CoCuMgAl-Mixed-Oxide-Based Catalysts with Fine-Tunable Composition for the Hydrogenation of Furan Compounds
Abstract
:1. Introduction
2. Materials and Methods
2.1. Catalyst Preparation
2.2. Structural Characterization of the Catalysts
2.3. Catalyst Testing
3. Results and Discussion
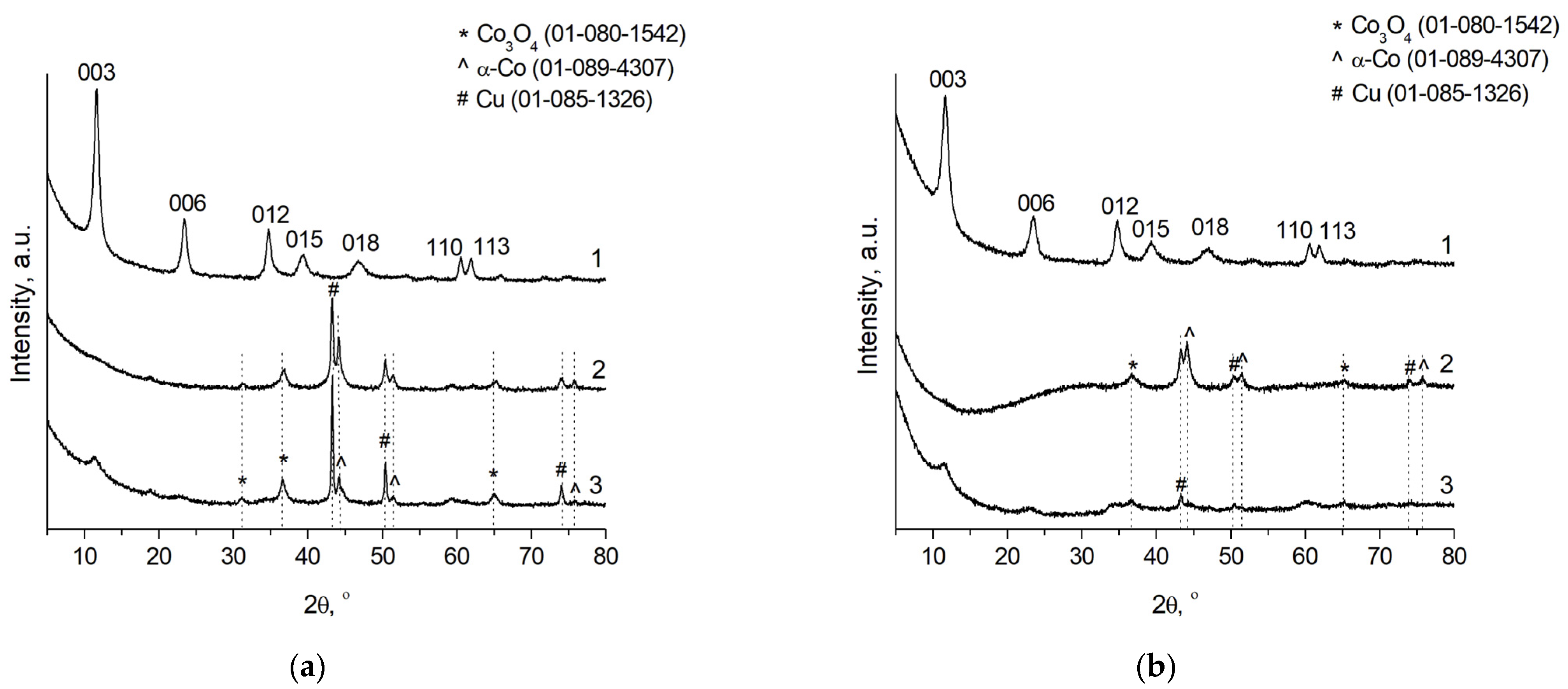
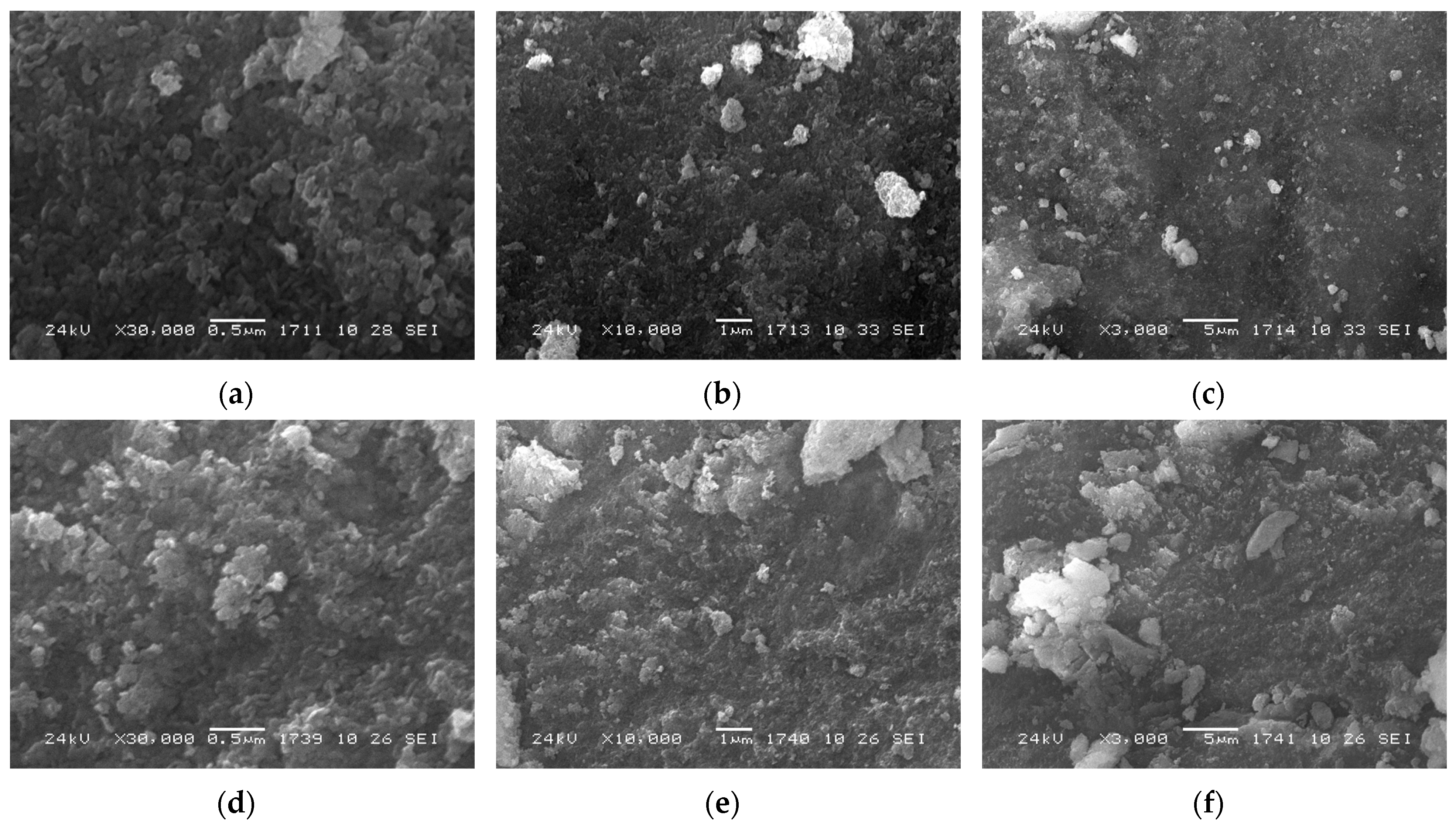
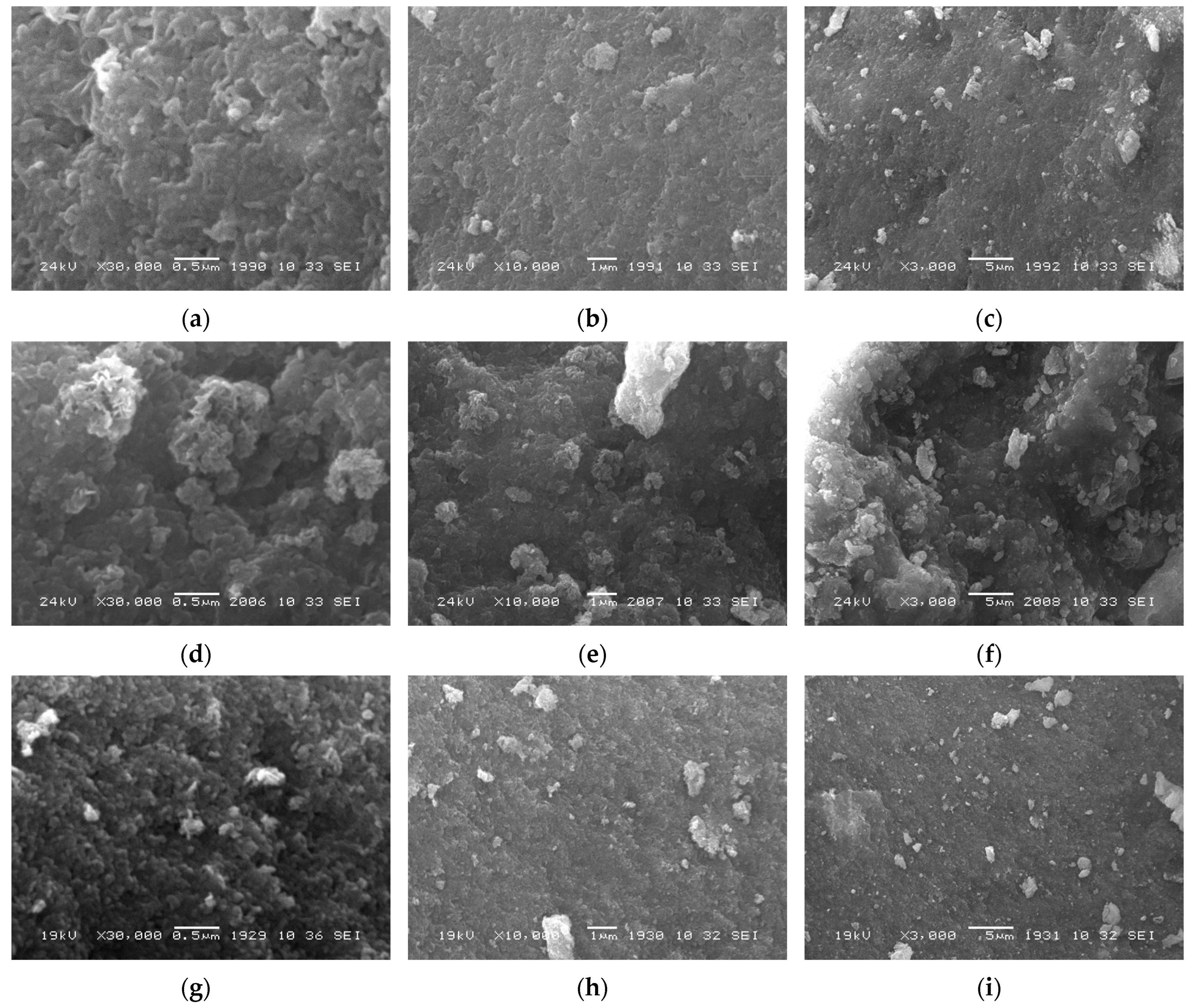
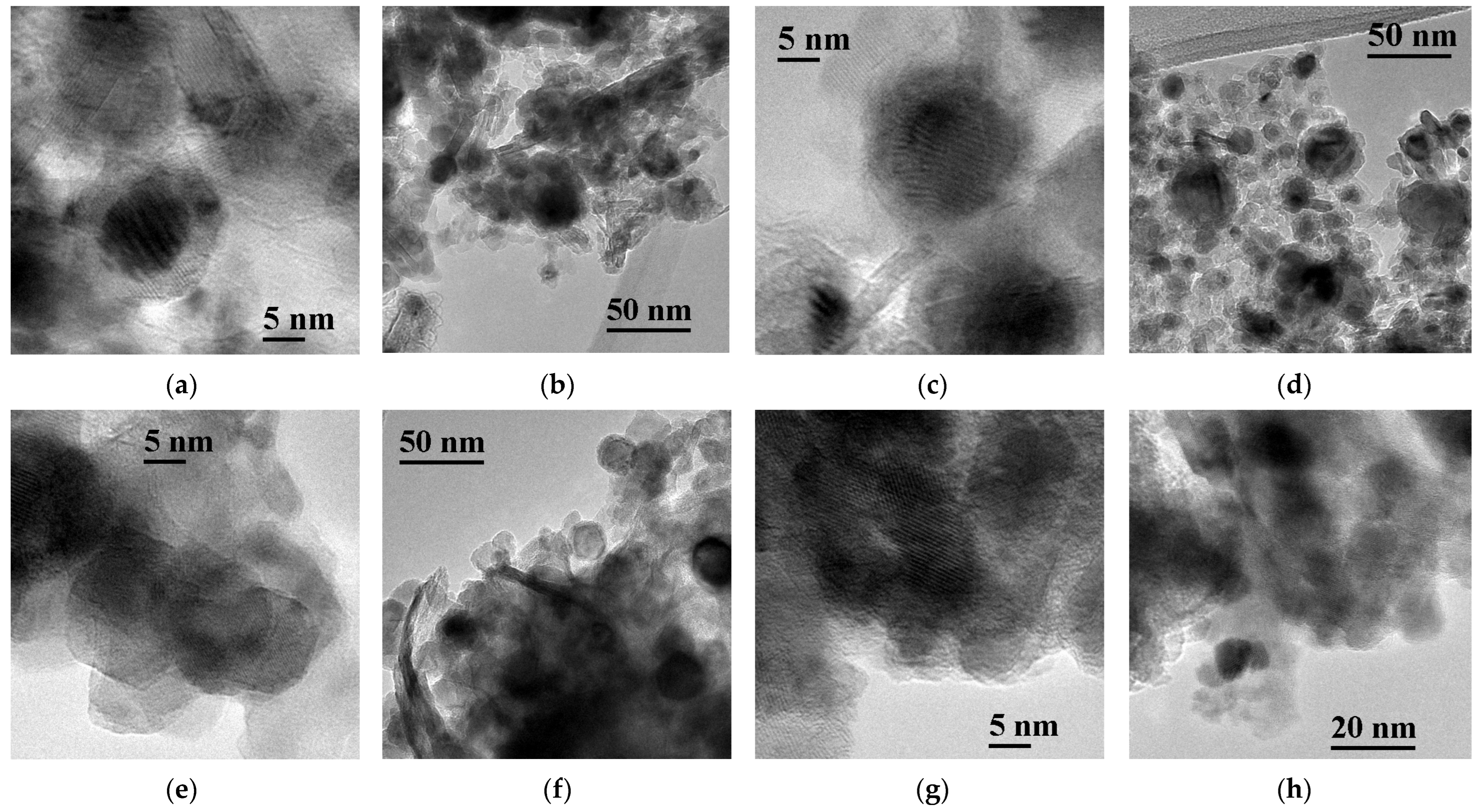
3.1. Structure and Morphology of the Catalysts Based on CoCuMgAl-LHs
3.2. Performance of the CoCuMgAl-Mixed Oxide Catalysts in Hydrogenations of FAL and 5-HMF
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Amjith, L.R.; Bavanish, B. A review on biomass and wind as renewable energy for sustainable environment. Chemosphere 2022, 293, 133579. [Google Scholar] [CrossRef]
- Sun, Y.; Wang, Z.; Liu, Y.; Meng, X.; Qu, J.; Liu, C.; Qu, B. A Review on the transformation of furfural residue for value-added products. Energies 2020, 13, 21. [Google Scholar] [CrossRef]
- Jiang, Z.; Zeng, Y.; Hu, D.; Guo, R.; Yan, K.; Luque, R. Chemical transformations of 5-hydroxymethylfurfural into highly added value products: Present and future. Green Chem. 2023, 25, 871–892. [Google Scholar] [CrossRef]
