Ultrasonic-Assisted Electrodeposition of Mn-Doped NiCo2O4 for Enhanced Photodegradation of Methyl Red, Hydrogen Production, and Supercapacitor Applications
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
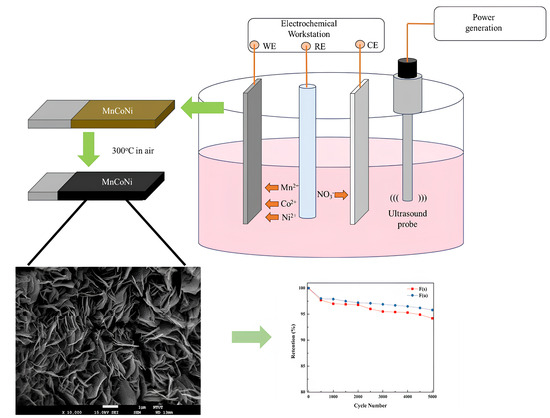
2.2. Preparation of Mn-Doped NiCo2O4 Electrodes
2.3. Characterization of the Mn-Doped NiCo2O4 Electrodes
2.4. Photocatalytic Performance Evaluation of the Mn-Doped NiCo2O4 Electrodes
2.5. Electrochemical Measurements
3. Results and Discussion
3.1. XRD Analysis
3.2. Morphology and XPS Analyses
3.3. UV–Vis Diffuse Reflectance Spectroscopy Analysis
3.4. Porosity and Surface Area Characterization
3.5. Photocatalytic Activity Evaluation
3.6. Electrochemical Performance of the Mn-Doped NiCo2O4 Electrodes
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- El Sharkawy, H.M.; Shawky, A.M.; Elshypany, R.; Selim, H. Efficient photocatalytic degradation of organic pollutants over TiO2 nanoparticles modified with nitrogen and MoS2 under visible light irradiation. Sci. Rep. 2023, 13, 8845. [Google Scholar] [CrossRef]
- Kale, G.; Arbuj, S.; Chothe, U.; Khore, S.; Nikam, L.; Kale, B. Highly Crystalline Ordered Cu-dopedTiO2Nanostructure by Paper Templated Method: Hydrogen Production and Dye Degradation under Natural Sunlight. J. Compos. Sci. 2020, 4, 48. [Google Scholar] [CrossRef]
- Pan, X.; Yang, M.-Q.; Fu, X.; Zhang, N.; Xu, Y.-J. Defective TiO2 with oxygen vacancies: Synthesis, properties and photocatalytic applications. Nanoscale 2013, 5, 3601–3614. [Google Scholar] [CrossRef]
- Bi, X.; Du, G.; Kalam, A.; Sun, D.; Yu, Y.; Su, Q.; Xu, B.; Al-Sehemi, A.G. Tuning oxygen vacancy content in TiO2 nanoparticles to enhance the photocatalytic performance. Chem. Eng. Sci. 2021, 234, 116440. [Google Scholar] [CrossRef]
- Dubey, R.S.; Jadkar, S.R.; Bhorde, A.B. Synthesis and characterization of various doped TiO2 nanocrystals for dye-sensitized solar cells. ACS Omega 2021, 6, 3470–3482. [Google Scholar] [CrossRef]
- Basavarajappa, P.S.; Patil, S.B.; Ganganagappa, N.; Reddy, K.R.; Raghu, A.V.; Reddy, C.V. Recent progress in metal-doped TiO2, non-metal doped/codoped TiO2 and TiO2 nanostructured hybrids for enhanced photocatalysis. Int. J. Hydrog. Energy 2020, 45, 7764–7778. [Google Scholar] [CrossRef]
- Erusappan, E.; Pan, G.-T.; Chung, H.-Y.; Chong, S.; Thiripuranthagan, S.; Yang, T.C.-K.; Huang, C.-M. Hierarchical nickel–cobalt oxide and glucose-based carbon electrodes for asymmetric supercapacitor with high energy density. J. Taiwan Inst. Chem. Eng. 2020, 112, 330–336. [Google Scholar] [CrossRef]
- Herrmann, J.-M.; Tahiri, H.; Ait-Ichou, Y.; Lassaletta, G.; Gonzalez-Elipe, A.; Fernandez, A. Characterization and photocatalytic activity in aqueous medium of TiO2 and Ag-TiO2 coatings on quartz. Appl. Catal. B Environ. 1997, 13, 219–228. [Google Scholar] [CrossRef]
- Moon, J.; Takagi, H.; Fujishiro, Y.; Awano, M. Preparation and characterization of the Sb-doped TiO2 photocatalysts. J. Mater. Sci. 2001, 36, 949–955. [Google Scholar] [CrossRef]
