. [Google Scholar] [CrossRef] [PubMed]
- Finlayson, C.E.; Baumberg, J.J. Generating Bulk-Scale Ordered Optical Materials Using Shear-Assembly in Viscoelastic Media. Materials 2017, 10. [Google Scholar] [CrossRef] [PubMed]
- Schneider, A.; Graillat, C.; Guyot, A.; McKenna, T.F. High solids content emulsions. III. Synthesis of concentrated latices by classic emulsion polymerization. J. Appl. Polym. Sci. 2002, 84, 1916–1934. [Google Scholar] [CrossRef]
- Boutti, S.; Graillat, C.; McKenna, T.F. New routes to high solid content latexes: A process for in situ particle nucleation and growth. Macromol. Symp. 2004, 206, 383–398. [Google Scholar] [CrossRef]
- Vijayendran, B.R. Polymer polarity and surfactant adsorption. J. Appl. Polym. Sci. 1979, 23, 733–742. [Google Scholar] [CrossRef]




| Sample | Name | Monomer (g) | KPS (g·L−1) | |||
|---|---|---|---|---|---|---|
| Methyl Methacrylate | Vinyl Pivalate | Acrylated Fatty Acid | Methacrylic Acid | |||
| 1 | TAFA5_a | 35 | 12.5 | 2.5 | - | 0.50 |
| 2 | TAFA5_b | 35 | 12.5 | 2.5 | - | 0.70 |
| 3 | TAFA5_c | 35 | 12.5 | 2.5 | - | 1.30 |
| 4 | TAFA5_d | 35 | 12.5 | 2.5 | - | 2.20 |
| 5 | TAFA10_a | 35 | 10.0 | 5.0 | - | 0.50 |
| 6 | TAFA10_d | 35 | 10.0 | 5.0 | - | 2.20 |
