Influence of High Energy Ball Milling and Dispersant on Capacitive Properties of Fe2O3—Carbon Nanotube Composites
Abstract
:1. Introduction
2. Materials and Methods
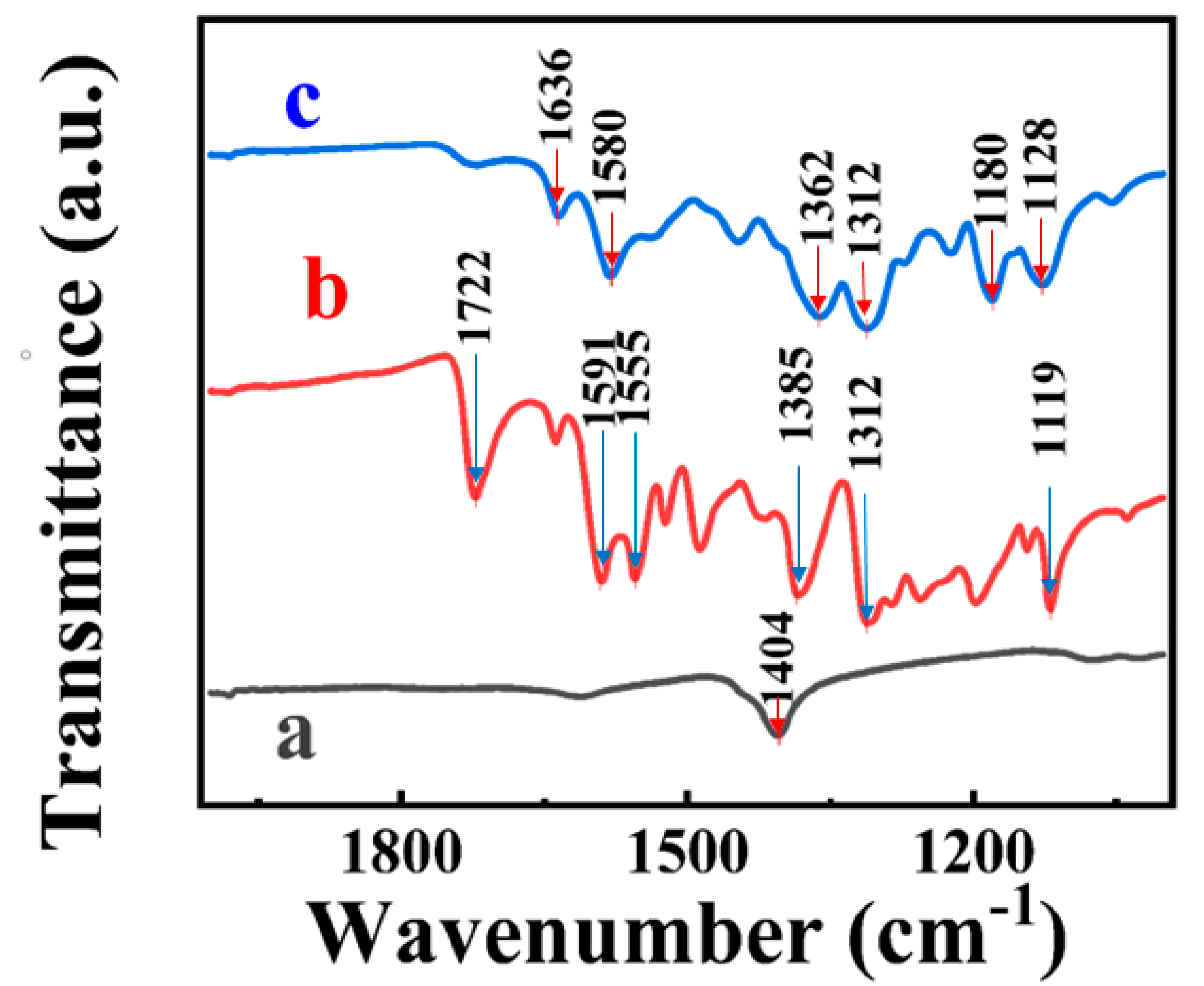
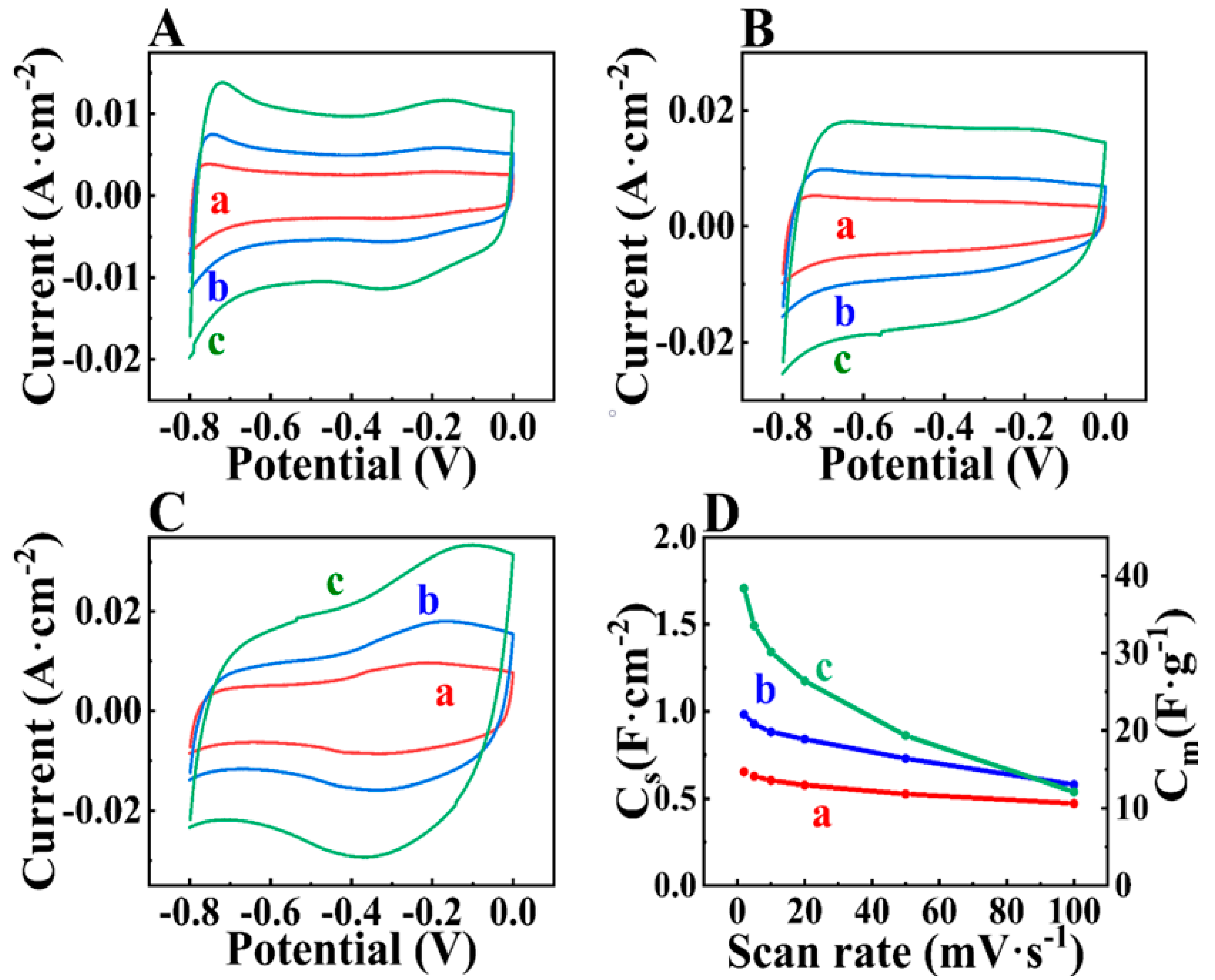
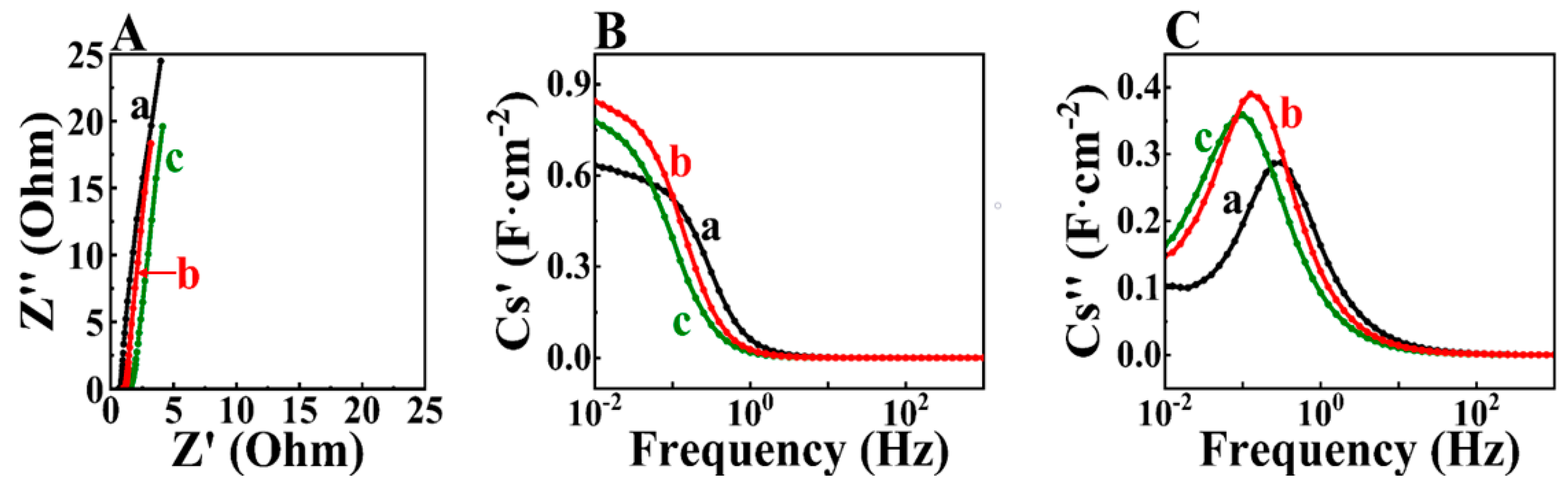
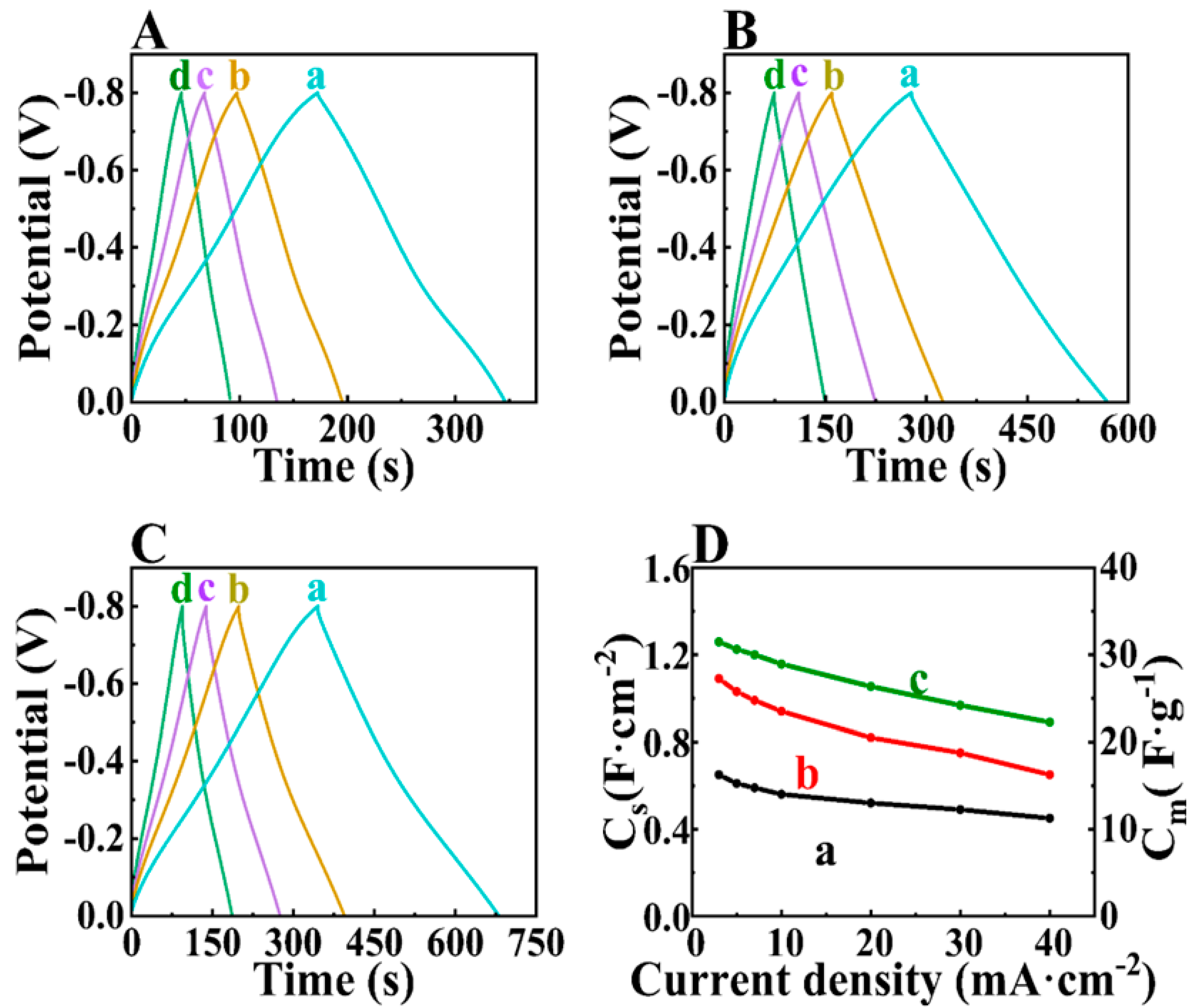
3. Results
| Material | Mass Loading | Voltage Window | CS | Reference |
|---|---|---|---|---|
| mg·cm−2 | V | mF cm−2 | ||
| α-Fe2O3/graphene | 1.0 | −1.0–0.0 | 286 | [59] |
| vs. SCE | at 2 mV·s−1 | |||
| α-Fe2O3/carbon | 2.4 | −1.0–0.0 | 862 | [60] |
| vs. SCE | at 1 mA·cm−2 | |||
| α-Fe2O3/carbon | 1.1 | −1.0–0.0 | 430.8 | [61] |
| nanoarray | vs. Ag/AgCl | at 1 mA·cm−2 | ||
| α-Fe2O3/carbon | N/A | −0.8–0.0 | 340 | [62] |
| vs. Ag/AgCl | at 1 mA·cm−2 | |||
| γ-Fe2O3/carbon | 40 | −0.8–0.0 | 1530 | this work |
| vs. SCE | at 2 mV·s−1 |
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Huang, S.-J.; Mose, M.P.; Kannaiyan, S. Artificial Intelligence Application in Solid State Mg-Based Hydrogen Energy Storage. J. Compos. Sci. 2021, 5, 145. [Google Scholar] [CrossRef]
- Kausar, A. Green Nanocomposites for Energy Storage. J. Compos. Sci. 2021, 5, 202. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, Y.; Wang, C.; Wang, Y. A Hierarchical Architecture of Functionalized Polyaniline/Manganese Dioxide Composite with Stable-Enhanced Electrochemical Performance. J. Compos. Sci. 2021, 5, 129. [Google Scholar] [CrossRef]
- Bharti; Ahmed, G.; Kumar, Y.; Bocchetta, P.; Sharma, S. Determination of Quantum Capacitance of Niobium Nitrides Nb2N and Nb4N3 for Supercapacitor Applications. J. Compos. Sci. 2021, 5, 85. [Google Scholar] [CrossRef]
- Sorkhi, L.; Farrokhi-Rad, M.; Shahrabi, T. Electrophoretic Deposition of Hydroxyapatite–Chitosan–Titania on Stainless Steel 316 L. Surfaces 2019, 2, 458–467. [Google Scholar] [CrossRef] [Green Version]