- Anpo, M.; Takeuchi, M. The design and development of highly reactive titanium oxide photocatalysts operating under visible light irradiation. J. Catal. 2003, 216, 505–516. [Google Scholar] [CrossRef]
- Umebayashi, T.; Yamaki, T.; Yamamoto, S.; Miyashita, A.; Tanaka, S.; Sumita, T.; Asai, K. Sulfur-doping of rutile-titanium dioxide by ion implantation: Photocurrent spectroscopy and first-principles band calculation studies. J. Appl. Phys. 2003, 93, 5156–5160. [Google Scholar] [CrossRef]
- Cui, B.; Lin, H.; Liu, Y.Z.; Li, J.B.; Sun, P.; Zhao, X.C.; Liu, C.J. Photophysical and photocatalytic properties of core-ring structured NiCo2O4 nanoplatelets. J. Phys. Chem. C 2009, 113, 14083–14087. [Google Scholar] [CrossRef]
- Wan, Y.; Chen, J.; Zhan, J.; Ma, Y. Facile synthesis of mesoporous NiCo2O4 fibers with enhanced photocatalytic performance for the degradation of methyl red under visible light irradiation. J. Environ. Chem. Eng. 2018, 6, 6079–6087. [Google Scholar] [CrossRef]
- Gaim, Y.T.; Yimanuh, S.M.; Kidanu, Z.G. Enhanced photocatalytic degradation of amoxicillin with MN-doped Cu2O under sunlight irradiation. J. Compos. Sci. 2022, 6, 317. [Google Scholar] [CrossRef]
- Omarova, A.Z.; Ayazbaev, T.; Yesdauletova, Z.S.; Aldabergen, S.A.; Kozlovskiy, A.L.; Moldabayeva, G.Z. Evaluation of the Applicability of Modifying CdSe Thin Films by the Addition of Cobalt and Nickel to Enhance the Efficiency of Photocatalytic Decomposition of Organic Dyes. J. Compos. Sci. 2023, 7, 460. [Google Scholar] [CrossRef]
- Han, X.; Song, L.; Ding, J.; Hu, L.; Xu, C.; Wang, Y. Design and preparation of Cu-doped NiCo2O4 nanosheets with intrinsic porosities for symmetric supercapacitors. Mater. Lett. 2020, 278, 128400. [Google Scholar] [CrossRef]
- Van Nguyen, T.; Van Thuy, V.; Thao, V.D.; Hatsukano, M.; Higashimine, K.; Maenosono, S.; Chun, S.E.; Thu, T.V. Facile synthesis of Mn-doped NiCo2O4 nanoparticles with enhanced electrochemical performance for a battery-type supercapacitor electrode. Dalton Trans. 2020, 49, 6718–6729. [Google Scholar] [CrossRef]
- Pradeepa, S.; Rajkumar, P.; Diwakar, K.; Sutharthani, K.; Subadevi, R.; Sivakumar, M. A Facile One-Pot Hydrothermal Synthesis of Zn, Mn Co-Doped NiCo2O4 as an Efficient Electrode for Supercapacitor Applications. Chem. Sel. 2021, 6, 6851–6862. [Google Scholar] [CrossRef]
- Liang, Z.; Tu, H.; Kong, Z.; Yao, X.; Xu, D.; Liu, S.; Shao, Y.; Wu, Y.; Hao, X. Urchin like inverse spinel manganese doped NiCo2O4 microspheres as high performances anode for lithium-ion batteries. J. Colloid Interface Sci. 2022, 616, 509–519. [Google Scholar] [CrossRef]
- Hong, Z.-Y.; Chen, L.-C.; Li, Y.-C.M.; Hsu, H.-L.; Huang, C.-M. Response Surface Methodology Optimization in High-Performance Solid-State Supercapattery Cells Using NiCo2S4–Graphene Hybrids. Molecules 2022, 27, 6867. [Google Scholar] [CrossRef]
- Hsieh, M.-C.; Chen, B.-H.; Hong, Z.-Y.; Liu, J.-K.; Huang, P.-C.; Huang, C.-M. Fabrication of 5 V High-Performance Solid-State Asymmetric Supercapacitor Device Based on MnO2/Graphene/Ni Electrodes. Catalysts 2022, 12, 572. [Google Scholar] [CrossRef]
