Mechanics and Crack Analysis of Irida Graphene Bilayer Composite: A Molecular Dynamics Study
Abstract
:1. Introduction
2. Materials and Methods
3. Results
3.1. Unbroken Analysis
3.1.1. Monolayer Comparison of GE and IG
3.1.2. Bilayer Comparison of GE/GE, IG/IG, and GE/IG
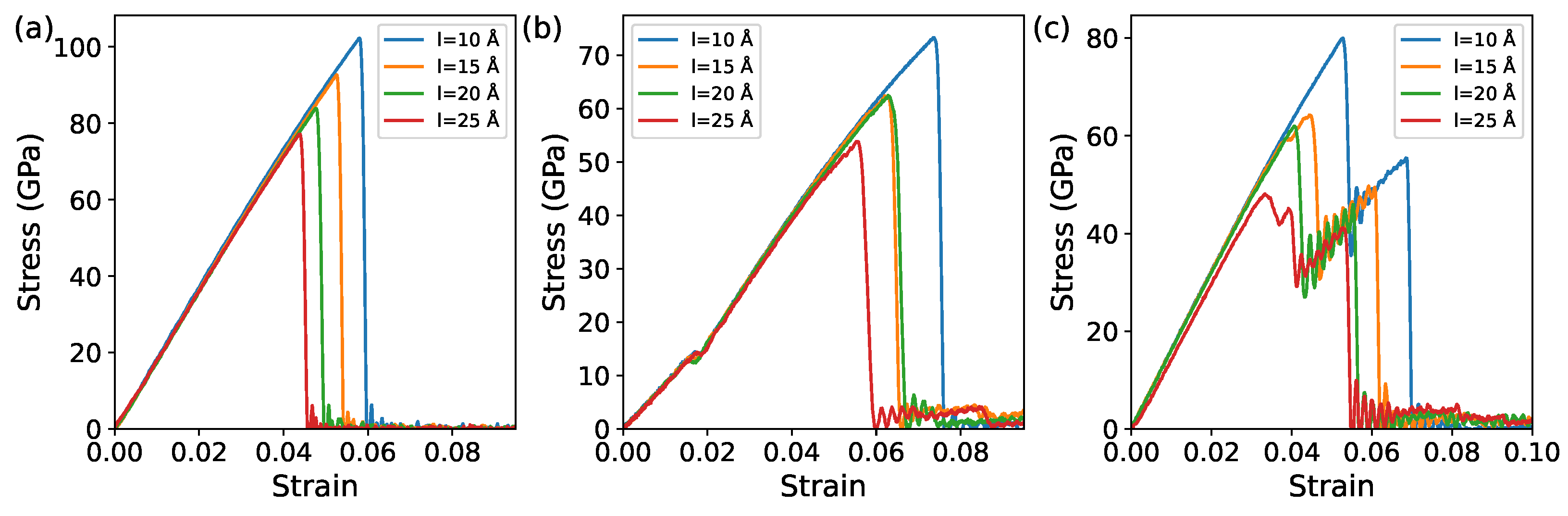
3.2. Cracks Analysis with Different Sizes
3.2.1. Monolayer Comparison of GE and IG
3.2.2. Bilayer Comparison of GE/GE, IG/IG, and GE/IG
3.3. Cracks Analysis with Different Crack Directions
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Novoselov, K.S.; Geim, A.K.; Morozov, S.V.; Jiang, D.; Zhang, Y.; Dubonos, S.V.; Grigorieva, I.V.; Firsov, A.A. Electric field effect in atomically thin carbon films. Science 2004, 306, 666–669. [Google Scholar] [CrossRef] [PubMed]
- Aliofkhazraei, M.; Ali, N.; Milne, W.I.; Ozkan, C.S.; Mitura, S.; Gervasoni, J.L. Mechanical properties of graphene. In Graphene Science Handbook; CRC Press: Boca Raton, FL, USA, 2016; pp. 19–32. [Google Scholar] [CrossRef]
- Agius Anastasi, A.; Konstantinos Ritos, G.C.; Borg, M.K. Mechanical properties of pristine and nanoporous graphene. Mol. Simul. 2016, 42, 1502–1511. [Google Scholar] [CrossRef]
- Dewapriya, M.A.N.; Srikantha Phani, A.; Rajapakse, R.K.N. Influence of temperature and free edges on the mechanical properties of graphene. Model. Simul. Mater. Sci. Eng. 2013, 21, 065017. [Google Scholar] [CrossRef]
- Zandiatashbar, A.; Lee, G.H.; An, S.J.; Lee, S.; Mathew, N.; Terrones, M.; Hayashi, T.; Picu, C.R.; Hone, J.; Koratkar, N. Effect of defects on the intrinsic strength and stiffness of graphene. Nat. Commun. 2014, 5, 3186. [Google Scholar] [CrossRef]
- Cheng, Y.; Zhou, S.; Hu, P.; Zhao, G.; Li, Y.; Zhang, X.; Han, W. Enhanced mechanical, thermal, and electric properties of graphene aerogels via supercritical ethanol drying and high-temperature thermal reduction. Sci. Rep. 2017, 7, 1439. [Google Scholar] [CrossRef] [PubMed]
- Ranjbartoreh, A.R.; Wang, B.; Shen, X.; Wang, G. Advanced mechanical properties of graphene paper. J. Appl. Phys. 2011, 109, 014306. [Google Scholar] [CrossRef]