| 7 | TMA25 | 35 | 2.5 | - | 12.5 | 0.50 |
| 8 | TMA20 | 35 | 5.0 | - | 10.0 | 0.50 |
| 9 | TMA15 | 35 | 7.5 | - | 7.5 | 0.29 |
| 10 | TMA10 | 35 | 10.0 | - | 5.0 | 0.29 |
| 11 | TMA5 | 35 | 12.5 | - | 2.5 | 0.29 |
| 12 | P(MMA-co-MA) | 35 | - | - | 15.0 | 0.50 |
| 13 | P(MMA-co-VPi) | 35 | 15.0 | - | - | 0.50 |
| 14 | PMMA | 50 | - | - | - | 0.29 |
| 15 | PVPi | - | 50.0 | - | - | 0.29 |
| Fatty Acids | Composition (%) | |
| 16:0 | Palmitoleic | 11.30 |
| 18:0 | Estearic | 3.70 |
| 20:0 | Arachidic | 0.70 |
| 22:0 | Benzoic | 0.43 |
| 24:0 | Lignoceric | 0.15 |
| Total of saturated | 16.28 | |
| 18:1 | Oleic | 21.80 |
| 18:2 | Linolenic | 54.56 |
| 18:3 | Linolenic | 6.15 |
| 20:1 | Eicosanoic | 0.20 |
| Total ofunsaturated | 82.71 | |
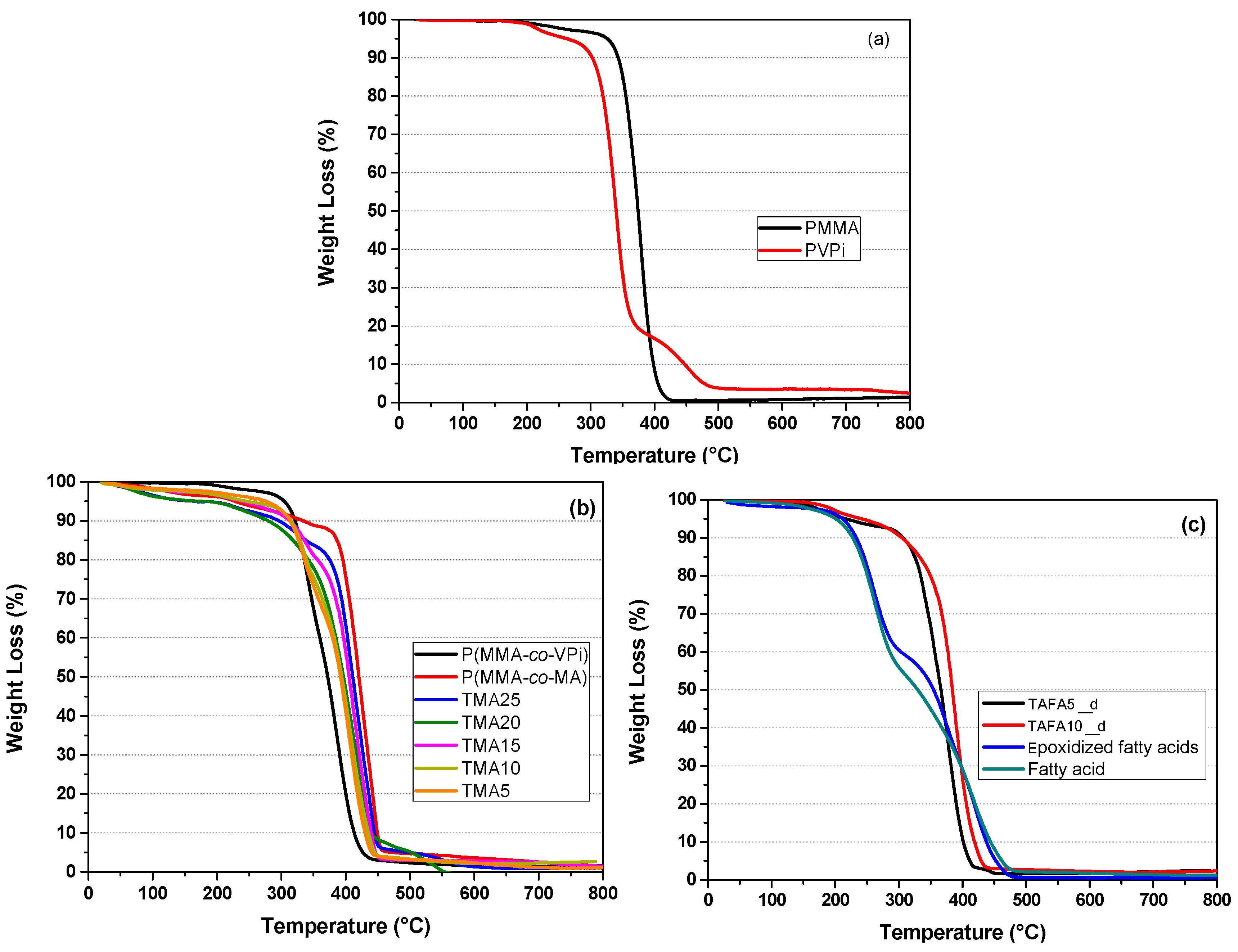
| Sample Name | Monomer Feed Fraction (wt%) | Tg (°C) | |||
|---|---|---|---|---|---|
| Methyl Methacrylate | Vinyl Pivalate | Acrylated Fatty Acid | Methacrylic Acid | ||
| PMMA | 100 | - | - | - | 120 |
| PVPi | - | 100 | - | - | 70 |
| P(MMA-co-MA) | 70 | - | - | 30 | 140 |
| P(MMA-co-VPi) | 70 | 30 | - | 110 | |
| TAFA5_d | 70 | 25 | 5 | - | 90 |
| TAFA10_d | 70 | 20 | 10 | - | 70 |
| TMA25 | 70 | 5 | - | 25 | 145 |
| TMA20 | 70 | 10 | - | 20 | 140 |