- Wang, Y.; Zhao, D.; Rodríguez-Padrón, D.; Len, C. Recent advances in catalytic hydrogenation of furfural. Catalysts 2019, 9, 796. [Google Scholar] [CrossRef]
- Endot, N.A.; Junid, R.; Jamil, M.S.S. Insight into biomass upgrade: A review on hydrogenation of 5-hydroxymethylfurfural (HMF) to 2,5-dimethylfuran (DMF). Molecules 2021, 26, 6848. [Google Scholar] [CrossRef] [PubMed]
- Iroegbu, A.O.; Hlangothi, S.P. Furfuryl alcohol a versatile, eco-sustainable compound in perspective. Chem. Afr. 2019, 2, 223–239. [Google Scholar] [CrossRef]
- Zhao, W.; Wang, F.; Zhao, K.; Liu, X.; Zhu, X.; Yan, L.; Yin, Y.; Xu, Q.; Yin, D. Recent advances in the catalytic production of bio-based diol 2,5-bis (hydroxymethyl)furan. Carbon Resour. Convers. 2023, 6, 116–131. [Google Scholar] [CrossRef]
- Gupta, K.; Rai, R.K.; Singh, S.K. Metal catalysts for the efficient transformation of biomass-derived HMF and furfural to value added chemicals. ChemCatChem 2018, 10, 2326–2349. [Google Scholar] [CrossRef]
- Zhang, M.; Yang, J.-H. Selective hydrogenation of furfural: Pure silica supported metal catalysts. Chem. Sel. 2022, 7, e202200013. [Google Scholar] [CrossRef]
- Esteves, L.M.; Brijaldo, M.H.; Oliveira, E.G.; Martinez, J.J.; Rojas, H.; Caytuero, A.; Passos, F.B. Effect of support on selective 5-hydroxymethylfurfural hydrogenation towards 2,5-dimethylfuran over copper catalysts. Fuel 2020, 270, 117524. [Google Scholar] [CrossRef]