- Li, X.; Guo, J. Numerical Investigation of the Fracture Properties of Pre-Cracked Monocrystalline/Polycrystalline Graphene Sheets. Materials 2019, 12, 263. [Google Scholar] [CrossRef]
- Akinwande, D.; Brennan, C.J.; Scott Bunch, J.; Egberts, P.; Felts, J.R.; Gao, H.; Huang, R.; Kim, J.S.; Li, T.; Li, Y.; et al. A review on mechanics and mechanical properties of 2D materials—Graphene and beyond. Extrem. Mech. Lett. 2017, 13, 42–77. [Google Scholar] [CrossRef]
- Cao, Q.; Geng, X.; Wang, H.; Wang, P.; Liu, A.; Lan, Y.; Peng, Q. A Review of Current Development of Graphene Mechanics. Crystals 2018, 8, 357. [Google Scholar] [CrossRef]
- Lee, G.H.; Cooper, R.C.; An, S.J.; Lee, S.; van der Zande, A.; Petrone, N.; Hammerberg, A.G.; Lee, C.; Crawford, B.; Oliver, W.; et al. High-strength chemical-vapor-deposited graphene and grain boundaries. Science 2013, 340, 1073–1076. [Google Scholar] [CrossRef]
- Neto, A.H.C.; Castro Neto, A.H.; Guinea, F.; Peres, N.M.R.; Novoselov, K.S.; Geim, A.K. The electronic properties of graphene. Rev. Mod. Phys. 2009, 81, 109. [Google Scholar] [CrossRef]
- Balandin, A.A.; Ghosh, S.; Bao, W.; Calizo, I.; Teweldebrhan, D.; Miao, F.; Lau, C.N. Superior thermal conductivity of single-layer graphene. Nano Lett. 2008, 8, 902–907. [Google Scholar] [CrossRef]
- Zhao, Q.; Nardelli, M.B.; Bernholc, J. Ultimate strength of carbon nanotubes: A theoretical study. Phys. Rev. B 2002, 65, 144105. [Google Scholar] [CrossRef]
- Pereira Júnior, M.L.; da Cunha, W.F.; Giozza, W.F.; de Sousa Junior, R.T.; Ribeiro Junior, L.A. Irida-graphene: A new 2D carbon allotrope. FlatChem 2023, 37, 100469. [Google Scholar] [CrossRef]
- Allen, M.J.; Tung, V.C.; Kaner, R.B. Honeycomb carbon: A review of graphene. Chem. Rev. 2010, 110, 132–145. [Google Scholar] [CrossRef] [PubMed]
- Deng, B.; Hou, J.; Zhu, H.; Liu, S.; Liu, E.; Shi, Y.; Peng, Q. The normal-auxeticity mechanical phase transition in graphene. 2D Mater. 2017, 4, 021020. [Google Scholar] [CrossRef]
