Morphological Effect on the Surface Activity and Hydrogen Evolution Catalytic Performance of Cu2O and Cu2O/rGO Composites
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Cu2O Microparticle Synthesis
2.3. rGO Synthesis
2.4. Cu2O@rGO Composites Synthesis
2.5. Characterization Techniques
2.6. Hydrogen Evolution Electrocatalysis
2.7. Compactional Details
3. Results and Discussion
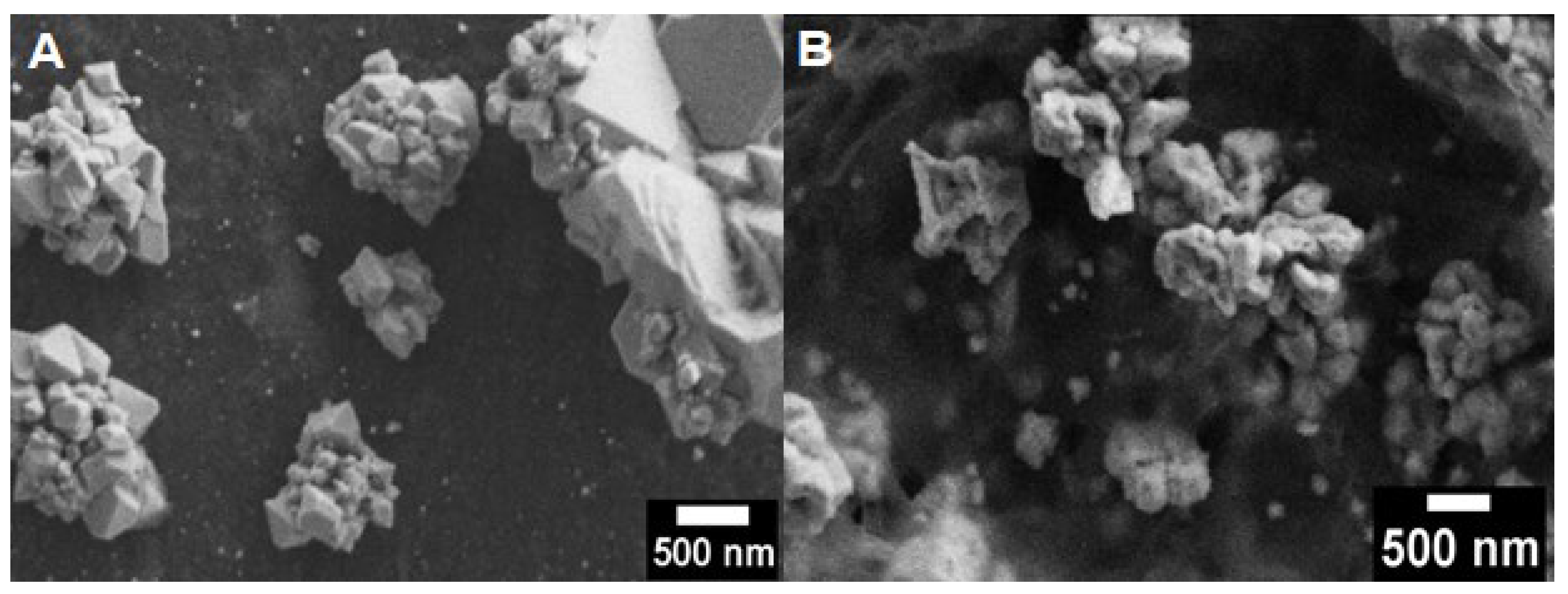
3.1. Morphology of Cu2O Particles and Cu2O@rGO
3.2. Morphology of Cu2O@rGO Composites
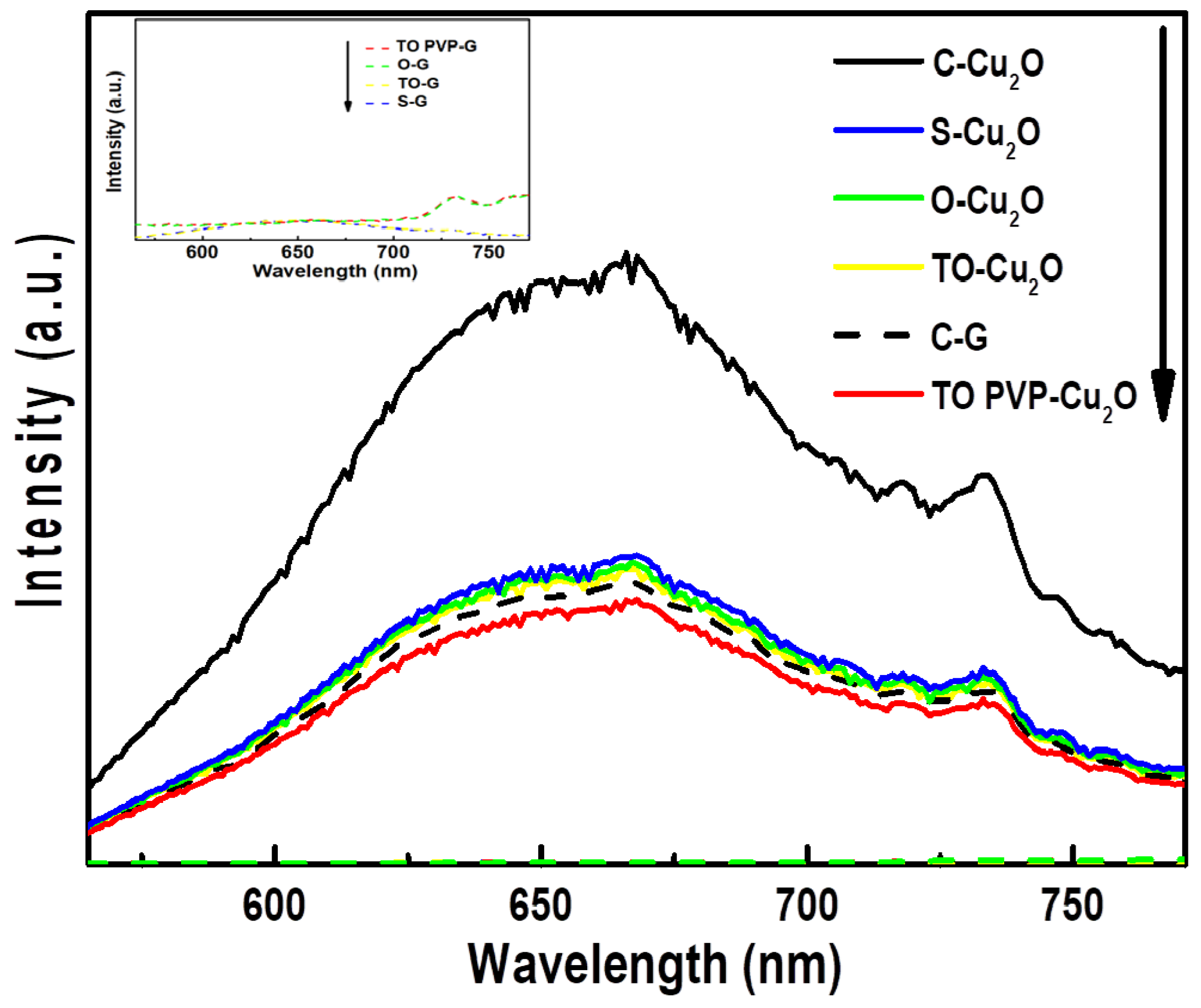
3.3. Electronic Properties of Cu2O Particles and Cu2O@rGO Composites
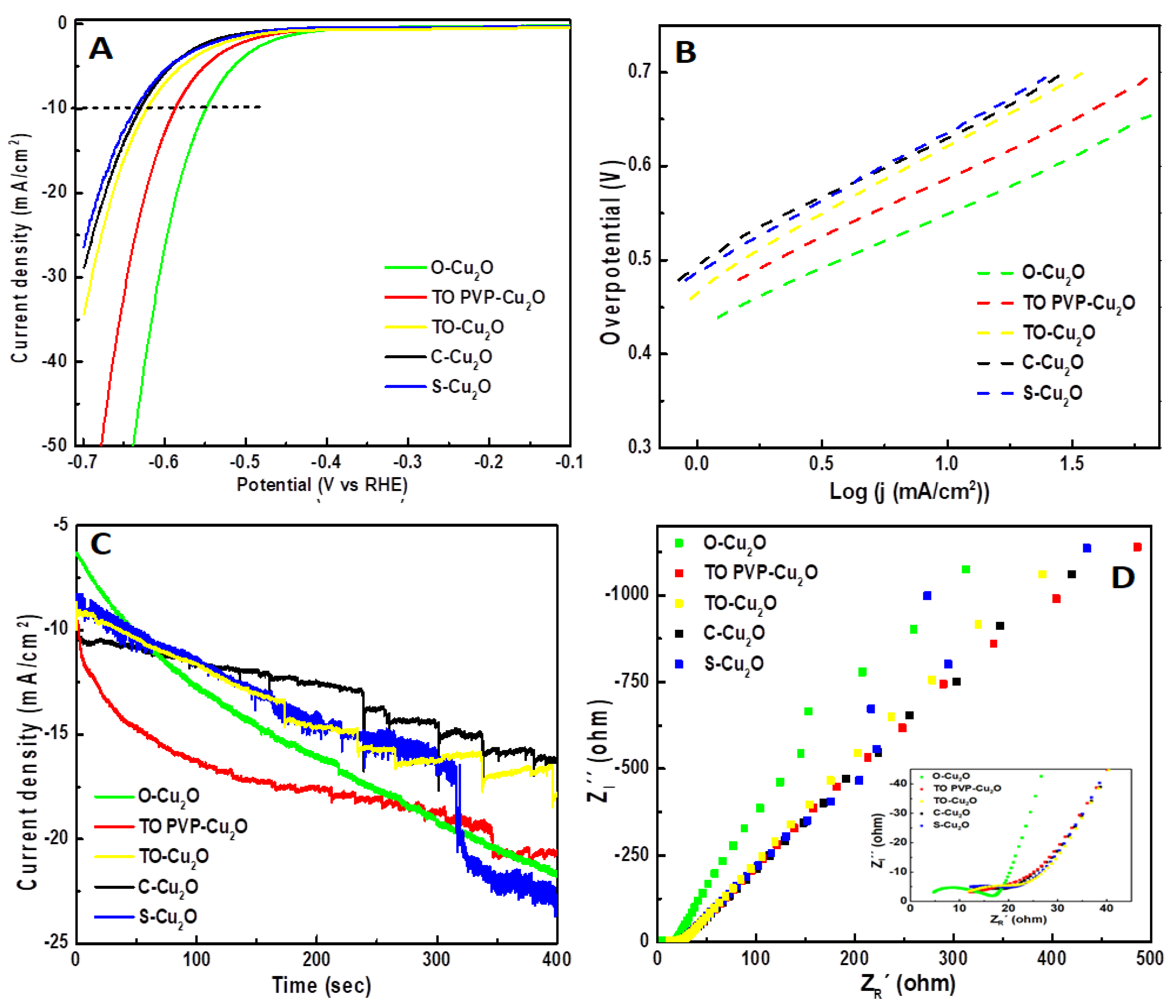
3.4. Electrochemical Hydrogen Evolution Reactions
3.4.1. HER Performance of Cu2O Geometrical Particles
3.4.2. HER Performance of Cu2O@rGO Composites
3.4.3. Density Functional Theory Calculations
3.4.4. After-Test Catalyst Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Heptonstall, P.J.; Gross, R.J.K. A systematic review of the costs and impacts of integrating variable renewables into power grids. Nat. Energy 2020, 6, 72–83. [Google Scholar] [CrossRef]
- Owusu, P.A.; Asumadu-Sarkodie, S.; Dubey, S. A review of renewable energy sources, sustainability issues and climate change mitigation. Cogent Eng. 2016, 3, 1167990. [Google Scholar] [CrossRef]
- Guan, D.; Xu, H.; Zhang, Q.; Huang, Y.C.; Shi, C.; Chang, Y.C.; Xu, X.; Tang, J.; Gu, Y.; Pao, C.W.; et al. Identifying A Universal Activity Descriptor and a Unifying Mechanism Concept on Perovskite Oxides for Green Hydrogen Production. Adv. Mater. 2023, 2305074. [Google Scholar] [CrossRef] [PubMed]
- Hara, M.; Komoda, M.; Hasei, H.; Yashima, M.; Ikeda, S.; Takata, T.; Kondo, J.N.; Domen, K. A Study of Mechano-Catalysts for Overall Water Splitting. J. Phys. Chem. B 2000, 104, 780–785. [Google Scholar] [CrossRef]
- Hara, M.; Kondo, T.; Komoda, M.; Ikeda, S.; Kondo, J.N.; Domen, K.; Hara, M.; Shinohara, K.; Tanaka, A. Cu2O as a photocatalyst for overall water splitting under visible light irradiation. Chem. Comm. 1998, 3, 357–358. [Google Scholar] [CrossRef]
- Bagal, I.V.; Chodankar, N.R.; Hassan, M.A.; Waseem, A.; Johar, M.A.; Kim, D.H.; Ryu, S.W. Cu2O as an emerging photocathode for solar water splitting—A status review. Int. J. Hydrogen Energy 2019, 44, 21351–21378. [Google Scholar] [CrossRef]
- Shang, Y.; Sun, D.; Shao, Y.; Zhang, D.; Guo, L.; Yang, S. A facile top-down etching to create a Cu2O jagged polyhedron covered with numerous {110} edges and {111} corners with enhanced photocatalytic activity. Chemistry 2012, 18, 14261–14266. [Google Scholar] [CrossRef]
- Yang, Z.; Sun, S.; Kong, C.; Song, X.; Ding, B. Designated-Tailoring on {100} Facets of Cu2O Nanostructures: From Octahedral to Its Different Truncated Forms. J. Nanomater. 2010, 2010, 710584. [Google Scholar] [CrossRef]
- Yao, K.X.; Yin, M.X.; Wang, T.W.; Zeng, H.C. Synthesis, Self-Assembly, Disassembly, and Reassembly of two types of Cu2O Nanocrystal Unifaceted with {001} or {110} Planes. J. Am. Chem. Soc. 2010, 132, 6131–6144. [Google Scholar] [CrossRef]
