Polyphenylenepyridines Based on Acetylaromatic Compounds
Abstract
:1. Introduction
2. Materials and Methods
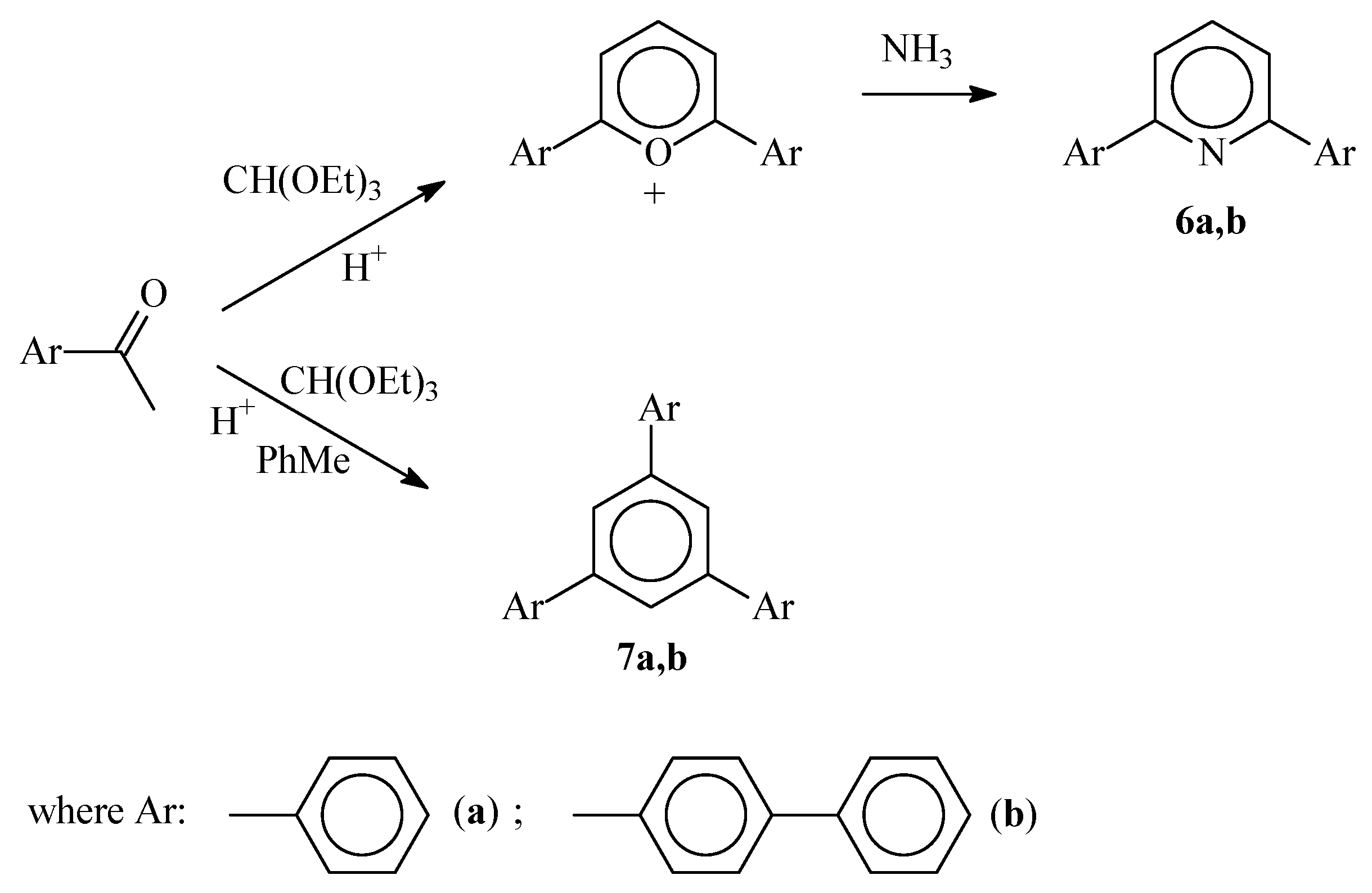
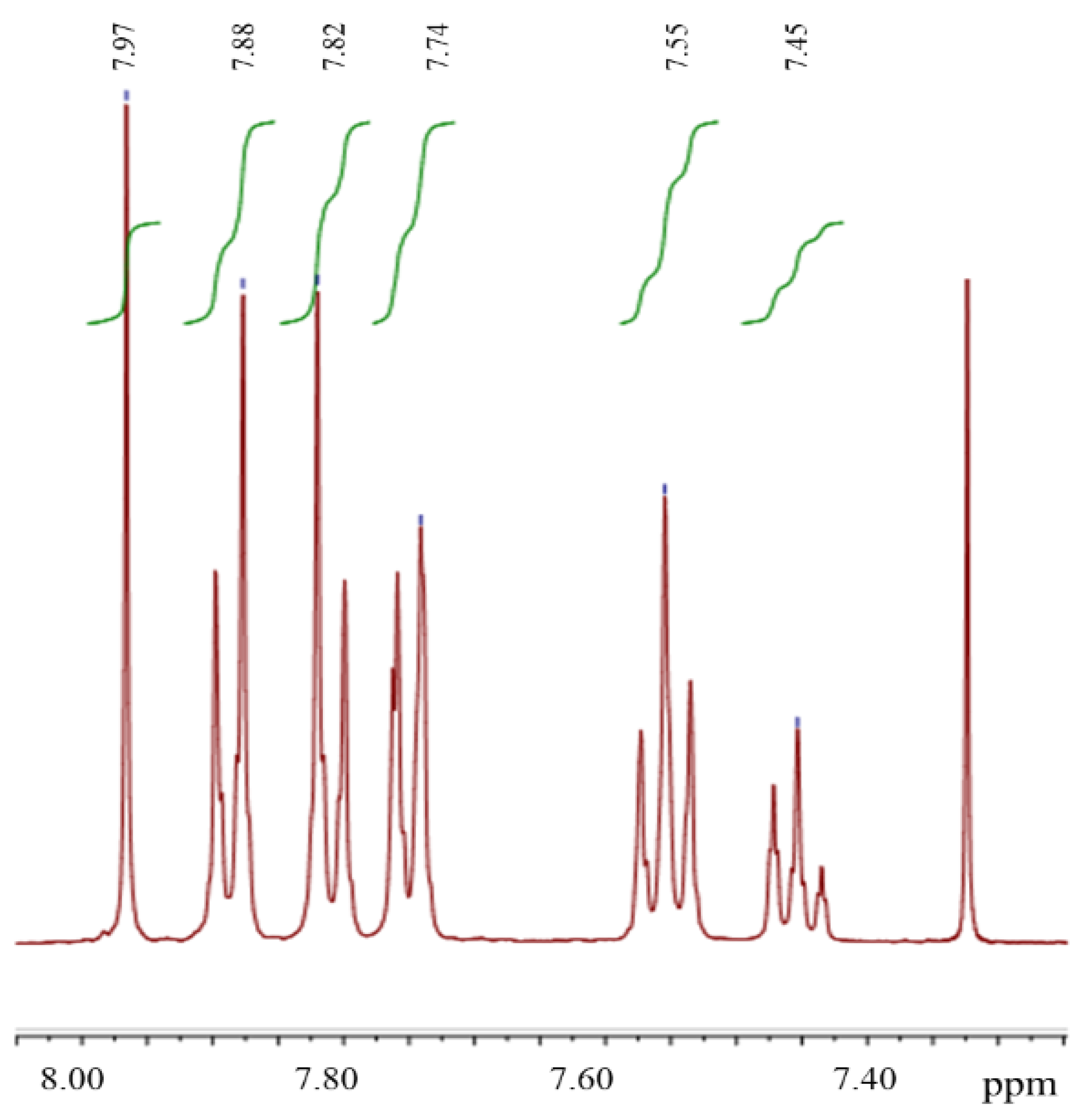
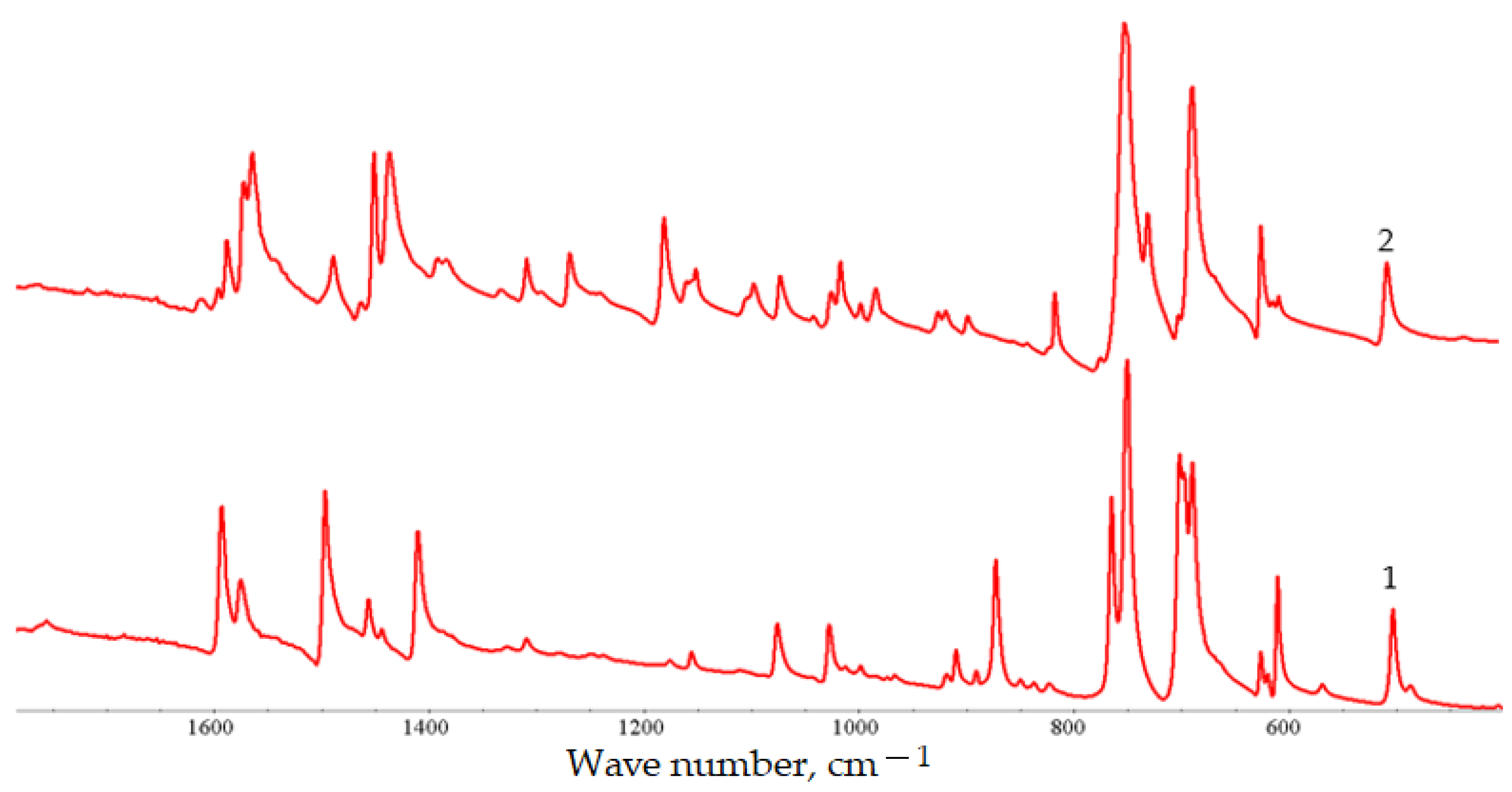
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Meng, N.; Ren, X.; Santagiuliana, G.; Ventura, L.; Zhang, H.; Wu, J.; Yan, H.; Reece, M.; Bilotti, E. Ultrahigh β-phase content poly(vinylidene fluoride) with relaxor-like ferroelectricity for high energy density capacitors. Nat. Commun. 2019, 10, 4535–4543. [Google Scholar] [CrossRef] [PubMed]
- AlOthman, Z. A Review: Fundamental aspects of silicate mesoporous materials. Materials 2012, 5, 2874–2902. [Google Scholar] [CrossRef]
- Shao, Y.; El-Kady, M.; Sun, J.; Li, Y.; Zhang, Q.; Zhu, M.; Wang, H.; Dunn, B.; Kaner, R.B. Design and mechanisms of asymmetric supercapacitors. Chem. Rev. 2018, 118, 9233–9280. [Google Scholar] [CrossRef] [PubMed]
- Simon, P.; Gogotsi, Y.; Dunn, B. Where do batteries end and supercapacitors begin? Science 2014, 343, 1210–1211. [Google Scholar] [CrossRef]
- Miller, J.R.; Simon, P. Materials science—Electrochemical capacitors for energy management. Science 2008, 321, 651–652. [Google Scholar] [CrossRef] [PubMed]
- Dubal, D.P.; Chodankar, N.R.; Kim, D.H.; Gomez-Romero, P. Towards flexible solid-state supercapacitors for smart and wearable electronics. Chem. Soc. Rev. 2018, 47, 2065–2129. [Google Scholar] [CrossRef]
- Lin, T.; Chen, I.; Liu, F.; Yang, C.; Bi, H.; Xu, F.; Huang, F. Nitrogen-doped mesoporous carbon of extraordinary capacitance for electrochemical energy storage. Science 2015, 350, 1508–1513. [Google Scholar] [CrossRef] [PubMed]