- Peng, Q.; Liang, C.; Ji, W.; De, S. A theoretical analysis of the effect of the hydrogenation of graphene to graphane on its mechanical properties. Phys. Chem. Chem. Phys. 2013, 15, 2003–2011. [Google Scholar] [CrossRef] [PubMed]
- Tozzini, V.; Pellegrini, V. Prospects for hydrogen storage in graphene. Phys. Chem. Chem. Phys. 2013, 15, 80–89. [Google Scholar] [CrossRef]
- Tan, Y.; Tao, X.; Ouyang, Y.; Peng, Q. Stable and 7.7 wt% hydrogen storage capacity of Ti decorated Irida-Graphene from first-principles calculations. Int. J. Hydrogen Energy 2023, in press. [Google Scholar] [CrossRef]
- Zhang, Y.F.; Guo, J. Li-decorated 2D Irida-graphene as a potential hydrogen storage material: A dispersion-corrected density functional theory calculations. Int. J. Hydrogen Energy 2023, in press. [Google Scholar] [CrossRef]
- Papageorgiou, D.G.; Kinloch, I.A.; Young, R.J. Mechanical properties of graphene and graphene-based nanocomposites. Prog. Mater. Sci. 2017, 90, 75–127. [Google Scholar] [CrossRef]
- Lee, C.; Wei, X.; Kysar, J.W.; Hone, J. Measurement of the elastic properties and intrinsic strength of monolayer graphene. Science 2008, 321, 385–388. [Google Scholar] [CrossRef] [PubMed]
- Roy, S.; Zhang, X.; Puthirath, A.B.; Meiyazhagan, A.; Bhattacharyya, S.; Rahman, M.M.; Babu, G.; Susarla, S.; Saju, S.K.; Tran, M.K.; et al. Structure, properties and applications of two-dimensional hexagonal boron nitride. Adv. Mater. 2021, 33, e2101589. [Google Scholar] [CrossRef] [PubMed]
- Imani Yengejeh, S.; Liu, J.; Kazemi, S.A.; Wen, W.; Wang, Y. Effect of structural phases on mechanical properties of molybdenum disulfide. ACS Omega 2020, 5, 5994–6002. [Google Scholar] [CrossRef]
- Sha, Z.D.; Pei, Q.X.; Ding, Z.; Jiang, J.W.; Zhang, Y.W. Mechanical properties and fracture behavior of single-layer phosphorene at finite temperatures. J. Phys. D Appl. Phys. 2015, 48, 395303. [Google Scholar] [CrossRef]
- Sun, Y.; Peng, Q.; Lu, G. Quantum mechanical modeling of hydrogen assisted cracking in aluminum. Phys. Rev. B Condens. Matter 2013, 88, 104109. [Google Scholar] [CrossRef]
