Characterization of Caseinate–Carboxymethyl Chitosan-Based Edible Films Formulated with and without Transglutaminase Enzyme
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Edible Film Formulations
2.2.1. Preparation of Films
2.2.2. Thickness Measurement
2.2.3. Film Solubility in Water
2.2.4. Moisture Content
2.2.5. Color
2.2.6. Opacity Index
2.2.7. Water Vapor Permeability (WVP)
2.2.8. Mechanical Properties (TS, EAB)
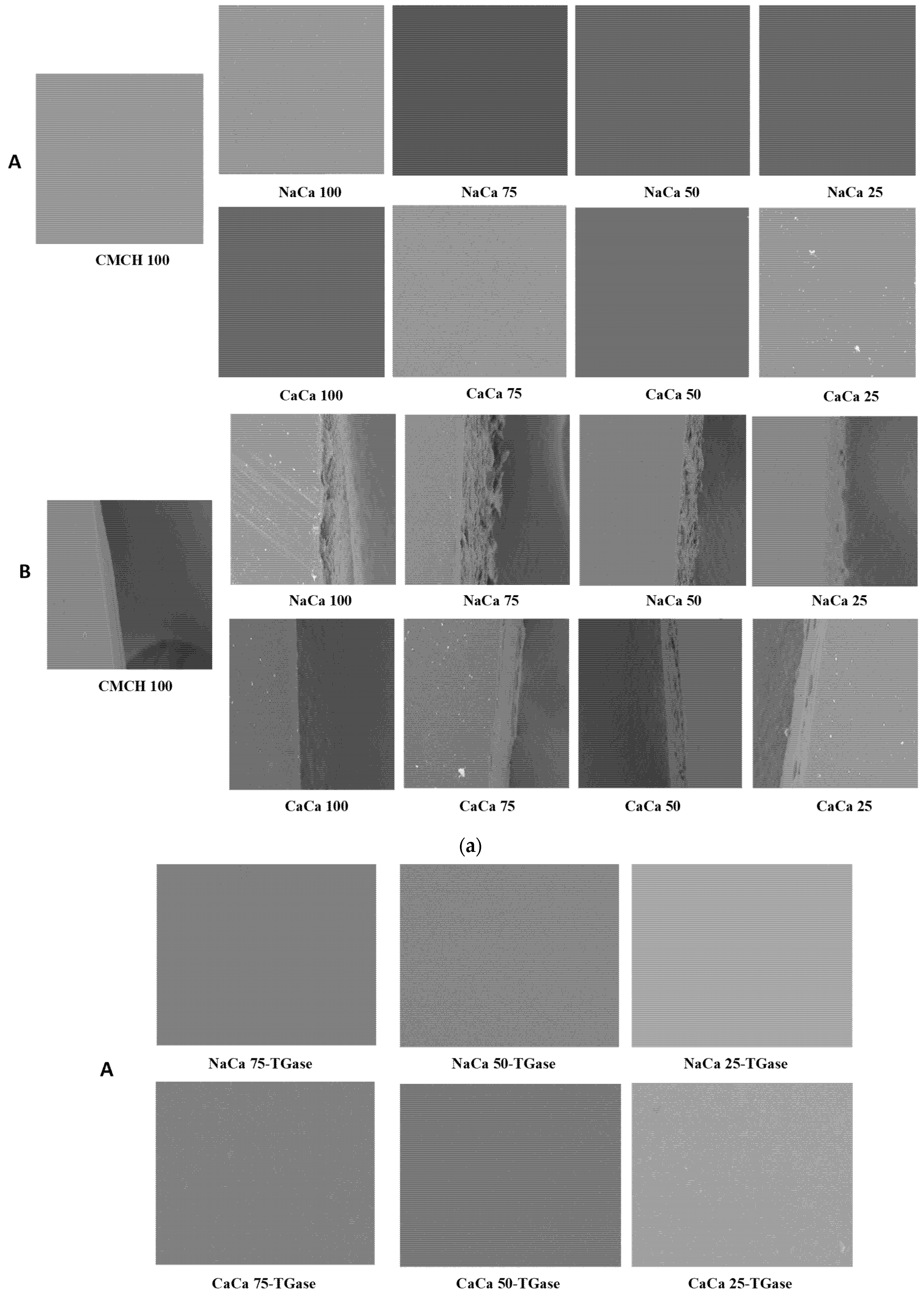
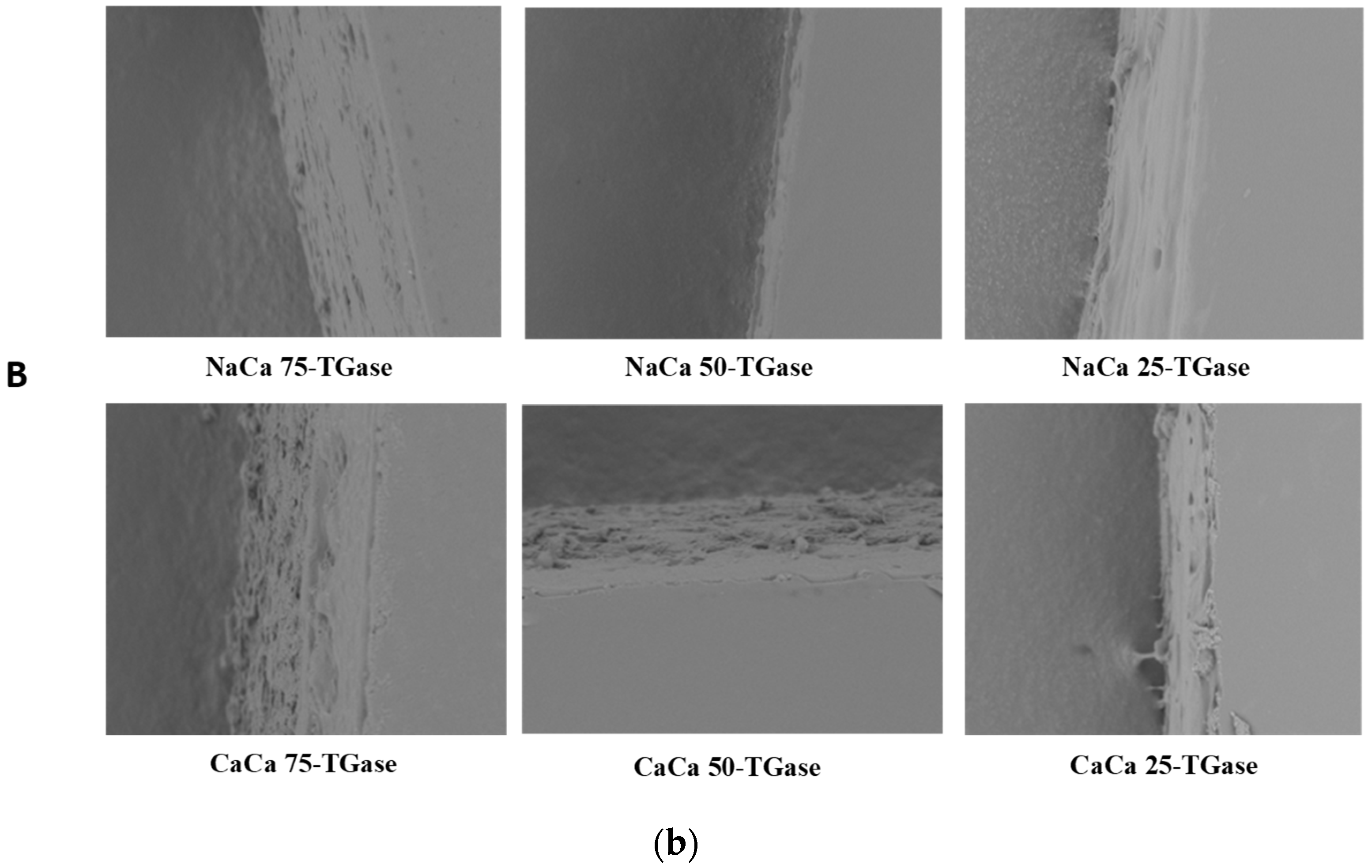
2.2.9. Scanning Electron Microscopy (SEM)
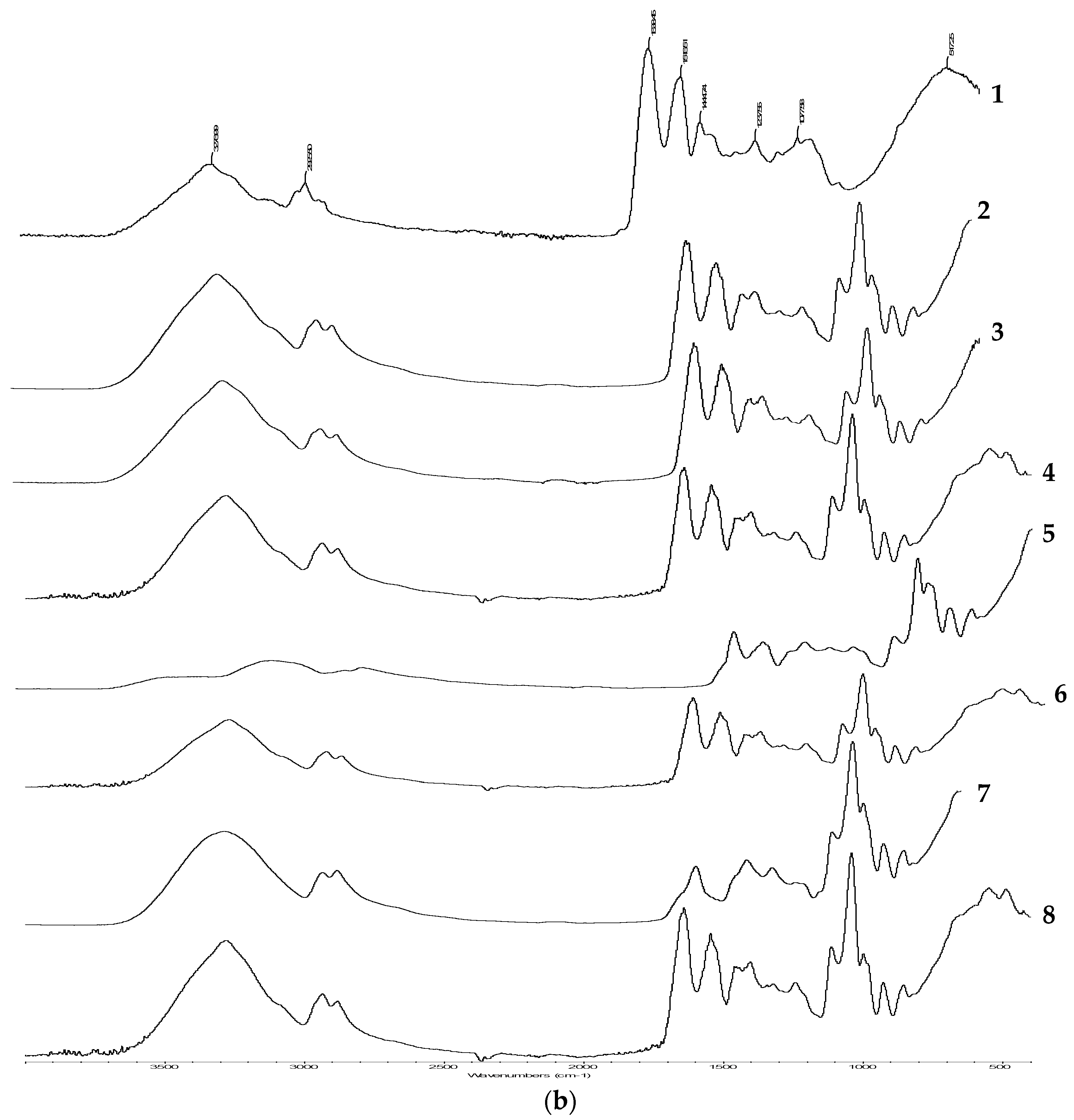
2.2.10. Fourier Transform Infrared Spectroscopy (FT-IR)
2.2.11. Statistical Analysis
3. Results and Discussion
3.1. Film Thickness
3.2. Water Solubility
3.3. Moisture Content
3.4. Color
3.5. Mechanical Properties
3.6. SEM
3.7. Water Vapor Permeability
3.8. FTIR
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Falco, I.; Flores-Meraz, P.L.; Randazzo, W.; Sanchez, G.; Lopez-Rubio, A.; Fabra, M.J. Antiviral activity of alginate-oleic acid based coatings incorporating green tea extract on strawberries and raspberries. Food Hydrocoll. 2019, 87, 611–618. [Google Scholar] [CrossRef] [Green Version]
- Lucey, J.A.; Srinivasan, M.; Singh, H.; Munro, P.A. Characterization of commercial and experimental sodium caseinates by multiangle laser light scattering and size exclusion chromatography. J. Agric. Food Chem. 2000, 48, 1610–1616. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Zhong, F.; Li, Y.; Shoemaker, C.F.; Xia, W.S. Preparation and characterization of pullulan-chitosan and pullulan-carboxymethyl chitosan blended films. Food Hydrocoll. 2013, 30, 82–91. [Google Scholar] [CrossRef]