- Ismail, M.M.; Hong, Z.-Y.; Arivanandhan, M.; Yang, T.C.-K.; Pan, G.-T.; Huang, C.-M. In Situ binder-free and hydrothermal growth of nanostructured NiCo2S4/Ni electrodes for solid-state hybrid supercapacitors. Energies 2021, 14, 7114. [Google Scholar] [CrossRef]
- MacDonald, M.; Zhitomirsky, I. Capacitive Properties of Ferrimagnetic NiFe2O4-Conductive Polypyrrole Nanocomposites. J. Compos. Sci. 2024, 8, 51. [Google Scholar] [CrossRef]
- Devendra, B.K.; Praveen, B.; Tripathi, V.; Nagaraju, G.; Prasanna, B.; Shashank, M. Development of rhodium coatings by electrodeposition for photocatalytic dye degradation. Vacuum 2022, 205, 111460. [Google Scholar] [CrossRef]
- Pandiyarajan, S.; Ganesan, M.; Liao, A.-H.; Manickaraj, S.S.M.; Huang, S.-T.; Chuang, H.-C. Ultrasonic-assisted supercritical-CO2 electrodeposition of Zn-Co film for high-performance corrosion inhibition: A greener approach. Ultrason. Sonochem. 2021, 72, 105463. [Google Scholar] [CrossRef]
- Hajnorouzi, A.; Modaresi, N. Direct sono electrochemical method for synthesizing Fe3O4 nanoparticles. J. Magn. Magn. Mater. 2020, 505, 166732. [Google Scholar] [CrossRef]
- Zhang, H.; Wang, J.; Chen, S.; Wang, H.; He, Y.; Ma, C. Ni–SiC composite coatings with improved wear and corrosion resistance synthesized via ultrasonic electrodeposition. Ceram. Int. 2021, 47, 9437–9446. [Google Scholar] [CrossRef]
- Mladenović, I.; Lamovec, J.; Radović, D.V.; Radojević, V.; Nikolić, N.D. Influence of intensity of ultrasound on morphology and hardness of copper coatings obtained by electrodeposition. J. Electrochem. Sci. Eng. 2022, 12, 603–615. [Google Scholar] [CrossRef]
- Costa, J.M.; de Almeida Neto, A.F. Ultrasound-assisted electrodeposition and synthesis of alloys and composite materials: A review. Ultrason. Sonochem. 2020, 68, 105193. [Google Scholar] [CrossRef]
- Duan, B.; Zhu, Z.; Sun, C.; Zhou, J.; Walsh, A. Preparing copper catalyst by ultrasound-assisted chemical precipitation method. Ultrason. Sonochem. 2020, 64, 105013. [Google Scholar]
- Bengoa, L.N.; Ispas, A.; Bengoa, J.F.; Bund, A.; Egli, W.A. Ultrasound assisted electrodeposition of Cu-SiO2 composite coatings: Effect of particle surface chemistry. J. Electrochem. Soc. 2019, 166, D244. [Google Scholar] [CrossRef]
- Zhang, H.; Wang, J.; Duan, H.; Ren, J.; Zhao, H.; Zhou, C.; Qi, J. Mn3+ partially substituting the Ni3+ of NiCo2O4 enhance the charge transfer kinetics and reaction activity for hybrid supercapacitor. Appl. Surf. Sci. 2022, 597, 153617. [Google Scholar] [CrossRef]
- Ádám, A.A.; Szabados, M.; Varga, G.; Papp, B.; Musza, K.; Kónya, Z.; Kukovecz, A.; Sipos, P.; Pálinkó, I. Ultrasound-assisted hydrazine reduction method for the preparation of nickel nanoparticles, physicochemical characterization and catalytic application in Suzuki-Miyaura cross-coupling reaction. Nanomaterials 2020, 10, 632. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Mei, T.; Chu, H.; Wang, J.; Du, S.; Miao, Y.; Zhang, W. Ultrasonic-assisted electrodeposition of Ni/diamond composite coatings and its structure and electrochemical properties. Ultrason. Sonochem. 2021, 73, 105475. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.; Wu, Q.; Wu, J.; Kou, H.; Zhu, Y.; Ning, C.; Dai, K. Ultrasound-assisted synthesis of nanocrystallized silicocarnotite biomaterial with improved sinterability and osteogenic activity. J. Mater. Chem. B 2020, 8, 3092–3103. [Google Scholar] [CrossRef] [PubMed]
