Influence of Copper-Based Fillers on Structural and Mechanical Properties of Polylactic Acid Composites
Abstract
:1. Introduction
2. Materials
- Copper (II) sulphate (CuSO4) powder (Component-Reagent LLC, Moscow, Russia);
- Copper (II) oxide (CuO) powder (Nanostructured and Amorphous Materials, Inc., Houston, TX, USA);
- Hollow copper (II) oxide microspheres (CuO shp) synthesized by the authors, not previously studied as antibacterial additives for polymers.
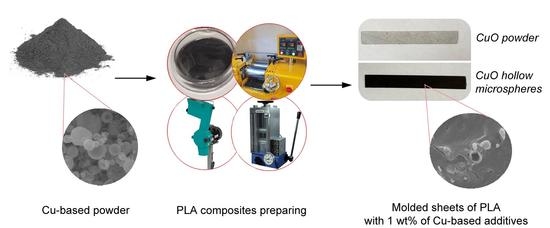
3. Methods
3.1. Determination of Chemical Structure and Dimensional Parameters of Particles of Antimicrobial Additives
3.2. Scanning Electron Microscopy (SEM)
3.3. Differential Scanning Calorimetry (DSC)
3.4. Tensile Strength Properties
3.5. Hydrostatic Weighing
4. Results and Discussion
4.1. Characterization of Antimicrobial Additives
4.2. Structure and Mechanical Properties of Composites
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Scaffaro, R.; Botta, L.; Maio, A.; Gallo, G. Incorporation of an Antibiotic in Poly(Lactic Acid) and Polypropylene by Melt Processing. J. Appl. Biomater. Funct. Mater. 2016, 14, e240–e247. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ruh, E.; Mammadov, E. Antibacterial Activity of Ciprofloxacin-Impregnated 3D-Printed Polylactic Acid Discs: An in Vitro Study. J. Infect. Dev. Ctries 2022, 16, 484–490. [Google Scholar] [CrossRef] [PubMed]
- Mahmoud Zaghloul, M.Y.; Yousry Zaghloul, M.M.; Yousry Zaghloul, M.M. Developments in Polyester Composite Materials—An in-Depth Review on Natural Fibres and Nano Fillers. Compos. Struct. 2021, 278, 114698. [Google Scholar] [CrossRef]
- Macocinschi, D.; Filip, D.; Vlad, S.; Tuchilus, C.G.; Cristian, A.F.; Barboiu, M. Polyurethane/β-Cyclodextrin/Ciprofloxacin Composite Films for Possible Medical Coatings with Antibacterial Properties. J. Mater. Chem. B 2014, 2, 681–690. [Google Scholar] [CrossRef] [PubMed]
- Can Suner, S.; Yildirim, Y.; Yurt, F.; Ozel, D.; Oral, A.; Ozturk, I. Antibiotic Loaded Electrospun Poly (Lactic Acid) Nanofiber Mats for Drug Delivery System. J. Drug Deliv. Sci. Technol. 2022, 71, 103263. [Google Scholar] [CrossRef]
- Youdhestar; Mahar, F.K.; Das, G.; Tajammul, A.; Ahmed, F.; Khatri, M.; Khan, S.; Khatri, Z. Fabrication of Ceftriaxone-Loaded Cellulose Acetate and Polyvinyl Alcohol Nanofibers and Their Antibacterial Evaluation. Antibiotics 2022, 11, 352. [Google Scholar] [CrossRef] [PubMed]
- Khan, F.; Katara, R.; Ramteke, S. Enhancement of Bioavailability of Cefpodoxime Proxetil Using Different Polymeric Microparticles. AAPS PharmSciTech 2010, 11, 1368–1375. [Google Scholar] [CrossRef] [Green Version]