- Zhang, D.F.; Zhang, H.; Guo, L.; Zheng, K.; Han, X.D.; Zhang, Z. Delicate control of crystallographic facet-oriented Cu2O nanocrystals and the correlated adsorPion ability. J. Mater. Chem. 2009, 19, 5220–5225. [Google Scholar] [CrossRef]
- Bai, Y.; Yang, T.; Gu, Q.; Cheng, G.; Zheng, R. Shape control mechanism of cuprous oxide nanoparticles in aqueous colloidal solutions. Powder Technol. 2012, 227, 35–42. [Google Scholar] [CrossRef]
- Shang, Y.; Guo, L. Facet-Controlled Synthetic Strategy of Cu2O-Based Crystals for Catalysis and Sensing. Adv. Sci. 2015, 2, 1500140. [Google Scholar] [CrossRef] [PubMed]
- Sun, S.; You, H.; Kong, C.; Song, X.; Ding, B.; Yang, Z. Etching-limited branching growth of cuprous oxide during ethanol-assisted solution synthesis. CrystEngComm 2011, 13, 2837–2840. [Google Scholar] [CrossRef]
- Wang, X.; Liu, C.; Zheng, B.; Jiang, Y.; Zhang, L.; Xie, Z.; Zheng, L. Controlled synthesis of concave Cu2O microcrystals enclosed by {hhl} high-index facets and enhanced catalytic activity. J. Mater. Chem. A 2013, 1, 282–287. [Google Scholar] [CrossRef]
- Yang, J.; Wan, X.; Tie, S.; Lan, S.; Gao, X. Crystal-facet-controllable synthesis of Cu2O micron crystals by one-step, surfactant- and capping agent-free method and the formation mechanism. Solid State Sci. 2020, 104, 106203. [Google Scholar] [CrossRef]
- Zhang, Z.; Che, H.; Gao, J.; Wang, Y.; She, X.; Sun, J.; Gunawan, P.; Zhong, Z.; Su, F. Shape-controlled synthesis of Cu2O microparticles and their catalytic performances in the Rochow reaction. Catal. Sci. Technol. 2012, 2, 1207–1212. [Google Scholar] [CrossRef]
- Zhao, H.Y.; Wang, Y.F.; Zeng, J.H. Hydrothermal Synthesis of Uniform Cuprous Oxide Microcrystals with Controlled Morphology. Cryst. Growth Des. 2008, 8, 3731–3734. [Google Scholar] [CrossRef]
- Takata, T.; Jiang, J.; Sakata, Y.; Nakabayashi, M.; Shibata, N.; Nandal, V.; Seki, K.; Hisatomi, T.; Domen, K. Photocatalytic water splitting with a quantum efficiency of almost unity. Nature 2020, 581, 411–414. [Google Scholar] [CrossRef]
- Pan, L.; Kim, J.H.; Mayer, M.T.; Son, M.-K.; Ummadisingu, A.; Lee, J.S.; Hagfeldt, A.; Luo, J.; Grätzel, M. Boosting the performance of Cu2O photocathodes for unassisted solar water splitting devices. Nat. Catal. 2018, 1, 412–420. [Google Scholar] [CrossRef]
- Paracchino, A.; Laporte, V.; Sivula, K.; Gratzel, M.; Thimsen, E. Highly active oxide photocathode for photoelectrochemical water reduction. Nat. Mater. 2011, 10, 456–461. [Google Scholar] [CrossRef]
- Luo, M.; Yao, W.; Huang, C.; Wu, Q.; Xu, Q. Shape effects of Pt nanoparticles on hydrogen production via Pt/CdS photocatalysts under visible light. J. Mat Chem. 2015, 3, 13884–13891. [Google Scholar] [CrossRef]
- Li, Q.; Xu, P.; Zhang, B.; Tsai, H.; Zheng, S.; Wu, G.; Wang, H.-L. Structure-Dependent Electrocatalytic Properties of Cu2O Nanocrystals for Oxygen Reduction Reaction. J. Phys. Chem. C 2013, 117, 13872–13878. [Google Scholar] [CrossRef]