- Fan, G.; Li, G.; Evans, D.G.; Duan, X. Catalytic applications of layered double hydroxides: Recent advances and perspectives. Chem. Soc. Rev. 2014, 43, 7040–7066. [Google Scholar] [CrossRef]
- Thongratkaew, S.; Luadthong, C.; Kiatphuengporn, S.; Khemthong, P.; Hirunsit, P.; Faungnawakij, K. Cu-Al spinel-oxide catalysts for selective hydrogenation of furfural to furfuryl alcohol. Catal. Today 2021, 367, 177–188. [Google Scholar] [CrossRef]
- Fu, X.; Ren, X.; Shen, J.; Jiang, Y.; Wang, Y.; Orooji, Y.; Xu, W.; Liang, J. Synergistic catalytic hydrogenation of furfural to 1,2-pentanediol and 1,5-pentanediol with LDO derived from CuMgAl hydrotalcite. Mol. Catal. 2021, 499, 111298. [Google Scholar] [CrossRef]
- Hai, X.; Tan, J.; He, J.; Yang, X.; Na, Y.; Wang, Y.; Zhao, Y. Hydrogenation of furfural to 1,5-pentanediol over CuCo bimetallic catalysts. J. Fuel Chem. Technol. 2023, 51, 959–969. [Google Scholar] [CrossRef]
- Villaverde, M.M.; Garetto, T.F.; Marchi, A.J. Liquid-phase transfer hydrogenation of furfural to furfuryl alcohol on Cu–Mg–Al catalysts. Catal. Commun. 2015, 58, 6–10. [Google Scholar] [CrossRef]