- Bouarab-Chibane, L.; Forquet, V.; Lantéri, P.; Clément, Y.; Léonard-Akkari, L.; Oulahal, N.; Degraeve, P.; Bordes, C. Antibacterial Properties of Polyphenols: Characterization and QSAR (Quantitative Structure-Activity Relationship) Models. Front. Microbiol. 2019, 10, 829. [Google Scholar] [CrossRef] [Green Version]
- Wen, Y.; Zhao, R.; Yin, X.; Shi, Y.; Fan, H.; Zhou, Y.; Tan, L. Antibacterial and Antioxidant Composite Fiber Prepared from Polyurethane and Polyacrylonitrile Containing Tea Polyphenols. Fibers Polym. 2020, 21, 103–110. [Google Scholar] [CrossRef]
- Liu, S.; Qin, S.; He, M.; Zhou, D.; Qin, Q.; Wang, H. Current Applications of Poly(Lactic Acid) Composites in Tissue Engineering and Drug Delivery. Compos. Part B Eng. 2020, 199, 108238. [Google Scholar] [CrossRef]
- Lemire, J.A.; Harrison, J.J.; Turner, R.J. Antimicrobial Activity of Metals: Mechanisms, Molecular Targets and Applications. Nat. Rev. Microbiol. 2013, 11, 371–384. [Google Scholar] [CrossRef] [PubMed]
- Cioffi, N.; Torsi, L.; Ditaranto, N.; Tantillo, G.; Ghibelli, L.; Sabbatini, L.; Bleve-Zacheo, T.; D’Alessio, M.; Zambonin, P.G.; Traversa, E. Copper Nanoparticle/Polymer Composites with Antifungal and Bacteriostatic Properties. Chem. Mater. 2005, 17, 5255–5262. [Google Scholar] [CrossRef]
- Hoque, J.; Yadav, V.; Prakash, R.G.; Sanyal, K.; Haldar, J. Dual-Function Polymer-Silver Nanocomposites for Rapid Killing of Microbes and Inhibiting Biofilms. ACS Biomater. Sci. Eng. 2019, 5, 81–91. [Google Scholar] [CrossRef] [PubMed]
- Sato, M.; Kawata, A.; Morito, S.; Sato, Y.; Yamaguchi, I. Preparation and Properties of Polymer/Zinc Oxide Nanocomposites Using Functionalized Zinc Oxide Quantum Dots. Eur. Polym. J. 2008, 44, 3430–3438. [Google Scholar] [CrossRef]
- Feng, S.; Zhang, F.; Ahmed, S.; Liu, Y. Physico-Mechanical and Antibacterial Properties of PLA/TiO2 Composite Materials Synthesized via Electrospinning and Solution Casting Processes. Coatings 2019, 9, 525. [Google Scholar] [CrossRef] [Green Version]
- Gurianov, Y.; Nakonechny, F.; Albo, Y.; Nisnevitch, M. Antibacterial Composites of Cuprous Oxide Nanoparticles and Polyethylene. Int. J. Mol. Sci. 2019, 20, 439. [Google Scholar] [CrossRef] [Green Version]
- Olkhov, A.A.; Karpova, S.G.; Tubayeva, P.M.; Lobanov, A.V.; Kurnosov, A.S.; Mastalygina, E.E.; Iordanskii, A.L. Supramolecular Structure of Electrospun Ultrathin Fibers Based on Poly-(3-Hydroxibutirate) with Zinc-Tetraphenylporphyrin Complex. AIP Conf. Proc. 2018, 2051, 020217. [Google Scholar] [CrossRef]
- Zaghloul, M.M.Y.M. Mechanical Properties of Linear Low-Density Polyethylene Fire-Retarded with Melamine Polyphosphate. J. Appl. Polym. Sci. 2018, 135, 46770. [Google Scholar] [CrossRef]
- Zaghloul, M.; Zaghloul, M.M.Y. Influence of Flame Retardant Magnesium Hydroxide on the Mechanical Properties of High Density Polyethylene Composites. J. Reinf. Plast. Compos. 2017, 36, 1802–1816. [Google Scholar] [CrossRef]