- Yun, H.J.; Lee, H.; Jool, J.B.; Kim, N.D.; Yi, J. Effect of TiO2 nanoparticle shape on hydrogen evolution via water splitting. J. Nanosci. Nanotechnol. 2011, 11, 1688–1691. [Google Scholar] [CrossRef] [PubMed]
- Dan, Y.; Lu, Y.; Kybert, N.J.; Luo, Z.; Johnson, A.T.C. Intrinsic Response of Graphene Vapor Sensors. Nano Lett. 2009, 9, 1472–1475. [Google Scholar] [CrossRef]
- Wu, L.; Tsui, L.-k.; Swami, N.; Zangari, G. Photoelectrochemical Stability of Electrodeposited Cu2O Films. J. Phys. Chem. C 2010, 114, 11551–11556. [Google Scholar] [CrossRef]
- Sowers, K.L.; Fillinger, A. Crystal Face Dependence of p-Cu2O Stability as Photocathode. J. Electrochem. Soc. 2009, 156, F80. [Google Scholar] [CrossRef]
- Meyer, B.K.; Polity, A.; Reppin, D.; Becker, M.; Hering, P.; Kramm, B.; Klar, P.J.; Sander, T.; Reindl, C.; Heiliger, C.; et al. The Physics of Copper Oxide (Cu2O); Elsevier: Amsterdam, The Netherlands, 2013; pp. 201–226. [Google Scholar]
- Lalitha, K.; Sadanandam, G.; Kumari, V.D.; Subrahmanyam, M.; Sreedhar, B.; Hebalkar, N.Y. Highly Stabilized and Finely Dispersed Cu2O/TiO2: A Promising Visible Sensitive Photocatalyst for Continuous Production of Hydrogen from Glycerol: Water Mixtures. J. Phys. Chem. C 2010, 114, 22181–22189. [Google Scholar] [CrossRef]
- Trang, T.N.Q.; Tu, L.T.N.; Man, T.V.; Mathesh, M.; Nam, N.D.; Thu, V.T.H. A high-efficiency photoelectrochemistry of Cu2O/TiO2 nanotubes based composite for hydrogen evolution under sunlight. Compos. B Eng. 2019, 174, 106969. [Google Scholar] [CrossRef]
- Salek, G.; Tenailleau, C.; Dufour, P.; Guillemet-Fritsch, S. Room temperature inorganic polycondensation of oxide (Cu2O and ZnO) nanoparticles and thin films preparation by the dip-coating technique. Thin Solid Films 2015, 589, 872–876. [Google Scholar] [CrossRef]
- Hou, F.; Shanmugasundaram, A.; Hassan, M.; Johar, M.; Ryu, S.-W.; Lee, D.-W. ZnO/Cu2O-decorated rGO: Heterojunction photoelectrode with improved solar water splitting performance. Int. J. Hydrogen Energy 2019, 44, 19177–19192. [Google Scholar] [CrossRef]
- Tran, P.D.; Batabyal, S.K.; Pramana, S.S.; Barber, J.; Wong, L.H.; Loo, S.C. A cuprous oxide-reduced graphene oxide (Cu2O-rGO) composite photocatalyst for hydrogen generation: Employing rGO as an electron acceptor to enhance the photocatalytic activity and stability of Cu2O. Nanoscale 2012, 4, 3875–3878. [Google Scholar] [CrossRef]
- Momeni, M.M.; Ghayeb, Y.; Menati, M. Fabrication, characterization and photoelectrochemical properties of cuprous oxide-reduced graphene oxide photocatalysts for hydrogen generation. J. Mat. Sci. Mater. Electron. 2017, 29, 4136–4146. [Google Scholar] [CrossRef]
- Roy, I.; Bhattacharyya, A.; Sarkar, G.; Saha, N.R.; Rana, D.; Ghosh, P.P.; Palit, M.; Das, A.R.; Chattopadhyay, D. In situ synthesis of a reduced graphene oxide/cuprous oxide nanocomposite: A reusable catalyst. RSC Adv. 2014, 4, 52044–52052. [Google Scholar] [CrossRef]