- Zhou, M.; Li, X.; Zhao, H.; Wang, J.; Zhao, Y.; Ge, F.; Cai, Z. Combined effect of nitrogen and oxygen heteroatoms and micropores of porous carbon frameworks from Schiff-base networks on their high supercapacitance. J. Mater. Chem. A 2018, 6, 1621–1629. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, X.; Yang, K.; Fan, X.; Tong, Y.; Zhang, Z.; Lu, X.; Mai, K.; Ni, Q.; Zhang, M.; et al. Ultrahigh energy fiber-shaped supercapacitors based on porous hollow conductive polymer composite fiber electrodes. J. Mater. Chem. A 2018, 6, 12250–12258. [Google Scholar] [CrossRef]
- Liu, T.; Zhang, L.; You, W.; Yu, J. Core–Shell Nitrogen-Doped Carbon Hollow Spheres/Co3O4 Nanosheets as Advanced Electrode for High-Performance Supercapacitor. Small 2018, 14, 1702407. [Google Scholar] [CrossRef]
- Bavatharani, C.; Muthusankar, E.; Wabaidur, S.; Alothman, Z.; Alsheetan, K.; AL-Anazy, M.; Ragupathy, D. Electrospun carbon nanofibers from biomass and biomass blends-current trends. Synth. Met. 2021, 271, 116609. [Google Scholar] [CrossRef]
- Fu, W.; Zhao, E.; Ren, X.; Magasinski, A.; Yushin, G. Hierarchical Fabric Decorated with Carbon Nanowire/Metal Oxide Nanocomposites for 1.6 V Wearable Aqueous Supercapacitors. Adv. Energy Mater. 2018, 8, 1703454. [Google Scholar] [CrossRef]
- Sirisinudomkit, P.; Senokos, E.; Rubio, N.; Shaffer, M.S.P. Reductive processing of single walled carbon nanotubes for high volumetric performance supercapacitors. Mater. Adv. 2021, 2, 1981–1992. [Google Scholar] [CrossRef]