- Zaghloul, M.M.Y.; Mohamed, Y.S.; El-Gamal, H. Fatigue and Tensile Behaviors of Fiber-Reinforced Thermosetting Composites Embedded with Nanoparticles. J. Compos. Mater. 2018, 53, 709–718. [Google Scholar] [CrossRef]
- Sedlarik, V. Antimicrobial Modifications of Polymers. In Biodegradation-Life of Science; IntechOpen: London, UK, 2013; pp. 187–204. [Google Scholar] [CrossRef] [Green Version]
- Cárdenas, G.; Díaz, V.J.; Meléndrez, M.F.; Cruzat, C.C.; García Cancino, A. Colloidal Cu Nanoparticles/Chitosan Composite Film Obtained by Microwave Heating for Food Package Applications. Polym. Bull. 2009, 62, 511–524. [Google Scholar] [CrossRef]
- Palza, H. Antimicrobial Polymers with Metal Nanoparticles. Int. J. Mol. Sci. 2015, 16, 2099–2116. [Google Scholar] [CrossRef] [Green Version]
- Llorens, A.; Lloret, E.; Picouet, P.A.; Trbojevich, R.; Fernandez, A. Metallic-Based Micro and Nanocomposites in Food Contact Materials and Active Food Packaging. Trends Food Sci. Technol. 2012, 24, 19–29. [Google Scholar] [CrossRef]
- Gurianov, Y.; Nakonechny, F.; Albo, Y.; Nisnevitch, M. LLDPE Composites with Nanosized Copper and Copper Oxides for Water Disinfection. Polymers 2020, 12, 1713. [Google Scholar] [CrossRef] [PubMed]
- Delgado, K.; Quijada, R.; Palma, R.; Palza, H. Polypropylene with Embedded Copper Metal or Copper Oxide Nanoparticles as a Novel Plastic Antimicrobial Agent. Lett. Appl. Microbiol. 2011, 53, 50–54. [Google Scholar] [CrossRef]
- Bezza, F.A.; Tichapondwa, S.M.; Chirwa, E.M.N. Fabrication of Monodispersed Copper Oxide Nanoparticles with Potential Application as Antimicrobial Agents. Sci. Rep. 2020, 10, 16680. [Google Scholar] [CrossRef] [PubMed]
- Chukhlanov, V.Y.; Selivanov, O.G.; Chukhlanova, N.V.; Mastalygina, E.E. Syntactic Foams for Filling Sealing Compositions Based on Epoxy Resin and Hollow Phenol-Formaldehyde Microspheres. Polym. Sci. Ser. D 2020, 13, 241–244. [Google Scholar] [CrossRef]
- Zolkin, A.L.; Galanskiy, S.A.; Kuzmin, A.M. Perspectives for Use of Composite and Polymer Materials in Aircraft Construction. IOP Conf. Ser. Mater. Sci. Eng. 2021, 1047, 012023. [Google Scholar] [CrossRef]
- Mastalygina, E.E.; Chukhlanov, V.Y. Dielectric Study of Syntactic Foams Based on Polydimethylsiloxane and Hollow Glass Microspheres at X-Band Microwave Frequency. IOP Conf. Ser. Mater. Sci. Eng. 2020, 896, 012101. [Google Scholar] [CrossRef]
- Logutenko, O.A.; Titkov, A.I.; Vorobyov, A.M.; Lyakhov, N.Z. A Novel Method to Prepare Copper Microspheres via Chemical Reduction Route. J. Mater. Res. Technol. 2021, 13, 1254–1265. [Google Scholar] [CrossRef]
- Zhu, H.; Wang, J.; Xu, G. Fast Synthesis of Cu2O Hollow Microspheres and Their Application in DNA Biosensor of Hepatitis B Virus. Cryst. Growth Des. 2009, 9, 633–638. [Google Scholar] [CrossRef]