- Song, Y.; Zhao, R.; Zhang, K.; Ding, J.; Lv, X.; Chen, M.; Xie, J. Facile synthesis of Mn3O4/double-walled carbon nanotube nanocomposites and its excellent supercapacitive behavior. Electrochim. Acta 2017, 230, 350–357. [Google Scholar] [CrossRef]
- Zhitomirsky, I.; Petric, A. Electrochemical deposition of yttrium oxide. J. Mater. Chem. 2000, 10, 1215–1218. [Google Scholar] [CrossRef]
- Pang, X.; Zhitomirsky, I.; Niewczas, M. Cathodic electrolytic deposition of zirconia films. Surf. Coat. Technol. 2005, 195, 138–146. [Google Scholar] [CrossRef]
- Beladi-Mousavi, S.M.; Sadaf, S.; Hennecke, A.K.; Klein, J.; Mahmood, A.M.; Rüttiger, C.; Gallei, M.; Fu, F.; Fouquet, E.; Ruiz, J. The Metallocene Battery: Ultrafast Electron Transfer Self Exchange Rate Accompanied by a Harmonic Height Breathing. Angew. Chem. 2021, 133, 13666–13670. [Google Scholar] [CrossRef]
- Beladi-Mousavi, S.M.; Sadaf, S.; Mahmood, A.M.; Walder, L. High performance poly (viologen)–graphene nanocomposite battery materials with puff paste architecture. ACS Nano 2017, 11, 8730–8740. [Google Scholar] [CrossRef]
- Beladi-Mousavi, S.M.; Klein, J.; Ciobanu, M.; Sadaf, S.; Mahmood, A.M.; Walder, L. Flexible, Self-Supported Anode for Organic Batteries with a Matched Hierarchical Current Collector System for Boosted Current Density. Small 2021, 17, 2103885. [Google Scholar] [CrossRef]
- Zhang, H.; Xu, D.; Wang, L.; Ye, Z.; Chen, B.; Pei, L.; Wang, Z.; Cao, Z.; Shen, J.; Ye, M. A Polymer/Graphene Composite Cathode with Active Carbonyls and Secondary Amine Moieties for High-Performance Aqueous Zn-Organic Batteries Involving Dual-Ion Mechanism. Small 2021, 17, 2100902. [Google Scholar] [CrossRef]
- Kausar, A.; Bocchetta, P. Poly(methyl methacrylate) Nanocomposite Foams Reinforced with Carbon and Inorganic Nanoparticles—State-of-the-Art. J. Compos. Sci. 2022, 6, 129. [Google Scholar] [CrossRef]