- Orliac, O.; Rouilly, A.; Silvestre, F.; Rigal, L. Effects of varoius plasticizers on the mechanical properties. water resistance and aging of thermo-moulded films made from sunflower proteins. Ind. Crops Prod. 2003, 18, 91–100. [Google Scholar] [CrossRef]
- Liu, J.; Liu, S.; Chen, Y.; Zhang, L.; Kan, J.; Jin, C. Physical, mechanical and antioxidant properties of chitosan films grafted with different hydroxybenzoic acids. Food Hydrocoll. 2017, 71, 176–186. [Google Scholar] [CrossRef]
- Dejong, C.A.H.; Koppelman, S.J. Transglutaminase catalyzed reactions. Impact on food applications. J. Food Sci. 2002, 67, 2798–2806. [Google Scholar] [CrossRef]
- Di Pierro, P.; Chico, B.; Villalonga, R.; Marinielle, L.; Damiano, A.E.; Masi, P. Chitosan-whey protein edible films produced in the absence of transgluaminase : Anaysis of their mechanical and barrier properties. Biomacromolecules 2006, 7, 744–749. [Google Scholar] [CrossRef] [Green Version]
- Di Pierro, P.; Chico, B.; Villalonga, R.; Marinielle, L.; Masi, P.; Porta, R. Transglutaminase-catalyzed preparation of chitosan-ovalbumin films. Enzym. Microb. Technol. 2007, 40, 437–441. [Google Scholar] [CrossRef] [Green Version]
- Díaz-Montes, E.; Castro-Muñoz, R. Edible Films and Coatings as Food-Quality Preservers: An Overview. Foods 2021, 10, 249. [Google Scholar] [CrossRef]
- Shahidi, F.; Hossain, A. Preservation of aquatic food using edible films and coatings containing essential oils: A review. Crit. Rev. Food Sci. Nutr. 2022, 62, 66–105. [Google Scholar] [CrossRef]
- Pereda, M.; Aranguren, M.I.; Marcovich, N.E. Characterization of chitosan/ caseinate films. J. Appl. Polym. Sci. 2008, 2, 1080–1090. [Google Scholar] [CrossRef]
- Volpe, S.; Cavella, S.; Masi, P.; Torrieri, E. Effect of solid concentration on structure and properties of chitosan-caseinate blend films. Food Packag. Shelf Life 2017, 13, 76–84. [Google Scholar] [CrossRef]