- Bavatharani, C.; Muthusankar, E.; Alothman, Z.; Wabaidur, S.; Ponnusamy, V.; Ragupathy, D. Ultra-high sensitive, selective, non-enzymatic dopamine sensor based on electrochemically active graphene decorated Polydiphenylamine-SiO2 nanohybrid composite. Ceram. Int. 2020, 46, 23276–23281. [Google Scholar] [CrossRef]
- Long, C.; Jiang, L.; Wu, X.; Jiang, Y.; Yang, D.; Wang, C.; Wei, T.; Fan, Z. Facile synthesis of functionalized porous carbon with three-dimensional interconnected pore structure for high volumetric performance supercapacitors. Carbon 2015, 93, 412–420. [Google Scholar] [CrossRef]
- Zhang, Y.; Tao, B.; Xing, W.; Zhang, L.; Xue, Q.; Yan, Z. Sandwich-like nitrogen-doped porous carbon/graphene nanoflakes with high-rate capacitive performance. Nanoscale 2016, 8, 7889–7898. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Wu, J.; Dai, F.; Li, C.M. 3D honeycomb-like carbon foam synthesized with biomass buckwheat flour for high-performance supercapacitor electrodes. Chem. Commun. 2019, 55, 9168. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.; Shi, H.; Das, P.; Qin, J.; Li, Y.; Wang, X.; Su, F.; Wen, P.; Li, S.; Lu, P.; et al. A review. The chemistry and promising applications of graphene and porous graphene materials. Adv. Funct. Mater. 2020, 30, 1909035. [Google Scholar] [CrossRef]
- Gao, Y.; Zhang, J.; Luo, X.; Wan, Y.; Zhao, Z.; Han, X.; Xia, Z. Energy density-enhancement mechanism and design principles for heteroatom-doped carbon supercapacitors. Nano Energy 2020, 72, 104666. [Google Scholar] [CrossRef]