- Rodriguez-Uicab, O.; Tayyarian, T.; Abot, J.L. Effect of Curing Temperature of Epoxy Matrix on the Electrical Response of Carbon Nanotube Yarn Monofilament Composites. J. Compos. Sci. 2022, 6, 43. [Google Scholar] [CrossRef]
- Lemartinel, A.; Castro, M.; Fouché, O.; De-Luca, J.-C.; Feller, J.-F. A Review of Nanocarbon-Based Solutions for the Structural Health Monitoring of Composite Parts Used in Renewable Energies. J. Compos. Sci. 2022, 6, 32. [Google Scholar] [CrossRef]
- Sikkema, R.; Baker, K.; Zhitomirsky, I. Electrophoretic deposition of polymers and proteins for biomedical applications. Adv. Colloid Interface Sci. 2020, 284, 102272. [Google Scholar] [CrossRef]
- Rajendran, D.; Ramalingame, R.; Adiraju, A.; Nouri, H.; Kanoun, O. Role of Solvent Polarity on Dispersion Quality and Stability of Functionalized Carbon Nanotubes. J. Compos. Sci. 2022, 6, 26. [Google Scholar] [CrossRef]
- Su, Y.; Zhitomirsky, I. Electrophoretic deposition of graphene, carbon nanotubes and composite films using methyl violet dye as a dispersing agent. Colloids Surf. A Physicochem. Eng. Asp. 2013, 436, 97–103. [Google Scholar] [CrossRef]
- Shi, K.; Zhitomirsky, I. Electrophoretic nanotechnology of graphene–carbon nanotube and graphene–polypyrrole nanofiber composites for electrochemical supercapacitors. J. Colloid Interface Sci. 2013, 407, 474–481. [Google Scholar] [CrossRef]
- Muñoz, J.E.; Cervantes, J.; Esparza, R.; Rosas, G. Iron nanoparticles produced by high-energy ball milling. J. Nanoparticle Res. 2007, 9, 945–950. [Google Scholar] [CrossRef]
- Indris, S.; Bork, D.; Heitjans, P. Nanocrystalline oxide ceramics prepared by high-energy ball milling. J. Mater. Synth. Processing 2000, 8, 245–250. [Google Scholar] [CrossRef]
- Helle, S.; Pedron, M.; Assouli, B.; Davis, B.; Guay, D.; Roué, L. Structure and high-temperature oxidation behaviour of Cu–Ni–Fe alloys prepared by high-energy ball milling for application as inert anodes in aluminium electrolysis. Corros. Sci. 2010, 52, 3348–3355. [Google Scholar] [CrossRef]