- Li, L.; Cheng, M.; Qin, L.; Almatrafi, E.; Yang, X.; Yang, L.; Tang, C.; Liu, S.; Yi, H.; Zhang, M.; et al. Enhancing hydrogen peroxide activation of Cusingle bondCo layered double hydroxide by compositing with biochar: Performance and mechanism. Sci. Total Environ. 2022, 828, 154188. [Google Scholar] [CrossRef] [PubMed]
- George, A.; Kundu, M. Exchanging anion in CuCo─carbonate double hydroxide for faradaic supercapacitors: A case study. ACS Omega 2023, 8, 17028–17042. [Google Scholar] [CrossRef] [PubMed]
- Cao, A.; Liu, G.; Yue, Y.; Zhang, L.; Liu, Y. Nanoparticles of Cu–Co alloy derived from layered double hydroxides and their catalytic performance for higher alcohol synthesis from syngas. RSC Adv. 2015, 5, 58804–58812. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, Y.; Shi, H.; Wang, M.; Cheng, S.H.-S.; Bian, H.; Kamruzzaman, M.; Cao, L.; Chung, C.Y.; Lu, Z. Cobalt-copper layered double hydroxide nanosheets as high performance bifunctional catalysts for rechargeable lithium-air batteries. J. Alloys Compd. 2016, 688, 380–387. [Google Scholar] [CrossRef]
- Pan, K.; Yu, F.; Liu, Z.; Zhou, X.; Sun, R.; Li, W.; Zhao, H.; Liu, M.; Guo, X.; Dai, B. Enhanced low-temperature CO-SCR denitration performance and mechanism of two-dimensional CuCoAl layered double oxide. J. Environ. Chem. Eng. 2022, 10, 108030. [Google Scholar] [CrossRef]