| TMA15 | 70 | 15 | - | 15 | 135 |
| TMA10 | 70 | 20 | - | 10 | 130 |
| TMA5 | 70 | 25 | - | 5 | 120 |
| Sample | Mole Fraction (%) | ||
|---|---|---|---|
| MMA | VPi | AFA | |
| TAFA5_d | 86.2 | 12.1 | 1.7 |
| TAFA10_d | 90.5 | 6.3 | 3.2 |
| P(MMA-co-VPi) | 76.9 | 23.1 | ---- |
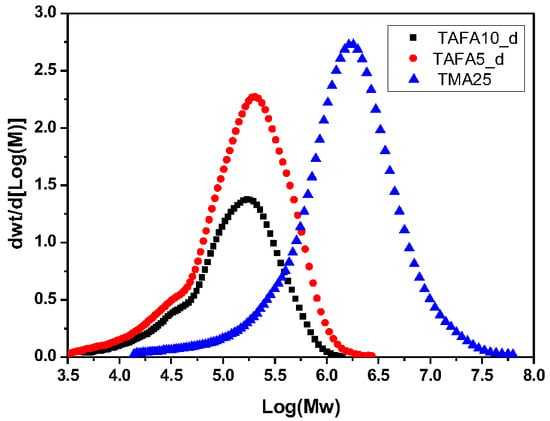
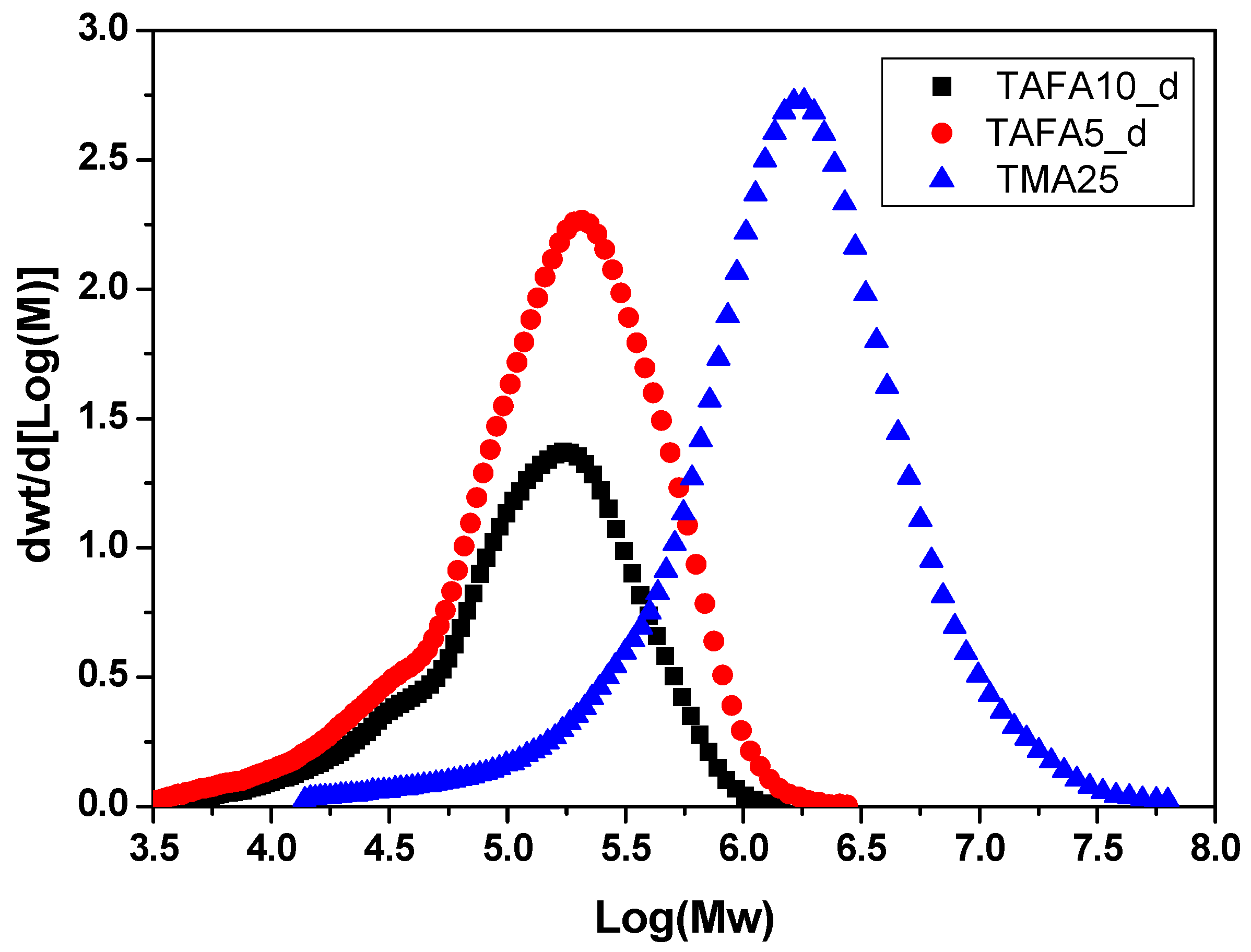
| Sample | Mn (kg∙mol−1) | Mw (kg∙mol−1) | ÐM |
|---|---|---|---|
| TAFA5_d | 62 | 225 | 3.6 |
| TAFA10_d | 61 | 181 | 2.9 |
| TMA25 | 47 | 2685 | 5.6 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Neves, J.S.; Valadares, L.F.; Machado, F. Tailoring Acrylated Soybean Oil-Containing Terpolymers through Emulsion Polymerization. Colloids Interfaces 2018, 2, 46. https://doi.org/10.3390/colloids2040046
Neves JS, Valadares LF, Machado F. Tailoring Acrylated Soybean Oil-Containing Terpolymers through Emulsion Polymerization. Colloids and Interfaces. 2018; 2(4):46. https://doi.org/10.3390/colloids2040046
Chicago/Turabian StyleNeves, Juliete Silva, Leonardo Fonseca Valadares, and Fabricio Machado. 2018. "Tailoring Acrylated Soybean Oil-Containing Terpolymers through Emulsion Polymerization" Colloids and Interfaces 2, no. 4: 46. https://doi.org/10.3390/colloids2040046