- Chen, L.; Zhang, Y.; Zhu, P.; Zhou, F.; Zeng, W.; Lu, D.D.; Sun, R.; Wong, C. Copper Salts Mediated Morphological Transformation of Cu2O from Cubes to Hierarchical Flower-like or Microspheres and Their Supercapacitors Performances. Sci. Rep. 2015, 5, 9672. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Savioli Lopes, M.; Jardini, A.L.; Maciel Filho, R. Poly (Lactic Acid) Production for Tissue Engineering Applications. Procedia Eng. 2012, 42, 1402–1413. [Google Scholar] [CrossRef] [Green Version]
- Yudin, A.; Shatrova, N.; Khaydarov, B.; Kuznetsov, D.; Dzidziguri, E.; Issi, J.P. Synthesis of Hollow Nanostructured Nickel Oxide Microspheres by Ultrasonic Spray Atomization. J. Aerosol Sci. 2016, 98, 30–40. [Google Scholar] [CrossRef]
- Shelenkov, P.G.; Pantyukhov, P.V.; Popov, A.A. Highly Filled Biocomposites Based on Ethylene-Vinyl Acetate Copolymer and Wood Flour. IOP Conf. Ser. Mater. Sci. Eng. 2018, 369, 012043. [Google Scholar] [CrossRef] [Green Version]
- Zare, Y. Study of Nanoparticles Aggregation/Agglomeration in Polymer Particulate Nanocomposites by Mechanical Properties. Compos. Part A Appl. Sci. Manuf. 2016, 84, 158–164. [Google Scholar] [CrossRef]
- Sarasini, F.; Luzi, F.; Dominici, F.; Maffei, G.; Iannone, A.; Zuorro, A.; Lavecchia, R.; Torre, L.; Carbonell-Verdu, A.; Balart, R.; et al. Effect of Different Compatibilizers on Sustainable Composites Based on a PHBV/PBAT Matrix Filled with Coffee Silverskin. Polymers 2018, 10, 1256. [Google Scholar] [CrossRef] [Green Version]
- Tertyshnaya, Y.V.; Karpova, S.G.; Podzorova, M.V.; Khvatov, A.V.; Moskovskiy, M.N. Thermal Properties and Dynamic Characteristics of Electrospun Polylactide/Natural Rubber Fibers during Disintegration in Soil. Polymers 2022, 14, 1058. [Google Scholar] [CrossRef]
- Ho, M.P.; Lau, K.T.; Wang, H.; Hui, D. Improvement on the Properties of Polylactic Acid (PLA) Using Bamboo Charcoal Particles. Compos. Part B Eng. 2015, 81, 14–25. [Google Scholar] [CrossRef]
- Rogovina, S.Z.; Aleksanyan, K.V.; Loginova, A.A.; Ivanushkina, N.E.; Vladimirov, L.V.; Prut, E.V.; Berlin, A.A. Influence of PEG on Mechanical Properties and Biodegradability of Composites Based on PLA and Starch. Starch Stärke 2018, 70, 1700268. [Google Scholar] [CrossRef]
- Liu, Z.; Fu, M.; Ling, F.; Sui, G.; Bai, H.; Zhang, Q.; Fu, Q. Stereocomplex-Type Polylactide with Bimodal Melting Temperature Distribution: Toward Desirable Melt-Processability and Thermomechanical Performance. Polymer 2019, 169, 21–28. [Google Scholar] [CrossRef]
- Liu, W.; Wu, X.; Chen, X.; Liu, S.; Zhang, C. Flexibly Controlling the Polycrystallinity and Improving the Foaming Behavior of Polylactic Acid via Three Strategies. ACS Omega 2022, 7, 6248–6260. [Google Scholar] [CrossRef]