- Tsuzuki, T.; Schäffel, F.; Muroi, M.; McCormick, P.G. α-Fe2O3 nano-platelets prepared by mechanochemical/thermal processing. Powder Technol. 2011, 210, 198–202. [Google Scholar] [CrossRef]
- André, B.; Coulet, M.-V.; Esposito, P.-H.; Rufino, B.; Denoyel, R. High-energy ball milling to enhance the reactivity of aluminum nanopowders. Mater. Lett. 2013, 110, 108–110. [Google Scholar] [CrossRef] [Green Version]
- Rogachev, A.; Shkodich, N.; Vadchenko, S.; Baras, F.; Kovalev, D.Y.; Rouvimov, S.; Nepapushev, A.; Mukasyan, A. Influence of the high energy ball milling on structure and reactivity of the Ni+ Al powder mixture. J. Alloy. Compd. 2013, 577, 600–605. [Google Scholar] [CrossRef]
- Kumar, M.; Xiong, X.; Wan, Z.; Sun, Y.; Tsang, D.C.; Gupta, J.; Gao, B.; Cao, X.; Tang, J.; Ok, Y.S. Ball milling as a mechanochemical technology for fabrication of novel biochar nanomaterials. Bioresour. Technol. 2020, 312, 123613. [Google Scholar] [CrossRef]
- Kong, L.; Ma, J.; Zhu, W.; Tan, O. Preparation of Bi4Ti3O12 ceramics via a high-energy ball milling process. Mater. Lett. 2001, 51, 108–114. [Google Scholar] [CrossRef]
- Bid, S.; Pradhan, S. Preparation of zinc ferrite by high-energy ball-milling and microstructure characterization by Rietveld’s analysis. Mater. Chem. Phys. 2003, 82, 27–37. [Google Scholar] [CrossRef]
- Hadef, F. Effect of high-energy ball milling on structure and properties of some intermetallic alloys: A mini review. Metallogr. Microstruct. Anal. 2019, 8, 430–444. [Google Scholar] [CrossRef]
- Rodríguez, V.P.; Rojas-Ayala, C.; Medina, J.M.; Cabrera, P.P.; Quispe-Marcatoma, J.; Landauro, C.; Tapia, J.R.; Baggio-Saitovitch, E.; Passamani, E. Fe50Ni50 synthesized by high energy ball milling: A systematic study using X-ray diffraction, EXAFS and Mössbauer methods. Mater. Charact. 2019, 149, 249–254. [Google Scholar] [CrossRef]