- Saberi, B.; Thakur, R.; Juong, Q.; Chockchaisawasdee, S.; Golding, J.; Scarlett, C.; Stathopoulos, C. Optimization of physical and optical properties of biodegrable edible film based on pea starch and guar gum. Ind. Crops Prod. 2016, 86, 342–352. [Google Scholar] [CrossRef]
- Popovic, S.; Pericin, D.; Vastag, Z.; Lazic, C.; Popovic, L. Pumpkin oil cake protein isolate films as potential gas barrier coating. J. Food Eng. 2012, 110, 374–379. [Google Scholar] [CrossRef]
- Ghasemlou, M.; Khodaiyan, F.; Oromiehie, A.; Yarmand, M.S. Development and characterisation of a new biodegradable edible film made from kefiran, an exopolysaccaride obtained from kefir grains. Food Chem. 2011, 127, 1496–1502. [Google Scholar] [CrossRef]
- Leceta, L.; Guerrero, P.; Caba, K. Functional properties of chitosan-based films. Carbohydr. Polym. 2013, 93, 339–346. [Google Scholar] [CrossRef]
- Wang, L.; Wang, Q.; Tong, J.; Zhou, J. physicochemical properties of chitosan films incorporated with honeysuckle flower extract for active food packaging. J. Food Process Eng. 2015, 40, 1745–4530. [Google Scholar] [CrossRef] [Green Version]
- Yu, Z.; Sun, L.; Wang, W.; Zeng, W.; Mustapha, A.; Lin, M. Soy protein-based films incorporated with cellulose nanocrystals and pine needle extract for active packaging. Ind. Crops Prod. 2018, 112, 412–419. [Google Scholar] [CrossRef]
- Castro-Muñoz, R.; González-Valdez, J. New trends in biopolymer-based membranes for pervaporation. Molecules 2019, 24, 3584. [Google Scholar] [CrossRef] [Green Version]