- Xu, Q.; Yu, X.; Liang, Q.; Bai, Y.; Huang, Z.; Kang, F. Nitrogen-doped hollow activated carbon nanofibers as high performance supercapacitor electrodes. J. Electroanal. Chem. 2015, 739, 84–88. [Google Scholar] [CrossRef]
- Zhu, D.; Wang, Y.; Gan, L.; Liu, M.; Cheng, K.; Zhao, Y.; Deng, X.; Sunb, D. Nitrogen-containing carbon microspheres for supercapacitor electrodes. Electrochim. Acta 2015, 158, 166–174. [Google Scholar] [CrossRef]
- Paek, E.; Pak, A.J.; Kweon, K.E.; Hwang, G.S. On the origin of the enhanced supercapacitor performance of nitrogen-doped graphene. J. Phys. Chem. C 2013, 117, 5610–5616. [Google Scholar] [CrossRef]
- Singh, S.K.; Takeyasu, K.; Nakamura, J. Active sites and mechanism of oxygen reduction reaction electrocatalysis on nitrogen-doped carbon materials. Adv. Mater. 2019, 31, 1804297. [Google Scholar] [CrossRef] [PubMed]
- Qian, W.; Sun, F.; Xu, Y.; Qiu, L.; Liu, C.; Wang, S.; Yan, F. Human hair-derived carbon flakes for electrochemical supercapacitors. Energy Environ. Sci. 2014, 7, 379–386. [Google Scholar] [CrossRef]
- Shang, M.; Zhang, J.; Liu, X.; Liu, Y.; Guo, S.; Yu, S.; Filatov, S.; Yi, X. N, S self-doped hollow-sphere porous carbon derived from puffball spores for high performance supercapacitors. Appl. Surf. Sci. 2021, 542, 148697. [Google Scholar] [CrossRef]
- Guo, W.; Chen, M.; Zhang, Y.; Yang, W.; Guo, Q.; Zhang, M.; Cheng, F. Generation three-dimensional nitrogen-doped graphene frameworks as advanced electrode for supercapacitors. J. Mater. Res. 2018, 33, 1131–1141. [Google Scholar] [CrossRef]