- Cabeza, M.; Feijoo, I.; Merino, P.; Pena, G.; Pérez, M.; Cruz, S.; Rey, P. Effect of high energy ball milling on the morphology, microstructure and properties of nano-sized TiC particle-reinforced 6005A aluminium alloy matrix composite. Powder Technol. 2017, 321, 31–43. [Google Scholar] [CrossRef]
- Révész, Á.; Gajdics, M. Improved H-Storage Performance of Novel Mg-Based Nanocomposites Prepared by High-Energy Ball Milling: A Review. Energies 2021, 14, 6400. [Google Scholar] [CrossRef]
- Liu, W.; Yue, M.; Cui, B.; Hadjipanayis, G.C. Permanent magnetic nanoparticles and nanoflakes prepared by surfactant-assisted high-energy ball milling. Rev. Nanosci. Nanotechnol. 2014, 3, 259–275. [Google Scholar] [CrossRef]
- Wang, G.; Xu, J.; Wen, M.; Cai, R.; Ran, R.; Shao, Z. Influence of high-energy ball milling of precursor on the morphology and electrochemical performance of Li4Ti5O12–ball-milling time. Solid State Ion. 2008, 179, 946–950. [Google Scholar] [CrossRef]
- Nandhini, R.; Mini, P.; Avinash, B.; Nair, S.; Subramanian, K. Supercapacitor electrodes using nanoscale activated carbon from graphite by ball milling. Mater. Lett. 2012, 87, 165–168. [Google Scholar] [CrossRef]
- Kakaei, K.; Akbarpour, M.R.; Liyaghi, Z. Synthesis of partially oxidized NiTi nanostructure by high energy ball milling and its application to supercapacitor. J. Mater. Sci. Mater. Electron. 2018, 29, 2222–2227. [Google Scholar] [CrossRef]
- Wang, Y.; Cao, J.; Zhou, Y.; Ouyang, J.-H.; Jia, D.; Guo, L. Ball-milled graphite as an electrode material for high voltage supercapacitor in neutral aqueous electrolyte. J. Electrochem. Soc. 2012, 159, A579. [Google Scholar] [CrossRef]
- Chen, H.; Wang, J.; Pan, T.; Xiao, H.; Zhang, J.; Cao, C. Effects of high-energy ball milling (HEBM) on the structure and electrochemical performance of nickel hydroxide. Int. J. Hydrog. Energy 2003, 28, 119–124. [Google Scholar] [CrossRef]