- Stepanova, L.N.; Mironenko, R.M.; Kobzar, E.O.; Leont’eva, N.N.; Gulyaeva, T.I.; Vasilevich, A.V.; Serkova, A.N.; Salanov, A.N.; Lavrenov, A.V. Synthesis of CuAl-, CoAl-, and CuCoAl-catalysts from layered hydroxides for furfural hydrogenation. Eng 2022, 3, 400–411. [Google Scholar] [CrossRef]
- Kobzar, E.O.; Stepanova, L.N.; Nepomniashchii, A.A.; Vasilevich, A.V.; Gulyaeva, T.I.; Trenikhin, M.V.; Lavrenov, A.V. CuCoMgAlOx mixed oxides as selective catalysts for the hydrogenation of furan compounds. Hydrogen 2023, 4, 644–657. [Google Scholar] [CrossRef]
- Bennett, E.; Wilson, T.; Murphy, P.J.; Refson, K.; Hannon, A.C.; Imberti, S.; Callear, S.K.; Chass, G.A.; Parker, S.F. How the surface structure determines the properties of CuH. Inorg. Chem. 2015, 54, 2213–2220. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Wang, H.; Li, W.; Wu, X.; Tang, W.; Chen, Y. Effect of Cu substitution on promoted benzene oxidation over porous CuCo-based catalysts derived from layered double hydroxide with resistance of water vapor. Appl. Catal. B. 2015, 166–167, 260–269. [Google Scholar] [CrossRef]
- Rives, V.; Dubey, A.; Kannan, S. Synthesis, characterization and catalytic hydroxylation of phenol over CuCoAl ternary hydrotalcites. Phys. Chem. Chem. Phys. 2001, 3, 4826–4836. [Google Scholar] [CrossRef]