- Huang, Y.; Yan, C.-F.; Guo, C.-Q.; Lu, Z.-X.; Shi, Y.; Wang, Z.-D. Synthesis of GO-modified Cu2O nanosphere and the photocatalytic mechanism of water splitting for hydrogen production. Int. J. Hydrogen Energy 2017, 42, 4007–4016. [Google Scholar] [CrossRef]
- Choi, S.; Kim, C.; Suh, J.M.; Jang, H.W. Reduced graphene oxide-based materials for electrochemical energy conversion reactions. Carbon Energy 2019, 1, 85–108. [Google Scholar] [CrossRef]
- Singh, V.; Joung, D.; Zhai, L.; Das, S.; Khondaker, S.I.; Seal, S. Graphene based materials: Past, present and future. Progr. Mater. Sci. 2011, 56, 1178–1271. [Google Scholar] [CrossRef]
- Chen, C.; Li, D.; Wang, A.; Guo, J.; Dong, S.; Chen, D.; Jiao, X.; Xia, Y. Interfacial enhancement for hydrogen radical transfer on hollow Cu2O/rGO nanohybrid with efficient catalytic reduction activity. Appl. Catal. A Gen. 2020, 590, 117331. [Google Scholar] [CrossRef]
- Kumar, J.S.; Jana, M.; Khanra, P.; Samanta, P.; Koo, H.; Murmu, N.C.; Kuila, T. One pot synthesis of Cu2O/RGO composite using mango bark extract and exploration of its electrochemical properties. Electrochim. Acta 2016, 193, 104–115. [Google Scholar] [CrossRef]
- Liu, M.; Liu, R.; Chen, W. Graphene wrapped Cu2O nanocubes: Non-enzymatic electrochemical sensors for the detection of glucose and hydrogen peroxide with enhanced stability. Biosens. Bioelectron. 2013, 45, 206–212. [Google Scholar] [CrossRef]
- Wang, M.; Huang, J.; Tong, Z.; Li, W.; Chen, J. Reduced graphene oxide–cuprous oxide composite via facial deposition for photocatalytic dye-degradation. J. Alloys Compd. 2013, 568, 26–35. [Google Scholar] [CrossRef]
- Nathan, D.; Boby, S.; Basu, P.; Mahesh, R.; Harish, S.; Joseph, S.; Sagayaraj, P. One-pot hydrothermal preparation of Cu2O-CuO/rGO nanocomposites with enhanced electrochemical performance for supercapacitor applications. Appl. Surf. Sci. 2018, 449, 474–484. [Google Scholar]
- Zhang, F.; Li, Y.; Gu, Y.-E.; Wang, Z.; Wang, C. One-pot solvothermal synthesis of a Cu2O/Graphene nanocomposite and its application in an electrochemical sensor for dopamine. Mikrochim. Acta 2011, 173, 103–109. [Google Scholar] [CrossRef]
- Zhang, W.; Li, X.; Yang, Z.; Tang, X.; Ma, Y.; Li, M.; Hu, N.; Wei, H.; Zhang, Y. In situ preparation of cubic Cu2O-RGO nanocomposites for enhanced visible-light degradation of methyl orange. Nat. Nanotechnol. 2016, 27, 265703. [Google Scholar] [CrossRef] [PubMed]
- Ramirez-Ubillus, M.A.; De Melo Costa-Serge, N.; Hammer, P.; Nogueira, R.F.P. A new approach on synergistic effect and chemical stability of graphene oxide-magnetic nanocomposite in the heterogeneous Fenton degradation of caffeine. Environ. Sci. Pollut. Res. Int. 2021, 28, 55014–55028. [Google Scholar] [CrossRef] [PubMed]
- Barrios, E.; Fox, D.; Li Sip, Y.Y.; Catarata, R.; Calderon, J.E.; Azim, N.; Afrin, S.; Zhang, Z.; Zhai, L. Nanomaterials in Advanced, High-Performance Aerogel Composites: A Review. Polymers 2019, 11, 726. [Google Scholar] [CrossRef] [PubMed]