- Kubel, C.; Gonzalez-Ronda, L.; Drummy, L.F.; Martin, D.C. Defect-Mediated Curvature and Twisting in Polymer Crystals. J. Phys. Org. Chem. 2000, 13, 816–829. [Google Scholar] [CrossRef]








| Name | Polylactic Acid (Poly-Lactide) | Polycapro-Lactone | Polyethylene Glycol | Copper (II) Sulfate Penta-Hydrate Powder | Copper (II) Oxide Powder | |
|---|---|---|---|---|---|---|
| Parameter | ||||||
| Abbreviation | PLA | PCL | PEG | CuSO4 | CuO | |
| Trade mark, producer | Ingeo 4043D, Nature Works, LLC (USA) | 600C, Shenzhen ESUN Industrial Co., Ltd (China) | NorPeg 400, LLC “Norkem” | Component-Reagent LLC, Moscow, Russia | Nanostructured and Amorphous Materials, Inc., Houston, TX, USA | |
| Description | thermoplastic aliphatic polyester | thermoplastic aliphatic polyester | low-molecular-weight grade of polyethylene glycol | CuSO4 × 5H2O, GOST 4165-78; purity: 98% | purity: 99% | |
| Molecular weight (g/mol) | 155,000–165,000 | 50,000–60,000 | 380–440 | 249.69 | 79.55 | |
| Melt flow index (g/10 min) | 5–7 (210 °C, 2.16 kg) | 11–12 (160 °C, 2.16 kg) | - | - | - | |
| Density (g/cm3) | 1.21–1.25 | 1.08–1.12 | 1.1–1.2 | 2.3–3.6 | 6.1–6.6 | |
| Melting point (°C) | 145–160 | 58–60 | 4–8 | - | - | |
| Sample Name | PLA, wt.% | Plasticizer, wt.% | Antimicrobial Additive, wt.% |
|---|---|---|---|
| PLA (neat) | 100.0 | - | - |
| PEG + 5 CuSO4 | 90.0 | PEG, 5.0 | CuSO4, 5.0 |
| PEG + 10 CuSO4 | 85.0 | PEG, 5.0 | CuSO4, 10.0 |
| PEG + PCL + 10 CuSO4 | 75.0 | PEG, 5.0; PCL, 10.0 | CuSO4, 10.0 |
| 0.5 CuO | 99.5 | - | CuO, 0.5 |
| 1 CuO | 99.0 | - | CuO, 1.0 |
| 2 CuO | 98.0 | - | CuO, 2.0 |
| 5 CuO | 95.0 | - | CuO, 5.0 |
| 0.5 CuO sph | 99.5 | - | CuO sph, 0.5 |
| 1 CuO sph | 99.0 | - | CuO sph, 1.0 |
| 2 CuO sph | 98.0 | - | CuO sph, 2.0 |
| 5 CuO sph | 95.0 | - | CuO sph, 5.0 |
| Sample | σmax * (MPa) | SD ** (MPa)/VaR *** (MPa2) | εb **** (%) | SD (%)/VaR (%) | E ***** (GPa) | SD (GPa)/VaR (GPa2) |
|---|---|---|---|---|---|---|
| PLA (neat) | 59.1 | 2.8/7.7 | 5.8 | 0.6/0.4 | 3.67 | 0.09/0.01 |
| PEG + 5 CuSO4 | 38.3 | 2.7/7.4 | 3.5 | 0.5/0.2 | 3.01 | 0.28/0.08 |
| PEG + 10 CuSO4 | 27.3 | 1.8/3.4 | 6.0 | 0.8/4.4 | 2.98 | 0.18/0.03 |
| PEG + PCL + 10 CuSO4 | 22.4 | 1.6/2.7 | 4.4 | 1.3/1.4 | 2.59 | 0.14/0.02 |
| 0.5 CuO | 52.3 | 1.8/3.2 | 4.8 | 0.37/0.2 | 3.45 | 0.13/0.02 |
| 1 CuO | 55.7 | 2.3/5.4 | 3.8 | 0.56/0.2 | 3.77 | 0.30/0.09 |
| 2 CuO | 49.6 | 2.3/5.4 | 2.9 | 0.20/0.1 | 3.59 | 0.24/0.06 |
| 5 CuO | 50.5 | 2.9/8.2 | 2.5 | 0.12/0.1 | 1.59 | 0.29/0.09 |
| 0.5 CuO sph | 58.8 | 1.4/2.1 | 5.6 | 0.32/0.1 | 3.58 | 0.17/0.03 |
| 1 CuO sph | 59.8 | 2.1/4.6 | 5.8 | 0.27/0.1 | 3.55 | 0.15/0.03 |
| 2 CuO sph | 55.0 | 2.4/5.8 | 4.5 | 0.36/0.2 | 3.52 | 0.40/0.16 |
| 5 CuO sph | 46.5 | 2.2/5.1 | 2.8 | 0.42/0.1 | 2.00 | 0.18/0.03 |
| Sample | ρ * (g/cm3, Δ ± 0.002 g/cm3) | SD ** (g/cm3) | VaR *** ((g/cm3)2) |
|---|---|---|---|