- Xu, F.; Wu, D.; Fu, R.; Wei, B. Design and preparation of porous carbons from conjugated polymer precursors. Mater. Today 2017, 20, 629–656. [Google Scholar] [CrossRef]
- Sun, H.; Zhou, P.; Ye, X.; Wang, J.; Tian, Z.; Zhu, Z.; Ma, C.; Liang, W.; Li, A. Nitrogen-doping hollow carbon nanospheres derived from conjugated microporous polymers toward oxygen reduction reaction. J. Colloid Interface Sci. 2022, 617, 11–19. [Google Scholar] [CrossRef]
- Meng, N.; Li, H.; Liu, Y.; Liao, Y. Self-templating synthesis of nitrogen-rich porous carbons using pyridyl functionalized conjugated microporous polytriphenylamine for electrochemical energy storage. Electrochim. Acta 2022, 402, 139531. [Google Scholar] [CrossRef]
- Mikroyannidis, J.A.; Vellis, P.D.; Yang, S.-H.; Hsu, C.-S. Synthesis, electroluminescence, and photovoltaic cells of new vinylene-copolymers with 4-(anthracene-10-yl)-2,6-diphenylpyridine segments. J. Appl. Polym. Sci. 2010, 115, 731–740. [Google Scholar] [CrossRef]
- Karastatiris, P.; Mikroyannidis, J.A.; Spiliopoulos, I.K.; Fakis, M.; Persephonis, P. Luminescent poly(phenylene vinylene) derivatives with m-terphenyl or 2,6-diphenylpyridine kinked segments along the main chain: Synthesis, characterization, and stimulated emission. J. Polym. Sci. Part A 2004, 42, 2214–2224. [Google Scholar] [CrossRef]
- Liu, X.; Du, J.; Ye, Y.; Liu, Y.; Wang, S.; Meng, X.; Song, X.; Liang, Z.; Yan, W. Boosting selective C2H2/CH4, C2H4/CH4 and CO2/CH4 adsorption performance via 1,2,3-triazole functionalized triazine-based porous organic polymers. Chin. J. Chem. Eng. 2022, 42, 64–72. [Google Scholar] [CrossRef]