- Shen, C.; Calderon, J.E.; Barrios, E.; Soliman, M.; Khater, A.; Jeyaranjan, A.; Tetard, L.; Gordon, A.; Seal, S.; Zhai, L. Anisotropic electrical conductivity in polymer derived ceramics induced by graphene aerogels. J. Mat. Chem. C 2017, 5, 11708–11716. [Google Scholar] [CrossRef]
- Cai, J.; Liu, W.; Li, Z. One-pot self-assembly of Cu2O/RGO composite aerogel for aqueous photocatalysis. Appl. Surf. Sci. 2015, 358, 146–151. [Google Scholar] [CrossRef]
- Celik Kazici, H.; Caglar, A.; Aydogmus, T.; Aktas, N.; Kivrak, H. Microstructured prealloyed Titanium-Nickel powder as a novel nonenzymatic hydrogen peroxide sensor. J. Colloid. Interface. Sci. 2018, 530, 353–360. [Google Scholar] [CrossRef]
- John, P.; Kieron, B.; Ernzerhof, M. Generalized Gradient Approximation Made Simple. Phys. Rev. Lett. 1996, 77, 3865–3868. [Google Scholar]
- Bloch, P.E. Projector augmented-wave method. Phys. Rev. B 1993, 50, 17953–17979. [Google Scholar] [CrossRef]
- Dudarev, S.L.; Botton, G.A.; Savrasov, S.Y.; Humphreys, C.J.; Sutton, A.P. Electron-energy-loss spectra and the structural stability of nickel oxide: An LSDA+U study. Phys. Rev. B 1998, 57, 1505–1509. [Google Scholar] [CrossRef]
- Grimme, S.; Ehrlich, S.; Goerigk, L. Effect of the damping function in dispersion corrected density functional theory. J. Comput. Chem. 2011, 32, 1456–1465. [Google Scholar] [CrossRef] [PubMed]
- Giannozzi, P.; Giannozzi, P.; Baroni, S.; Bonini, N.; Calandra, M.; Car, R.; Cavazzoni, C.; Ceresoli, D.C.; Cococcioni, G.L.M. Advanced capabilities for materials modelling with Quantum ESPRESSO. J. Phys. Condens. Matter. 2017, 46, 465901. [Google Scholar] [CrossRef]
- Giannozzi, P.; Baroni, S.; Bonini, N.; Calandra, M.; Car, R.; Cavazzoni, C.; Ceresoli, D.; Chiarotti, G.L.; Cococcioni, M.; Dabo, I.; et al. QUANTUM ESPRESSO: A modular and open-source software project for quantum simulations of materials. J. Phys. Condens. Matter. 2009, 21, 395502. [Google Scholar] [CrossRef] [PubMed]
- Momma, K.; Izumi, F. VESTA 3for three-dimensional visualization of crystal, volumetric and morphology data. J. Appl. Crystallogr. 2011, 44, 1272–1276. [Google Scholar] [CrossRef]
- Soldemo, M.; Stenlid, J.H.; Besharat, Z.; Ghadami Yazdi, M.; Önsten, A.; Leygraf, C.; Göthelid, M.; Brinck, T.; Weissenrieder, J. The Surface Structure of Cu2O(100). J. Phys Chem. C 2016, 120, 4373–4381. [Google Scholar] [CrossRef]
- Kokalj, A.; Peljhan, S. Density Functional Theory Study of Adsorption of Benzotriazole on Cu2O Surfaces. J. Phys. Chem. C 2015, 119, 11625–11635. [Google Scholar] [CrossRef]
- Sompech, S.; Srion, A.; Nuntiya, A. The Effect of Ultrasonic Treatment on the Particle Size and Specific Surface Area of LaCoO3. Procedia Eng. 2012, 32, 1012–1018. [Google Scholar] [CrossRef]
- Dalapati, G.K.; Masudy-Panah, S.; Moakhar, R.S.; Chakrabortty, S.; Ghosh, S.; Kushwaha, A.; Katal, R.; Chua, C.S.; Xiao, G.; Tripathy, S.; et al. Nanoengineered Advanced Materials for Enabling Hydrogen Economy: Functionalized Graphene-Incorporated Cupric Oxide Catalyst for Efficient Solar Hydrogen Production. Global Chall. 2020, 4, 1900087. [Google Scholar] [CrossRef]