| PLA (neat) | 1.248 | 0.011 | 0.0001 |
| PEG + 5 CuSO4 | 1.276 | 0.009 | 0.0001 |
| PEG + 10 CuSO4 | 1.329 | 0.008 | 0.0001 |
| PEG + PCL + 10 CuSO4 | 1.317 | 0.007 | 0.0001 |
| 0.5 CuO | 1.257 | 0.003 | 0.0001 |
| 1 CuO | 1.255 | 0.004 | 0.0001 |
| 2 CuO | 1.259 | 0.035 | 0.0001 |
| 5 CuO | 1.293 | 0.016 | 0.0001 |
| 0.5 CuO sph | 1.261 | 0.007 | 0.0001 |
| 1 CuO sph | 1.263 | 0.002 | 0.0001 |
| 2 CuO sph | 1.258 | 0.014 | 0.0001 |
| 5 CuO sph | 1.273 | 0.022 | 0.0001 |
| Sample | Glass Transition | Cold Crystallization | Melting | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| T * (°C) | SD *** | T * (°C) | SD | ΔH ** (J/g) | SD | T * (°C) | SD | ΔH ** (J/g) | SD | |
| PLA (neat) | 58.7 | 0.4 | - | - | - | - | 148.7 | 0.6 | 13 | 2.5 |
| PEG + 5 CuSO4 | 48.9 | 0.7 | 83.5 | 0.6 | 31 | 2.1 | 142.7/129.4 | 1.0 | 29 | 2.6 |
| PEG + 10 CuSO4 | 50.1 | 0.8 | 89.5 | 0.9 | 34 | 3.3 | 147.8/135.7 | 0.8 | 27 | 2.4 |
| PEG + PCL + 10 CuSO4 | 59.1 | 0.7 | 88.6 | 1.0 | 25 | 2.5 | 136.5/148.5 | 1.1 | 27 | 2.0 |
| 0.5 CuO | 57.5 | 0.5 | 101.5 | 0.7 | 33 | 1.4 | 143.2/150.4 | 0.4 | 30 | 1.8 |
| 1 CuO | 58.4 | 0.7 | 103.7 | 0.9 | 29 | 2.0 | 143.2/149.6 | 0.7 | 26 | 1.6 |
| 2 CuO | 59.0 | 0.6 | 114.1 | 1.1 | 30 | 2.6 | 146.9/149.5 | 0.6 | 30 | 1.9 |
| 5 CuO | 58.6 | 0.9 | 106.7 | 0.8 | 32 | 2.3 | 143.6/149.1 | 0.9 | 28 | 2.1 |
| 0.5 CuO sph | 59.0 | 0.3 | 106.3 | 0.7 | 24 | 2.8 | 145.0/148.6 | 0.8 | 22 | 2.0 |
| 1 CuO sph | 57.5 | 0.5 | 102.6 | 0.6 | 25 | 1.2 | 143.2/150.3 | 0.6 | 26 | 1.8 |
| 2 CuO sph | 58.4 | 0.4 | 103.5 | 1.1 | 33 | 1.7 | 143.1/151.0 | 0.5 | 30 | 2.1 |
| 5 CuO sph | 59.1 | 0.5 | - | - | - | - | 145.0/151.0 | 0.9 | 24 | 2.7 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mastalygina, E.E.; Olkhov, A.A.; Vorontsov, N.V.; Kiselev, N.V.; Khaidarov, T.B.; Khaydarov, B.B.; Kolesnikov, E.A.; Burmistrov, I.N. Influence of Copper-Based Fillers on Structural and Mechanical Properties of Polylactic Acid Composites. J. Compos. Sci. 2022, 6, 386. https://doi.org/10.3390/jcs6120386
Mastalygina EE, Olkhov AA, Vorontsov NV, Kiselev NV, Khaidarov TB, Khaydarov BB, Kolesnikov EA, Burmistrov IN. Influence of Copper-Based Fillers on Structural and Mechanical Properties of Polylactic Acid Composites. Journal of Composites Science. 2022; 6(12):386. https://doi.org/10.3390/jcs6120386
Chicago/Turabian StyleMastalygina, Elena Evgenyevna, Anatoly Aleksandrovich Olkhov, Nikolay Vladimirovich Vorontsov, Nikolay Vitalievich Kiselev, Timur Bakhtierovich Khaidarov, Bekzod Bakhtierovich Khaydarov, Evgeniy Aleksandrovich Kolesnikov, and Igor Nikolaevich Burmistrov. 2022. "Influence of Copper-Based Fillers on Structural and Mechanical Properties of Polylactic Acid Composites" Journal of Composites Science 6, no. 12: 386. https://doi.org/10.3390/jcs6120386