- Takashi, M.; Yoshiaki, S.; Takeshi, S.; Masami, S.; Tsutomu, F. Palladium-catalyzed Sonogashira and Hiyama reactions using phosphine-free hydrazone ligands. J. Org. Chem. 2006, 71, 9499–9502. [Google Scholar] [CrossRef]
- Molander, G.A.; Biolatto, B. Efficient ligandless palladium-catalyzed Suzuki reactions of potassium aryltrifluoroborates. Org. Lett. 2002, 4, 1867–1870. [Google Scholar] [CrossRef]
- Khotina, I.A.; Kovalev, A.I.; Kushakova, N.S.; Babushkina, M.A.; Vasiliev, Y.V.; Peregudov, A.S. Structures of branched oligophenylenes studied by NMR spectroscopy. Russ. Chem. Bull. 2013, 62, 2234–2244. [Google Scholar] [CrossRef]
- Available online: https://sdbs.db.aist.go.jp/sdbs/cgi-bin/direct_frame_top.cgi (accessed on 1 August 2023).
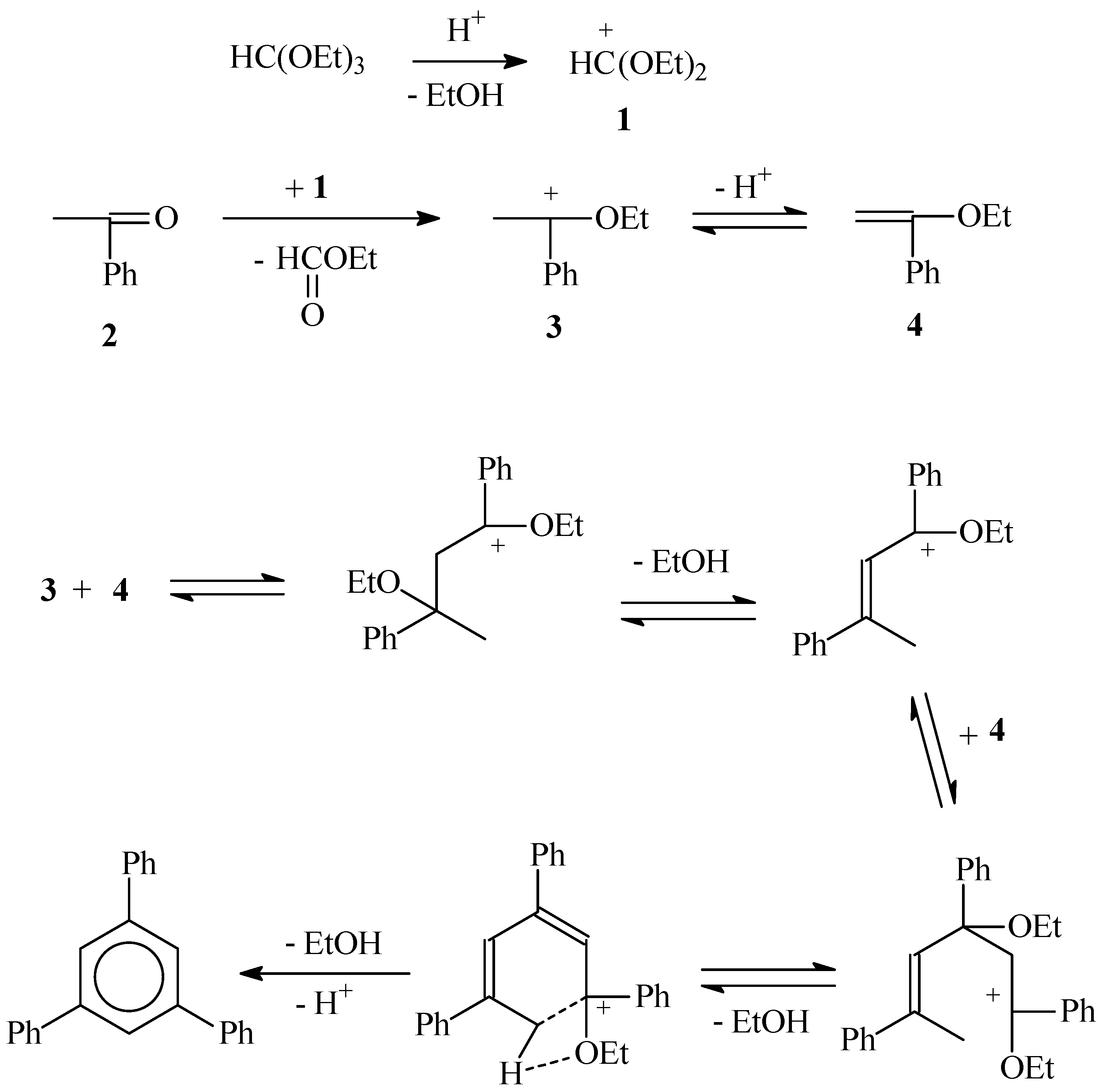
- Kovaleva, M.A.; Kovalev, A.I.; Khotina, I.A. Alternative pathways of the reaction between acetophenone and triethyl orthoformate. Russ. Chem. Bull. 2023, 72, 1186–1191. [Google Scholar] [CrossRef]
- Mróz, W.; Kovalev, A.I.; Babushkina-Lebedeva, M.A.; Kushakova, N.S.; Vercelli, B.; Squeo, B.M.; Botta, C.; Pasini, M.; Destri, S.; Giovanella, U.; et al. Branched oligophenylenes with phenylene–ethynylene fragments as anode interfacial layer for solution processed optoelectronics. Macromol. Chem. Phys. 2019, 220, 1900036. [Google Scholar] [CrossRef]
- Kovalev, A.I.; Mart’yanova, E.S.; Khotina, I.A.; Klemenkova, Z.S.; Blinnikova, Z.K.; Volchkova, E.V.; Loginova, T.P.; Ponomarev, I.I. Polyphenylene gels. Polym. Sci. Ser. B 2018, 60, 675–679. [Google Scholar] [CrossRef]