- Zhang, Y.-H.; Cai, X.-L.; Guo, D.-Y.; Zhang, H.-J.; Zhou, N.; Fang, S.-M.; Chen, J.-L.; Zhang, H.-L. Oxygen vacancies in concave cubes Cu2O-reduced graphene oxide heterojunction with enhanced photocatalytic H2 production. J. Mat. Sci. Mater. Electron. 2019, 30, 7182–7193. [Google Scholar] [CrossRef]
- Wang, C.; Tissot, H.; Escudero, C.; Pérez-Dieste, V.; Stacchiola, D.; Weissenrieder, J. Redox Properties of Cu2O(100) and (111) Surfaces. J. Phys Chem. C 2018, 122, 28684–28691. [Google Scholar] [CrossRef]
- An, X.; Li, K.; Tang, J. Cu2O/reduced graphene oxide composites for the photocatalytic conversion of CO2. ChemSusChem 2014, 7, 1086–1093. [Google Scholar] [CrossRef] [PubMed]
- Ho, W.C.J.; Tay, Q.; Qi, H.; Huang, Z.; Li, J.; Chen, Z. Photocatalytic and Adsorption Performances of Faceted Cuprous Oxide (Cu2O) Particles for the Removal of Methyl Orange (MO) from Aqueous Media. Molecules 2017, 22, 677. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Mei, Z.; Liu, L.; Liang, H.; Azarov, A.; Kuznetsov, A.; Liu, Y.; Ji, A.; Meng, Q.; Du, X. Probing defects in nitrogen-doped Cu2O. Sci. Rep. 2014, 4, 7240. [Google Scholar] [CrossRef] [PubMed]
- Engel, C.; Polson, T.; Spado, J.; Bell, J.; Fillinger, A. Photoelectrochemistry of Porous p-Cu2O Films. J. Electrochem. Soc. 2008, 155, F37–F42. [Google Scholar] [CrossRef]
- Grosse, P.; Yoon, A.; Rettenmaier, C.; Herzog, A.; Chee, S.W.; Roldan-Cuenya, B. Dynamic transformation of cubic copper catalysts during CO2 electroreduction and its impact on catalytic selectivity. Nat. Commun. 2021, 12, 6736. [Google Scholar] [CrossRef]
- Wu, N.; Wang, J.; Tafen, D.N.; Wang, H.; Zheng, J.G.; Lewis, J.P.; Liu, X.; Leonard, S.S.; Manivannan, A. Shape-enhanced photocatalytic activity of single-crystalline anatase TiO2 (101) nanobelts. J. Am. Chem. Soc. 2010, 132, 6679–6685. [Google Scholar] [CrossRef]
- Ehrnst, Y.; Sherrell, P.C.; Rezk, A.R.; Yeo, L.Y. Acoustically-Induced Water Frustration for Enhanced Hydrogen Evolution Reaction in Neutral Electrolytes. Adv. Energy Mat. 2022, 13, 2203164. [Google Scholar] [CrossRef]
- Shinagawa, T.; Garcia-Esparza, A.T.; Takanabe, K. Insight on Tafel slopes from a microkinetic analysis of aqueous electrocatalysis for energy conversion. Sci. Rep. 2015, 5, 13801. [Google Scholar] [CrossRef]
- Mei, B.-A.; Munteshari, O.; Lau, J.; Dunn, B.; Pilon, L. Physical Interpretations of Nyquist Plots for EDLC Electrodes and Devices. J. Phys. Chem. C 2017, 122, 194–206. [Google Scholar] [CrossRef]
- Wang, L.; Ge, J.; Wang, A.; Deng, M.; Wang, X.; Bai, S.; Li, R.; Jiang, J.; Zhang, Q.; Luo, Y.; et al. Designing p-Type Semiconductor–Metal Hybrid Structures for Improved Photocatalysis. Angew. Chem. Int. Ed. 2014, 53, 5107–5111. [Google Scholar] [CrossRef] [PubMed]
- Ekspong, J.; Gracia-Espino, E.; Wågberg, T. Hydrogen Evolution Reaction Activity of Heterogeneous Materials: A Theoretical Model. J. Phys. Chem. C 2020, 124, 20911–20921. [Google Scholar] [CrossRef]