- Maryam Adilah, Z.A.; Jamilah, B.; Nur Hanani, Z.A. Functional and antioxidant properties of protein-based films incorporated with mango kernel extract for active packaging. Food Hydrocoll. 2018, 74, 207–218. [Google Scholar] [CrossRef]
- Jiang, S.-J.; Zhang, X.; Ma, Y.; Tuo, Y.; Qian, F.; Fu, W.; Mu, G. Characterization of whey protein-carboxymethylated chitosan composite films with and without transglutaminase treatment. Carbohydr. Polym. 2016, 153, 153–159. [Google Scholar] [CrossRef] [PubMed]
- Leuangsukrerk, M.; Phupksakul, T.; Tananuwong, K.; Boromichaichartkul, C.; Janjarasskul, T. Properties of konjac glucomannan-whey protein isolate blend films. Food Sci. Technol. 2014, 59, 94–100. [Google Scholar] [CrossRef]
- Carpine, D.; Dagostin, J.L.A.; Bertan, L.C.; Mafra, M.R. Development and characterization of soy protein isolate emulsion-based edible films with added coconut oil for olive oil packaging: Barrier, mechanical, and thermal properties. Food Bioprocess Technol. 2015, 8, 1811–1823. [Google Scholar] [CrossRef]
- Chambi, H.; Grosso, C. Edible films produced with gelatin and casein cross-linked with transgluaminase. Int. Food Res. J. 2006, 39, 458–466. [Google Scholar] [CrossRef]
- Dniels, C.A. Polymers: Structure and Propertes; Technomic Publishing Co., Inc.: Lancaster, CA, USA, 1989. [Google Scholar]
- Arrieta, M.P.; Peltzer, M.A.; Garrigos, M.; Jimenez, A. Structure and mechanical properties of sodium and calcium caseinate edible active films with carvacrol. J. Food Eng. 2013, 114, 486–494. [Google Scholar] [CrossRef] [Green Version]
- Barreto, P.L.M.; Pires, A.T.N.; Soldi, V. Thermal degradation of edible films based on milk proteins and gelatin in inert atmosphere. Polym. Degrad. Stab. 2003, 1, 147–152. [Google Scholar] [CrossRef]