- Min, J.W.; Yim, C.J.; Im, W.B. Preparation and electrochemical characterization of flower-like Li1.2Ni0.17Co0.17Mn0.5O2 microstructure cathode by electrospinning. Ceram. Int. 2014, 40, 2029–2034. [Google Scholar] [CrossRef]
- Ding, Q.; Dou, Y.; Liao, Y.; Huang, S.; Wang, R.; Min, W.; Chen, X.; Wu, C.; Yuan, D.; Liu, H.K.; et al. Oxygen Vacancy-Rich Ultrathin Co3O4 Nanosheets as Nanofillers in Solid-Polymer Electrolyte for High-Performance Lithium Metal Batteries. Catalysts 2023, 13, 711. [Google Scholar] [CrossRef]
- Liu, G.; Liu, Y.; Luo, J.; Wang, Y.; He, J.; Shi, X.; Nie, Z.; Lu, X. Surface Corrosion Engineering Endows Mn-Doped NiCo2O4 Nanosheets with High Capacity and Long Life for Alkaline Zn-Based Batteries. ACS Appl. Energy Mater. 2024, 7, 2234–2240. [Google Scholar] [CrossRef]
- Xing, Z.; Ju, Z.; Zhao, Y.; Wan, J.; Zhu, Y.; Qiang, Y.; Qian, Y. One-pot hydrothermal synthesis of Nitrogen-doped graphene as high-performance anode materials for lithium ion batteries. Sci. Rep. 2016, 6, 26146. [Google Scholar] [CrossRef]
- Dillinger, B.; Suchicital, C.; Clark, D. The effects of ultrasonic cavitation on the dissolution of lithium disilicate glass. Sci. Rep. 2022, 12, 20398. [Google Scholar] [CrossRef]
- Salam, M.A.; Imdadulhaq, E.S.; Al-Romaizan, A.N.; Saleh, T.S.; Mostafa, M.M.M. Ultrasound-Assisted 1, 3-Dipolar Cycloadditions Reaction Utilizing Ni-Mg-Fe LDH: A Green and Sustainable Perspective. Catalysts 2023, 13, 650. [Google Scholar] [CrossRef]
- Tsai, Y.-C.; Yang, W.-D.; Lee, K.-C.; Huang, C.-M. An effective electrodeposition mode for porous MnO2/Ni foam composite for asymmetric supercapacitors. Materials 2016, 9, 246. [Google Scholar] [CrossRef] [PubMed]
- Vazhayil, A.; Thomas, J.; Thomas, T.; Hasan, I.; Thomas, N. Mn-substituted NiCo2O4/rGO composite electrode for supercapacitors and their electrochemical performance boost by redox additive in alkaline electrolyte. J. Energy Storage 2024, 84, 110789. [Google Scholar]
- Raman, V.; Mohan, N.V.; Balakrishnan, B.; Rajmohan, R.; Rajangam, V.; Selvaraj, A.; Kim, H.J. Porous shiitake mushroom carbon composite with NiCo2O4 nanorod electrochemical characteristics for efficient supercapacitor applications. Ionics 2020, 26, 345–354. [Google Scholar] [CrossRef]















| Samples | Mole Ratios of Ni:Co:Mn | pH Value | Ultrasound Power (W) | Annealed Temperature (°C) |
|---|---|---|---|---|
| A(u) | 1:1:0.0 | 7 | - | 500 |
| B(u) | 1:1:0.2 | 7 | - | 500 |
| C(u) | 1:1:0.4 | 7 | - | 500 |
| D(u) | 1:1:0.6 | 7 | - | 500 |
| E(u) | 1:1:0.8 | 7 | - | 500 |
| F(u) | 1:1:1.0 | 7 | - | 500 |
| A(s) | 1:1:0.0 | 7 | 100 | 500 |