- Ouiram, T.; Moonla, C.; Preechaworapun, A.; Tangkuaram, T. Enzyme-free Cu2O@MnO2/GCE for Hydrogen Peroxide Sensing. Electroanalysis 2019, 31, 1356–1362. [Google Scholar] [CrossRef]
- Koolen, C.D.; Luo, W.; Züttel, A. From Single Crystal to Single Atom Catalysts: Structural Factors Influencing the Performance of Metal Catalysts for CO2 Electroreduction. ACS Catal. 2022, 13, 948–973. [Google Scholar] [CrossRef]
- Liu, L.; Yang, W.; Sun, W.; Li, Q.; Shang, J.K. Creation of Cu2O@TiO2 composite photocatalysts with p-n heterojunctions formed on exposed Cu2O facets, their energy band alignment study, and their enhanced photocatalytic activity under illumination with visible light. ACS. Appl. Mater. Interfaces 2015, 7, 1465–1476. [Google Scholar] [CrossRef]
- Sterlin Leo Hudson, M.; Raghubanshi, H.; Awasthi, S.; Sadhasivam, T.; Bhatnager, A.; Simizu, S.; Sankar, S.G.; Srivastava, O.N. Hydrogen uptake of reduced graphene oxide and graphene sheets decorated with Fe nanoclusters. Int. J. Hydrogen Energy 2014, 39, 8311–8320. [Google Scholar] [CrossRef]
- Zheng, Y.; Jiao, Y.; Li, L.; Chen, Y.; Jaroniec, M.; Qiao, S. Toward Design of Synergistically Active Carbon Based Catalysts for Electrocatalytic Hydrogen Evolution. ACS Nano 2014, 8, 5290–5296. [Google Scholar] [CrossRef]
- Kareem, A.; Kunhiraman, A.K.; Maiyalagan, T. Hydrogen evolution reaction catalyzed by microstructured SrMoO4 decorated on three-dimensional nanostructured rGO/f-MWCNT in acidic medium. Ionics 2020, 26, 5055–5064. [Google Scholar] [CrossRef]
- Han, J.; Zong, X.; Zhou, X.; Li, C. Cu2O/CuO photocathode with improved stability for photoelectrochemical water reduction. RSC Adv. 2015, 5, 10790–10794. [Google Scholar] [CrossRef]
- Yu, X.; Zhang, X.; Wang, H.; Wang, Z.; Feng, G. High-Coverage H2 Adsorption on the Reconstructed Cu2O(111) Surface. J. Phys. Chem. C 2017, 121, 22081–22091. [Google Scholar] [CrossRef]
- Lai, W.; Ma, Z.; Zhang, J.; Yuan, Y.; Qiao, Y.; Huang, H. Dynamic Evolution of Active Sites in Electrocatalytic CO2 Reduction Reaction: Fundamental Understanding and Recent Progress. Adv. Funct. Mat. 2022, 32, 2111193. [Google Scholar] [CrossRef]
- Deng, Y.; Handoko, A.D.; Du, Y.; Xi, S.; Yeo, B.S. In Situ Raman Spectroscopy of Copper and Copper Oxide Surfaces during Electrochemical Oxygen Evolution Reaction: Identification of CuIII Oxides as Catalytically Active Species. ACS Catal. 2016, 6, 2473–2481. [Google Scholar] [CrossRef]
- Fan, M.; Gao, R.; Zou, Y.C.; Wang, D.; Bai, N.; Li, G.D.; Zou, X. An efficient nanostructured copper(I) sulfide-based hydrogen evolution electrocatalyst at neutral pH. Electrochim. Acta 2016, 215, 366–373. [Google Scholar] [CrossRef]
- Zhou, M.; Lian, C.; Xu, Z.; Pi, T.; Yang, K.; Chi, B.; Li, Z. Active copper(II) and copper(I) reviving repeatedly in situ on copper wire electrode for full water splitting at ultra-low potential. Appl. Surf. Sci. 2020, 505, 144653. [Google Scholar] [CrossRef]