- Pelissari, F.M.; Grossmann, M.V.E.; Yamashita, F.; Pineda, E.A.G. Antimicrobial, mechanical, and barrier properties of cassava starch–chitosan films incorporated with oregano essential oil. J. Agric. Food Chem. 2009, 16, 7499–7504. [Google Scholar] [CrossRef]




| Sample | Thickness (mm) | Water Solubility (%) | Moisture Content (%) |
|---|---|---|---|
| CMCH 100 | 0.123 ± 0.09 | 55.93 ± 0.33 | 22.58 ± 0.19 |
| NaCA 100 | 0.167 ± 0.07 | 39.04 ± 0.40 | 38.50 ± 0.40 |
| NaCA 75 | 0.163 ± 0.08 | 33.84 ± 0.51 | 24.19 ± 0.26 |
| NaCA 75-TGase | 0.151 ± 0.01 | 40.17 ± 0.57 | 66.48 ± 0.40 |
| NaCA 50 | 0.155 ± 0.21 | 37.60 ± 2.00 | 21.32 ± 0.27 |
| NaCA 50-TGase | 0.165 ± 0.11 | 29.98 ± 0.06 | 58.88 ± 0.67 |
| NaCA 25 | 0.130 ± 0.07 | 31.88 ± 1.44 | 17.69 ± 0.16 |
| NaCA 25-TGase | 0.148 ± 0.34 | 30.00 ± 1.79 | 48.25 ± 0.68 |
| CaCA 100 | 0.162 ± 0.01 | 36.48 ± 0.35 | 33.37 ± 0.23 |
| CaCA 75 | 0.168 ± 0.05 | 33.39 ± 0.31 | 22.98 ± 0.25 |
| CaCA 75-TGase | 0.159 ± 0.02 | 44.48 ± 0.37 | 71.06 ± 0.71 |
| CaCA 50 | 0.150 ± 0.01 | 30.34 ± 0.14 | 17.07 ± 0.80 |
| CaCA 50-TGase | 0.143 ± 0.08 | 30.79 ± 0.68 | 64.92 ± 0.86 |
| CaCA 25 | 0.138 ± 0.01 | 41.89 ± 1.32 | 18.12 ± 0.20 |
| CaCA 25-TGase | 0.139 ± 0.62 | 31.82 ± 1.51 | 50.24 ± 0.17 |
| Sample | L* | a* | b* | WI | Opacity | |
|---|---|---|---|---|---|---|
| CMCH 100 | 90.3 ± 0.67 | −0.72 ± 0.01 | 6.59 ± 0.10 | 9.35 ± 0.48 | 88.2 ± 0.62 | 0.58 ± 0.84 |
| NaCA 100 | 96.0 ± 0.62 | −0.45 ± 0.16 | 2.18 ± 0.21 | 2.60 ± 0.17 | 95.4 ± 0.47 | 0.22 ± 0.17 |
| NaCA 75 | 90.4 ± 0.48 | −0.94 ± 0.04 | 5.49 ± 0.66 | 8.49 ± 0.08 | 88.9 ± 0.10 | 0.82 ± 0.21 |
| NaCA75-Gase | 90.6 ± 0.06 | −1.01 ± 0.04 | 4.56 ± 0.02 | 7.68 ± 0.05 | 89.6 ± 0.05 | 0.48 ± 0.02 |
| NaCA 50 | 91.2 ± 0.31 | −1.14 ± 0.41 | 4.75 ± 0.58 | 7.45 ± 0.72 | 89.9 ± 0.63 | 0.62 ± 0.12 |