- Kovalev, A.I.; Khotina, I.A. Microporous polymers based on triacetylarenes. Mendeleev Commun. 2022, 32, 244–246. [Google Scholar] [CrossRef]
- Kovalev, A.I.; Babich, S.A.; Kovaleva, M.A.; Kushakova, N.S.; Klemenkova, Z.S.; Blinnikova, Z.K.; Popov, A.Y.; Khotina, I.A. Microporous polymers based on rigid-chain di- and triacetyl aromatic compounds. Polym. Sci. Ser. B 2022, 64, 155–160. [Google Scholar] [CrossRef]
- Khotina, I.A.; Filippov, O.A.; Kovalev, A.I. Microporous polyphenylenes based on diacetylaromatic compounds. Mendeleev Commun. 2020, 30, 366–368. [Google Scholar] [CrossRef]
- Kovalev, A.I.; Pastukhov, A.V.; Tkachenko, E.S.; Klemenkova, Z.S.; Kuvshinov, I.R.; Khotina, I.A. Polyphenylenes with phen-1,3,5-triyl branching moieties based on p-diacetylbenzene: Synthesis and study of porosity and heat resistance. Polym. Sci. Ser. C 2020, 62, 205–213. [Google Scholar] [CrossRef]
- Beamson, G.; Briggs, D. High Resolution XPS of Organic Polymers: The Scienta ESCA 300 Database; Wiley: Chichester, UK, 1992; 280p, ISBN 0471 935921. [Google Scholar]
- Hales, B.J.; Case, E.E. Immobilized radicals. IV. Biological semiquinone anions and neutral semiquinones. Biochim. Biophys. Acta Bioenerg. 1981, 637, 291–302. [Google Scholar] [CrossRef]






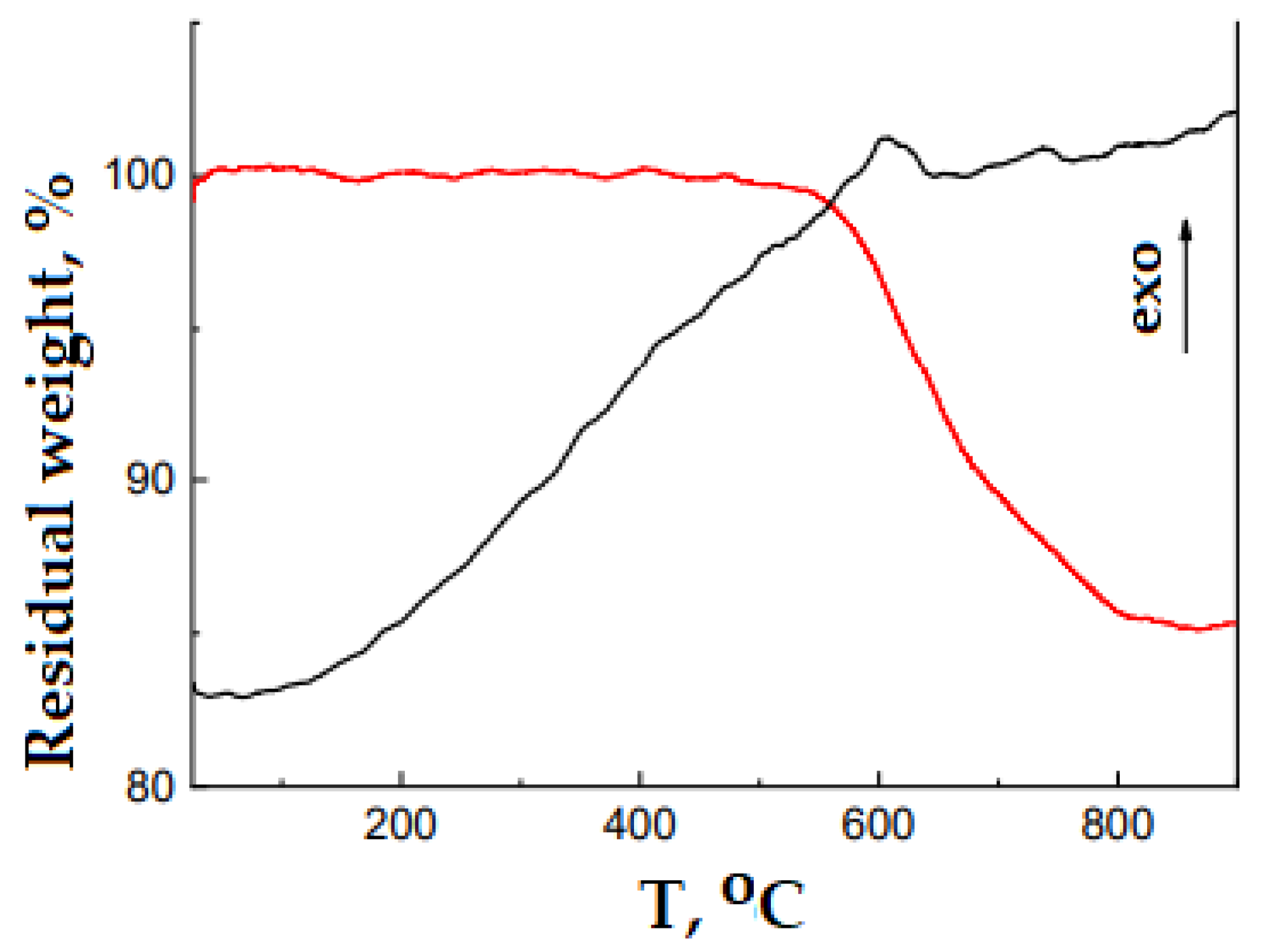
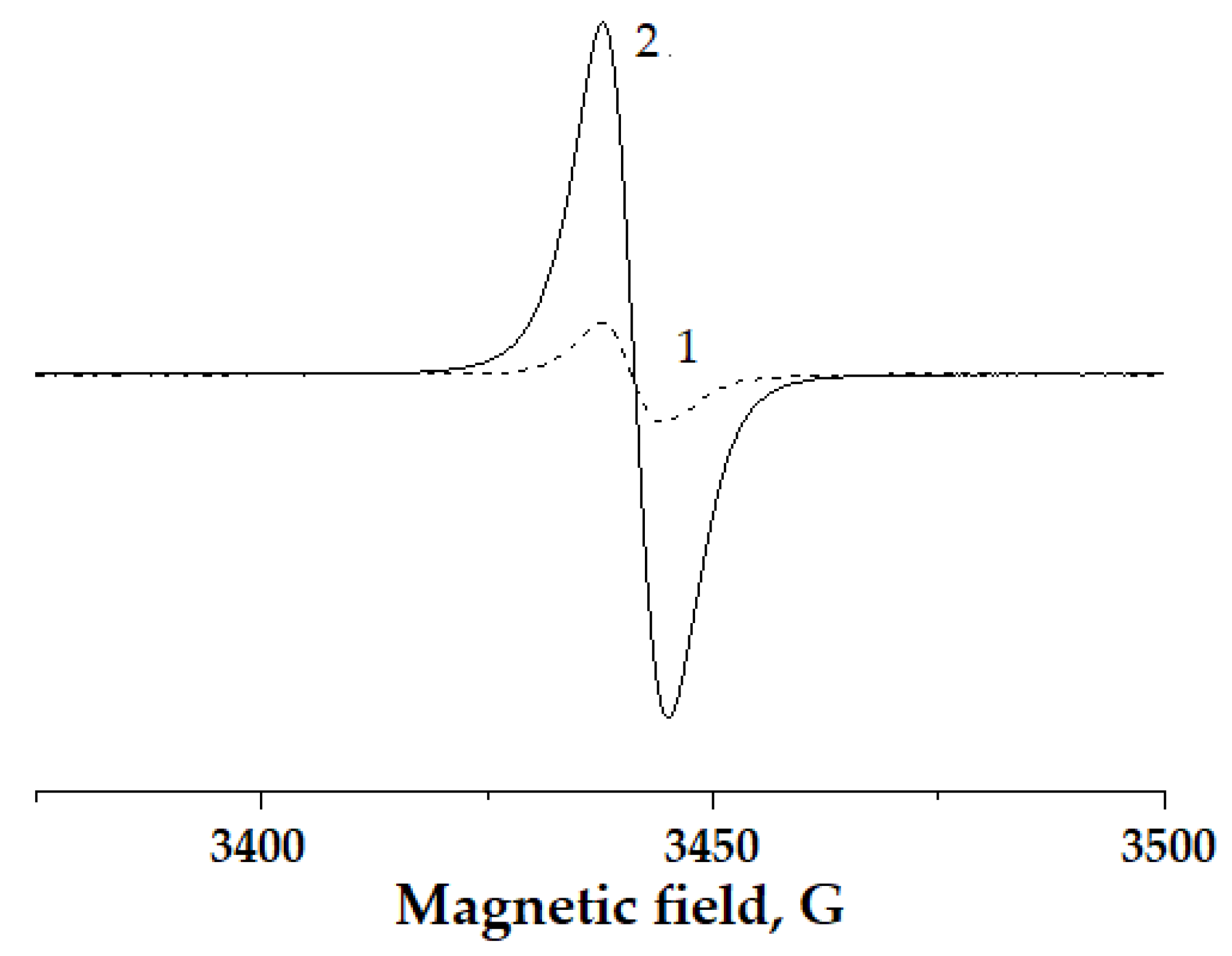
| Synthesis Conditions | Properties of the Polymers | |||||||
|---|---|---|---|---|---|---|---|---|