- Ribet, S.; Tichit, D.; Coq, B.; Ducourant, B.; Morato, F. Synthesis and activation of Co–Mg–Al layered double hydroxides. J. Solid State Chem. 1999, 142, 382–392. [Google Scholar] [CrossRef]
- Chen, S.; Wojcieszak, R.; Dumeignil, F.; Marceau, E.; Royer, S. How catalysts and experimental conditions determine the selective hydroconversion of furfural and 5-hydroxymethylfurfural. Chem. Rev. 2018, 118, 11023–11117. [Google Scholar] [CrossRef] [PubMed]

| No. | Co/Cu Molar Ratio | Sample | Preparation Conditions |
|---|---|---|---|
| 1 | 1 | CoCuMgAl-LH | Coprecipitation from an aqueous solution of Co(NO3)2, Cu(NO3)2, Mg(NO3)2, and Al(NO3)3 with Na2CO3 |
| 2 | 2 | ||
| 3 | 1 | CoCuMgAlOx | Calcination of CoCuMgAl-LH at 550 °C |
| 4 | 2 | ||
| 5 | 1 | CoCuMgAlOx-R | Calcination of CoCuMgAl-LH at 550 °C and subsequent reductive treatment of CoCuMgAlOx with H2 at 800 °C |
| 6 | 2 |
| Sample | Co/Cu | Phase Composition |
|---|---|---|
| CoCuMgAl-LH | 1 | hydrotalcite-type phase |
| 2 | ||
| CoCuMgAlOx-R | 1 | Co3O4, α-Co, Cu |
| 2 | ||
| CoCuMgAlOx-R after the FAL hydrogenation | 1 | Co3O4, α-Co, Cu |
| 2 | X-ray amorphous |
| Co/Cu Molar Ratio | Reaction Temperature and Total Pressure a | FAL Conversion, mol.% | Selectivity to FOL, mol.% |
|---|---|---|---|
| 1 | 90 °C, 20 bar | 10 | 95 b |
| 1 | 150 °C, 30 bar | 71 | 82 c |