| NaCA50-Gase | 91.0 ± 0.88 | −0.77 ± 0.13 | 2.92 ± 0.25 | 6.43 ± 0.83 | 90.5 ± 0.88 | 0.39 ± 0.32 |
| NaCA 25 | 91.1 ± 0.76 | −1.09 ± 0.12 | 4.94 ± 0.86 | 7.69 ± 0.39 | 89.7 ± 0.43 | 0.54 ± 0.11 |
| NaCA25-Gase | 92.0 ± 0.35 | −0.65 ± 0.02 | 2.21 ± 0.30 | 5.27 ± 0.16 | 91.6 ± 0.25 | 0.84 ± 0.26 |
| CaCA 100 | 96.2 ± 0.70 | −0.81 ± 0.4 | 2.79 ± 0.25 | 3.11 ± 0.18 | 95.6 ± 0.65 | 0.83 ± 0.04 |
| CaCA 75 | 90.7 ± 0.31 | −1.45 ± 0.13 | 4.93 ± 0.53 | 8.24 ± 0.48 | 89.1 ± 0.43 | 0.23 ± 0.05 |
| CaCA75-Gase | 89.9 ± 0.24 | −1.30 ± 0.02 | 4.76 ± 0.32 | 8.44 ± 0.16 | 88.8 ± 0.16 | 0.54 ± 0.02 |
| CaCA 50 | 91.8 ± 0.20 | −0.95 ± 0.05 | 3.44 ± 0.09 | 6.09 ± 0.10 | 91.1 ± 0.15 | 0.37 ± 0.03 |
| CaCA50-Gase | 90.2 ± 0.62 | −0.99 ± 0.06 | 3.85 ± 0.25 | 7.58 ± 0.31 | 89.5 ± 0.36 | 0.58 ± 0.03 |
| CaCA 25 | 87.8 ± 0.14 | −1.40 ± 0.04 | 7.63 ± 0.07 | 11.9 ± 0.82 | 85.5 ± 0.66 | 0.45 ± 0.07 |
| CaCA25-Gase | 90.4 ± 0.79 | −0.75 ± 0.09 | 2.41 ± 0.25 | 6.74 ± 0.63 | 90.1 ± 0.71 | 0.68 ± 0.04 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mohamed, A.; Ramaswamy, H.S. Characterization of Caseinate–Carboxymethyl Chitosan-Based Edible Films Formulated with and without Transglutaminase Enzyme. J. Compos. Sci. 2022, 6, 216. https://doi.org/10.3390/jcs6070216
Mohamed A, Ramaswamy HS. Characterization of Caseinate–Carboxymethyl Chitosan-Based Edible Films Formulated with and without Transglutaminase Enzyme. Journal of Composites Science. 2022; 6(7):216. https://doi.org/10.3390/jcs6070216
Chicago/Turabian StyleMohamed, Amal, and Hosahalli S. Ramaswamy. 2022. "Characterization of Caseinate–Carboxymethyl Chitosan-Based Edible Films Formulated with and without Transglutaminase Enzyme" Journal of Composites Science 6, no. 7: 216. https://doi.org/10.3390/jcs6070216