- Johny, J.; Guzman, S.S.; Krishnan, B.; Martinez, J.A.; Avellaneda, D.; Shaji, S. SnS2 nanoparticles by liquid phase laser ablation: Effects of laser fluence, temperature and post irradiation on morphology and hydrogen evolution reaction. Appl. Surf. Sci. 2019, 470, 276–288. [Google Scholar] [CrossRef]
- Kong, F.; Qie, Y.; Liu, Y.; Yang, Z.; Shi, Z.; Yang, H. Magnetic properties and electrocatalytic properties of Fe5C2 particles with different morphologies. J. Mater. Sci. Mater. Electron. 2021, 33, 884–893. [Google Scholar] [CrossRef]
- Mukurala, N.; Mokurala, K.; Mohapatra, L.; Suman, S.; Kushwaha, A.K. Surface controlled synthesis of Cu2FeSnS4 particles for enhanced hydrogen evolution reaction. Int. J. Hydrogen Energy 2021, 46, 34689–34700. [Google Scholar] [CrossRef]



| Cu2O Particle | Label | 100/111 Ratio (r) | Size (µm) |
|---|---|---|---|
| Cube | C-Cu2O | 100 | 1.1 ± 0.1 |
| Octahedron | O-Cu2O | 0 | 2.0 ± 0.5 |
| Truncated Octahedron PVP | TO PVP-Cu2O | 0.26 | 1.0 ± 0.3 |
| Truncated Octahedron | TO-Cu2O | 0.09 | 1.0 ± 0.3 |
| Star | S-Cu2O | - | 2.4 ± 0.5 |
| Cu2O Particle | Onset (mV) | Overpotential (mV) at 10 mA/cm2 | Tafel Slope (mV/dec) | ESA (mm2) |
|---|---|---|---|---|
| O-Cu2O | −330 | −549 | 126 | 5.9 |
| TO PVP-Cu2O | −375 | −587 | 127 | 7.0 |
| TO-Cu2O | −419 | −620 | 148 | 4.0 |
| C-Cu2O | −417 | −629 | 135 | 4.9 |
| S-Cu2O | −410 | −634 | 147 | 4.7 |
| Cu2O@rGO | Onset (mV) | Overpotential (mV) at 10 mA/cm2 | Tafel Slope (mV/dec) | ESA (mm2) |
|---|---|---|---|---|
| O-G | −295 | −500 | 210 | 9.2 |
| TO PVP-G | −357 | −598 | 181 | 15.8 |
| TO-G | −315 | −482 | 205 | 24.2 |
| C-G | −355 | −533 | 156 | 16.3 |
| S-G | −310 | −458 | 200 | 13.0 |
| Catalyst | Electrolyte | Overpotential (mV) at 10 mA/cm2 | Reference |
|---|---|---|---|
| Cu2O/copper foam | 1 M Phosphate buffer solution | −255 | [84] |
| Cu2O-CuO/copper wire | 1 M KOH | −350 | [85] |
| SnS2/GCE | 1 M H2SO4 | −800 (16.5) | [86] |
| Fe5C2/GCE | 1 M KOH | −365 to −225 | [87] |
| Cu2FeSnS4/GCE | 0.5 M H2SO4 | −470 to −421 | [88] |
| Cu2O/rGO/GCE | 0.5 M H2SO4 | −598 to −458 | This work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ramirez-Ubillus, M.A.; Wang, A.; Zou, S.; Chumbimuni-Torres, K.Y.; Zhai, L. Morphological Effect on the Surface Activity and Hydrogen Evolution Catalytic Performance of Cu2O and Cu2O/rGO Composites. J. Compos. Sci. 2023, 7, 403. https://doi.org/10.3390/jcs7090403
Ramirez-Ubillus MA, Wang A, Zou S, Chumbimuni-Torres KY, Zhai L. Morphological Effect on the Surface Activity and Hydrogen Evolution Catalytic Performance of Cu2O and Cu2O/rGO Composites. Journal of Composites Science. 2023; 7(9):403. https://doi.org/10.3390/jcs7090403
Chicago/Turabian StyleRamirez-Ubillus, Manuel A., Ankai Wang, Shengli Zou, Karin Y. Chumbimuni-Torres, and Lei Zhai. 2023. "Morphological Effect on the Surface Activity and Hydrogen Evolution Catalytic Performance of Cu2O and Cu2O/rGO Composites" Journal of Composites Science 7, no. 9: 403. https://doi.org/10.3390/jcs7090403