| № | Monomer | The Ratio of TEOF and a Monomer mol/mol | Percentage of TEOF in the Initial Mixture, TEOF-Toluene, % | Polymer | C, % | H, % | N, % | Yield, % |
| 1 | 8a | 2.4 | 3.6 | 9a | 91.84 | 5.20 | 0 | 70 |
| 2 | 8a | 3.6 | 5.5 | 10a | 91.09 | 5.27 | 0.99 | 86 |
| 3 | 8a | 7 | 11 | 10a | 90.26 | 5.08 | 2.2 | 78 |
| 4 | 8a | 12 | 18 | 10a | 88.72 | 4.94 | 2.7 | 68 |
| 5 | 8a | 16.8 | 25 | 10a | 88.02 | 5.08 | 3.96 | 60 |
| 6 | 8a | 33 | 50 | 10a | 87.06 | 4.96 | 3.86 | 54 |
| 7 | 8b | 2.4 | 3.6 | 9b | 92.34 | 5.37 | 0 | 76 |
| 8 | 8b | 3.6 | 5.5 | 10b | 91.76 | 5.29 | 0.76 | 88 |
| 9 | 8b | 7 | 11 | 10b | 91.06 | 5.29 | 1.26 | 84 |
| 10 | 8b | 12 | 18 | 10b | 90.14 | 5.17 | 2.22 | 83 |
| 11 | 8b | 16.8 | 25 | 10b | 89.71 | 5.17 | 2.25 | 67 |
| 12 | 8b | 33 | 50 | 10b | 89.67 | 5.05 | 1.87 | 62 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kovalev, A.I.; Khotina, I.A.; Kovaleva, M.A.; Naumkin, A.V.; Ionova, I.S.; Mezhuev, Y.O. Polyphenylenepyridines Based on Acetylaromatic Compounds. J. Compos. Sci. 2023, 7, 359. https://doi.org/10.3390/jcs7090359
Kovalev AI, Khotina IA, Kovaleva MA, Naumkin AV, Ionova IS, Mezhuev YO. Polyphenylenepyridines Based on Acetylaromatic Compounds. Journal of Composites Science. 2023; 7(9):359. https://doi.org/10.3390/jcs7090359
Chicago/Turabian StyleKovalev, Alexey I., Irina A. Khotina, Maria A. Kovaleva, Alexander V. Naumkin, Irina S. Ionova, and Yaroslav O. Mezhuev. 2023. "Polyphenylenepyridines Based on Acetylaromatic Compounds" Journal of Composites Science 7, no. 9: 359. https://doi.org/10.3390/jcs7090359