- Zhu, H.; Fan, T.; Peng, Q.; Zhang, D. Giant Thermal Expansion in 2D and 3D Cellular Materials. Adv. Mater. 2018, 30, e1705048. [Google Scholar] [CrossRef]
- Peng, Q.; Ji, W.; De, S. Mechanical properties of the hexagonal boron nitride monolayer: Ab initio study. Comput. Mater. Sci. 2012, 56, 11–17. [Google Scholar] [CrossRef]
- Peng, Q.; Lu, G. A comparative study of fracture in Al: Quantum mechanical vs. empirical atomistic description. J. Mech. Phys. Solids 2011, 59, 775–786. [Google Scholar] [CrossRef]
- Peng, Q.; Zhang, X.; Hung, L.; Carter, E.A.; Lu, G. Quantum simulation of materials at micron scales and beyond. Phys. Rev. B Condens. Matter 2008, 78, 054118. [Google Scholar] [CrossRef]
- Hou, J.; Deng, B.; Zhu, H.; Lan, Y.; Shi, Y.; De, S.; Liu, L.; Chakraborty, P.; Gao, F.; Peng, Q. Magic auxeticity angle of graphene. Carbon 2019, 149, 350–354. [Google Scholar] [CrossRef]
- Peng, Q.; Meng, F.; Yang, Y.; Lu, C.; Deng, H.; Wang, L.; De, S.; Gao, F. Shockwave generates dislocation loops in bcc iron. Nat. Commun. 2018, 9, 4880. [Google Scholar] [CrossRef] [PubMed]
- Plimpton, S. Fast parallel algorithms for short-range molecular dynamics. J. Comput. Phys. 1995, 117, 1–19. [Google Scholar] [CrossRef]
- Stukowski, A. Visualization and analysis of atomistic simulation data with OVITO–the Open Visualization Tool. Model. Simul. Mater. Sci. Eng. 2009, 18, 015012. [Google Scholar] [CrossRef]
- Humphrey, W.; Dalke, A.; Schulten, K. VMD: Visual molecular dynamics. J. Mol. Graph. 1996, 14, 33–38. [Google Scholar] [CrossRef]
- Stuart, S.J.; Tutein, A.B.; Harrison, J.A. A reactive potential for hydrocarbons with intermolecular interactions. J. Chem. Phys. 2000, 112, 6472–6486. [Google Scholar] [CrossRef]
- Yang, X.; Wu, S.; Xu, J.; Cao, B.; To, A.C. Spurious heat conduction behavior of finite-size graphene nanoribbon under extreme uniaxial strain caused by the AIREBO potential. Phys. E Low Dimens. Syst. Nanostruct. 2018, 96, 46–53. [Google Scholar] [CrossRef]