- Hashiba, M.; Okamoto, H.; Nurishi, Y.; Hiramatsu, K. The zeta-potential measurement for concentrated aqueous suspension by improved electrophoretic mass transport apparatus—application to Al2O3, ZrO3 and SiC suspensions. J. Mater. Sci. 1988, 23, 2893–2896. [Google Scholar] [CrossRef]
- Chen, R.; Yu, M.; Sahu, R.P.; Puri, I.K.; Zhitomirsky, I. The Development of Pseudocapacitor Electrodes and Devices with High Active Mass Loading. Adv. Energy Mater. 2020, 10, 1903848. [Google Scholar] [CrossRef]
- Nawwar, M.; Poon, R.; Chen, R.; Sahu, R.P.; Puri, I.K.; Zhitomirsky, I. High areal capacitance of Fe3O4-decorated carbon nanotubes for supercapacitor electrodes. Carbon Energy 2019, 1, 124–133. [Google Scholar] [CrossRef] [Green Version]
- Wang, L.-L.; Jiang, J.-S. Preparation of α-Fe2O3 nanoparticles by high-energy ball milling. Phys. B Condens. Matter 2007, 390, 23–27. [Google Scholar] [CrossRef]
- Nazari, M.; Ghasemi, N.; Maddah, H.; Motlagh, M.M. Synthesis and characterization of maghemite nanopowders by chemical precipitation method. J. Nanostructure Chem. 2014, 4, 99. [Google Scholar] [CrossRef] [Green Version]
- Talgatov, E.T.; Auyezkhanova, A.S.; Seitkalieva, K.S.; Tumabayev, N.Z.; Akhmetova, S.N.; Zharmagambetova, A.K. Co-precipitation synthesis of mesoporous maghemite for catalysis application. J. Porous Mater. 2020, 27, 919–927. [Google Scholar] [CrossRef]
- Lee, B.P.; Messersmith, P.B.; Israelachvili, J.N.; Waite, J.H. Mussel-inspired adhesives and coatings. Annu. Rev. Mater. Res. 2011, 41, 99–132. [Google Scholar] [CrossRef] [Green Version]
- Lee, H.; Lee, B.P.; Messersmith, P.B. A reversible wet/dry adhesive inspired by mussels and geckos. Nature 2007, 448, 338–341. [Google Scholar] [CrossRef]
- Xu, N.; Li, Y.; Zheng, T.; Xiao, L.; Liu, Y.; Chen, S.; Zhang, D. A mussel-inspired strategy for CNT/carbon fiber reinforced epoxy composite by hierarchical surface modification. Colloids Surf. A Physicochem. Eng. Asp. 2022, 635, 128085. [Google Scholar] [CrossRef]