| 2 | 90 °C, 20 bar | 21 | >99 |
| 2 | 150 °C, 30 bar | 77 | 86 c |
| Co/Cu Molar Ratio | Reaction Temperature and Total Pressure a | 5-HMF Conversion, mol.% | Selectivity to BHMF, mol.% b |
|---|---|---|---|
| 1 | 90 °C, 20 bar | 13 | 59 |
| 1 | 150 °C, 30 bar | 27 | 63 |
| 2 | 90 °C, 20 bar | 7 | 75 |
| 2 | 150 °C, 30 bar | 9 | 61 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stepanova, L.N.; Mironenko, R.M.; Trenikhin, M.V.; Serkova, A.N.; Salanov, A.N.; Lavrenov, A.V. CoCuMgAl-Mixed-Oxide-Based Catalysts with Fine-Tunable Composition for the Hydrogenation of Furan Compounds. J. Compos. Sci. 2024, 8, 57. https://doi.org/10.3390/jcs8020057
Stepanova LN, Mironenko RM, Trenikhin MV, Serkova AN, Salanov AN, Lavrenov AV. CoCuMgAl-Mixed-Oxide-Based Catalysts with Fine-Tunable Composition for the Hydrogenation of Furan Compounds. Journal of Composites Science. 2024; 8(2):57. https://doi.org/10.3390/jcs8020057
Chicago/Turabian StyleStepanova, Liudmila N., Roman M. Mironenko, Mikhail V. Trenikhin, Aleksandra N. Serkova, Aleksei N. Salanov, and Aleksandr V. Lavrenov. 2024. "CoCuMgAl-Mixed-Oxide-Based Catalysts with Fine-Tunable Composition for the Hydrogenation of Furan Compounds" Journal of Composites Science 8, no. 2: 57. https://doi.org/10.3390/jcs8020057