- Wang, X.; Hong, Y.; Ma, D.; Zhang, J. Molecular dynamics study of thermal transport in a nitrogenated holey graphene bilayer. J. Mater. Chem. C Mater. Opt. Electron. Devices 2017, 5, 5119–5127. [Google Scholar] [CrossRef]
- Her, S.C.; Zhang, K.C. Mode I fracture toughness of graphene reinforced nanocomposite film on Al substrate. Nanomaterials 2021, 11, 1743. [Google Scholar] [CrossRef]
- Yu, T.; Li, J.; Han, M.; Zhang, Y.; Li, H.; Peng, Q.; Tang, H.K. Enhancing the Mechanical Stability of 2D Fullerene with a Graphene Substrate and Encapsulation. Nanomaterials 2023, 13, 1936. [Google Scholar] [CrossRef]
- Yu, T.; Li, J.; Yang, Z.; Li, H.; Peng, Q.; Tang, H.K. Effects of Crack Formation on the Mechanical Properties of Bilayer Graphene: A Comparative Analysis. Crystals 2023, 13, 584. [Google Scholar] [CrossRef]

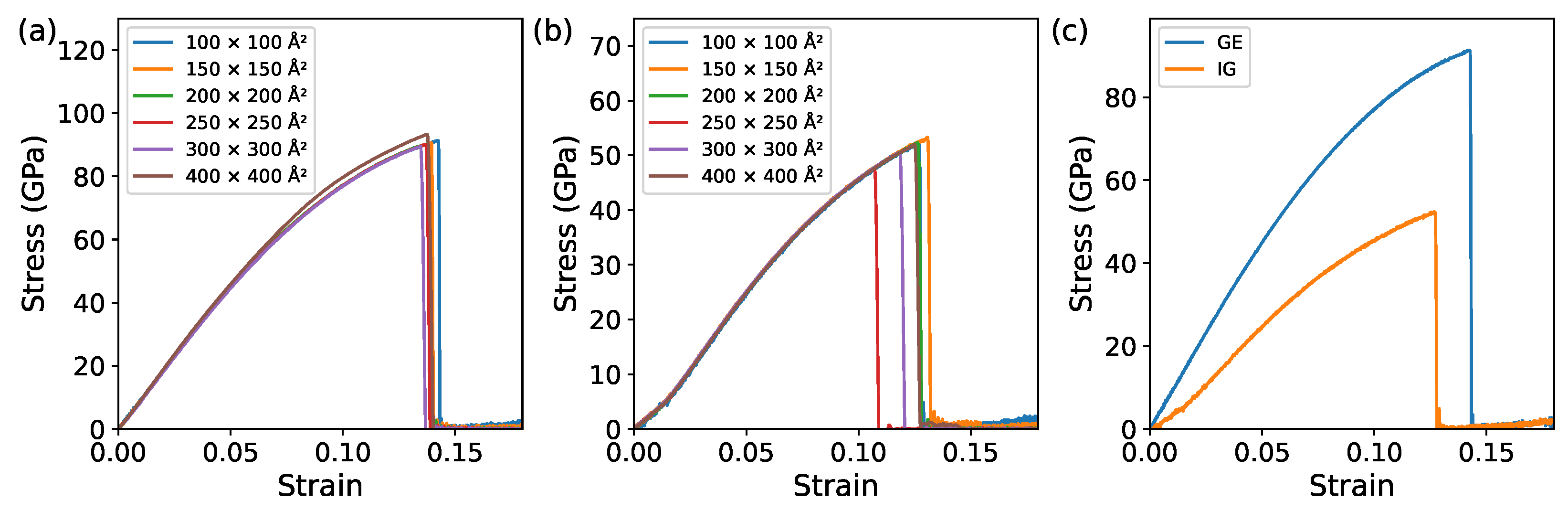







| Strcture | Fracture Stress (GPa) | Strain Energy (J/m) | Young’s Modulus (GPa) |
|---|---|---|---|
| GE | 90.1 | 7.6 | 911.2 |
| IG | 52.3 | 3.7 | 520.4 |
| qTPC | 17.6 | 1.1 | 134.7 |
| qHPC | 24.5 | 1.6 | 191.6 |
| Strcture | Fracture Stress (GPa) | Strain Energy (J/m) | Young’s Modulus (GPa) |
|---|---|---|---|
| GE/GE | 91.5 | 7.8 | 932.3 |
| IG/IG | 51.4 | 3.4 | 543.5 |
| GE/IG | 67.3/48.5 | 5.9 | 791.8 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, J.; Han, M.; Zhao, S.; Li, T.; Yu, T.; Zhang, Y.; Tang, H.-K.; Peng, Q. Mechanics and Crack Analysis of Irida Graphene Bilayer Composite: A Molecular Dynamics Study. J. Compos. Sci. 2023, 7, 490. https://doi.org/10.3390/jcs7120490
Li J, Han M, Zhao S, Li T, Yu T, Zhang Y, Tang H-K, Peng Q. Mechanics and Crack Analysis of Irida Graphene Bilayer Composite: A Molecular Dynamics Study. Journal of Composites Science. 2023; 7(12):490. https://doi.org/10.3390/jcs7120490
Chicago/Turabian StyleLi, Jianyu, Mingjun Han, Shuai Zhao, Teng Li, Taotao Yu, Yinghe Zhang, Ho-Kin Tang, and Qing Peng. 2023. "Mechanics and Crack Analysis of Irida Graphene Bilayer Composite: A Molecular Dynamics Study" Journal of Composites Science 7, no. 12: 490. https://doi.org/10.3390/jcs7120490