- Ata, M.; Liu, Y.; Zhitomirsky, I. A review of new methods of surface chemical modification, dispersion and electrophoretic deposition of metal oxide particles. Rsc. Adv. 2014, 4, 22716–22732. [Google Scholar] [CrossRef]
- Wu, K.; Wang, Y.; Zhitomirsky, I. Electrophoretic deposition of TiO2 and composite TiO2–MnO2 films using benzoic acid and phenolic molecules as charging additives. J. Colloid Interface Sci. 2010, 352, 371–378. [Google Scholar] [CrossRef]
- Wang, Y.; Zhitomirsky, I. Bio-inspired catechol chemistry for electrophoretic nanotechnology of oxide films. J. Colloid Interface Sci. 2012, 380, 8–15. [Google Scholar] [CrossRef]
- Chernyshova, I.V.; Ponnurangam, S.; Somasundaran, P. Linking interfacial chemistry of CO2 to surface structures of hydrated metal oxide nanoparticles: Hematite. Phys. Chem. Chem. Phys. 2013, 15, 6953–6964. [Google Scholar] [CrossRef] [PubMed]
- Ulusoy, H.İ.; Şimşek, S. Removal of uranyl ions in aquatic mediums by using a new material: Gallocyanine grafted hydrogel. J. Hazard. Mater. 2013, 254, 397–405. [Google Scholar] [CrossRef] [PubMed]
- Ahangaran, F.; Hassanzadeh, A.; Nouri, S. Surface modification of Fe3O4@ SiO2 microsphere by silane coupling agent. Int. Nano Lett. 2013, 3, 23. [Google Scholar] [CrossRef]
- Reddy, R.N.; Reddy, R.G. Sol–gel MnO2 as an electrode material for electrochemical capacitors. J. Power Sources 2003, 124, 330–337. [Google Scholar] [CrossRef]
- Jeong, Y.; Manthiram, A. Nanocrystalline manganese oxides for electrochemical capacitors with neutral electrolytes. J. Electrochem. Soc. 2002, 149, A1419. [Google Scholar] [CrossRef]
- Dong, W.; Rolison, D.R.; Dunn, B. Electrochemical properties of high surface area vanadium oxide aerogels. Electrochem. Solid State Lett. 2000, 3, 457. [Google Scholar] [CrossRef]
- Dong, W.; Sakamoto, J.S.; Dunn, B. Electrochemical properties of vanadium oxide aerogels. Sci. Technol. Adv. Mater. 2003, 4, 3–11. [Google Scholar] [CrossRef] [Green Version]