| B(s) | 1:1:0.2 | 7 | 100 | 500 |
| C(s) | 1:1:0.4 | 7 | 100 | 500 |
| D(s) | 1:1:0.6 | 7 | 100 | 500 |
| E(s) | 1:1:0.8 | 7 | 100 | 500 |
| F(s) | 1:1:1.0 | 7 | 100 | 500 |
| Samples | Particle Size (nm) |
|---|---|
| A(u) | 16.92 |
| B(u) | 16.11 |
| C(u) | 15.85 |
| D(u) | 15.21 |
| E(u) | 14.57 |
| F(u) | 14.13 |
| A(s) | 15.23 |
| B(s) | 14.50 |
| C(s) | 14.31 |
| D(s) | 13.62 |
| E(s) | 13.11 |
| F(s) | 12.75 |
| Sample | Mole Ratios of Ni:Co:Mn | Atomic Ratios of Ni:Co:O:Mn |
|---|---|---|
| A(u) | 1:1:0.0 | 1:3.88:5.55:0.000 |
| B(u) | 1:1:0.2 | 1:3.91:5.45:0.005 |
| C(u) | 1:1:0.4 | 1:3.96:5.31:0.007 |
| D(u) | 1:1:0.6 | 1:4.05:5.21:0.009 |
| E(u) | 1:1:0.8 | 1:4.11:5.12:0.012 |
| F(u) | 1:1:1.0 | 1:4.21:5.10:0.015 |
| A(s) | 1:1:0.0 | 1:3.90:5.53:0.000 |
| B(s) | 1:1:0.2 | 1:3.93:5.49:0.005 |
| C(s) | 1:1:0.4 | 1:4.01:5.35:0.008 |
| D(s) | 1:1:0.6 | 1:4.07:5.21:0.010 |
| E(s) | 1:1:0.8 | 1:4.13:5.15:0.013 |
| F(s) | 1:1:1.0 | 1:4.19:5.09:0.015 |
| Samples | Surface Area (m²/g) | Pore Volume (cm³/g) | Pore Size (Å) |
|---|---|---|---|
| A(u) | 65.11 | 0.49 | 120 |
| B(u) | 70.32 | 0.51 | 135 |
| C(u) | 80.56 | 0.62 | 146 |
| D(u) | 95.35 | 0.69 | 150 |
| E(u) | 102.36 | 0.75 | 155 |
| F(u) | 112.45 | 0.82 | 157 |
| A(s) | 84.12 | 0.56 | 145 |
| B(s) | 90.23 | 0.62 | 153 |
| C(s) | 98.54 | 0.73 | 162 |
| D(s) | 112.35 | 0.78 | 166 |
| E(s) | 126.54 | 0.82 | 170 |
| F(s) | 130.21 | 0.91 | 173 |
| Samples | First-Order Rate Constant (min−1) |
|---|---|
| A(u) | 0.0016 |
| B(u) | 0.0023 |
| C(u) | 0.0030 |
| D(u) | 0.0037 |
| E(u) | 0.0048 |
| F(u) | 0.0055 |
| A(s) | 0.0018 |
| B(s) | 0.0025 |
| C(s) | 0.0035 |
| D(s) | 0.0041 |
| E(s) | 0.0054 |
| F(s) | 0.0063 |
| Samples | Scan Rate (V/s) | Capacity (F/g) | Intrinsic Resistance of Electrode (Ω) | Retention Rate after 5000 Cycles Test |
|---|---|---|---|---|
| F(u) | 0.05 | 192 | 1.3 | 94% |
| F(s) | 0.05 | 260 | 0.8 | 96% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, K.-C.; Tiong, T.J.; Pan, G.-T.; Yang, T.C.-K.; Uma, K.; Tseng, Z.-L.; Nikoloski, A.N.; Huang, C.-M. Ultrasonic-Assisted Electrodeposition of Mn-Doped NiCo2O4 for Enhanced Photodegradation of Methyl Red, Hydrogen Production, and Supercapacitor Applications. J. Compos. Sci. 2024, 8, 164. https://doi.org/10.3390/jcs8050164
Lee K-C, Tiong TJ, Pan G-T, Yang TC-K, Uma K, Tseng Z-L, Nikoloski AN, Huang C-M. Ultrasonic-Assisted Electrodeposition of Mn-Doped NiCo2O4 for Enhanced Photodegradation of Methyl Red, Hydrogen Production, and Supercapacitor Applications. Journal of Composites Science. 2024; 8(5):164. https://doi.org/10.3390/jcs8050164
Chicago/Turabian StyleLee, Kuan-Ching, Timm Joyce Tiong, Guan-Ting Pan, Thomas Chung-Kuang Yang, Kasimayan Uma, Zong-Liang Tseng, Aleksandar N. Nikoloski, and Chao-Ming Huang. 2024. "Ultrasonic-Assisted Electrodeposition of Mn-Doped NiCo2O4 for Enhanced Photodegradation of Methyl Red, Hydrogen Production, and Supercapacitor Applications" Journal of Composites Science 8, no. 5: 164. https://doi.org/10.3390/jcs8050164