- Venevtsev, Y.N.; Gagulin, V.V.; Zhitomirsky, I.D. Material science aspects of seignette-magnetism problem. Ferroelectrics 1987, 73, 221–248. [Google Scholar] [CrossRef]
- Liu, Y.; Luo, D.; Shi, K.; Michaud, X.; Zhitomirsky, I. Asymmetric supercapacitor based on MnO2 and Fe2O3 nanotube active materials and graphene current collectors. Nano-Struct. Nano-Objects 2018, 15, 98–106. [Google Scholar] [CrossRef]
- Kang, M.; Zhou, S.; Zhang, J.; Ning, F.; Ma, C.; Qiu, Z. Facile fabrication of oxygen vacancy-rich α-Fe2O3 microspheres on carbon cloth as negative electrode for supercapacitors. Electrochim. Acta 2020, 338, 135820. [Google Scholar] [CrossRef]
- Chen, D.; Zhou, S.; Quan, H.; Zou, R.; Gao, W.; Luo, X.; Guo, L. Tetsubo-like α-Fe2O3/C nanoarrays on carbon cloth as negative electrode for high-performance asymmetric supercapacitors. Chem. Eng. J. 2018, 341, 102–111. [Google Scholar] [CrossRef]
- Liang, H.; Xia, C.; Emwas, A.-H.; Anjum, D.H.; Miao, X.; Alshareef, H.N. Phosphine plasma activation of α-Fe2O3 for high energy asymmetric supercapacitors. Nano Energy 2018, 49, 155–162. [Google Scholar] [CrossRef]





Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, C.; Zhitomirsky, I. Influence of High Energy Ball Milling and Dispersant on Capacitive Properties of Fe2O3—Carbon Nanotube Composites. J. Compos. Sci. 2022, 6, 177. https://doi.org/10.3390/jcs6060177
Zhang C, Zhitomirsky I. Influence of High Energy Ball Milling and Dispersant on Capacitive Properties of Fe2O3—Carbon Nanotube Composites. Journal of Composites Science. 2022; 6(6):177. https://doi.org/10.3390/jcs6060177
Chicago/Turabian StyleZhang, Chengwei, and Igor Zhitomirsky. 2022. "Influence of High Energy Ball Milling and Dispersant on Capacitive Properties of Fe2O3—Carbon Nanotube Composites" Journal of Composites Science 6, no. 6: 177. https://doi.org/10.3390/jcs6060177






