Uptake of Methylene Blue from Aqueous Solution by Pectin–Chitosan Binary Composites
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Equipment
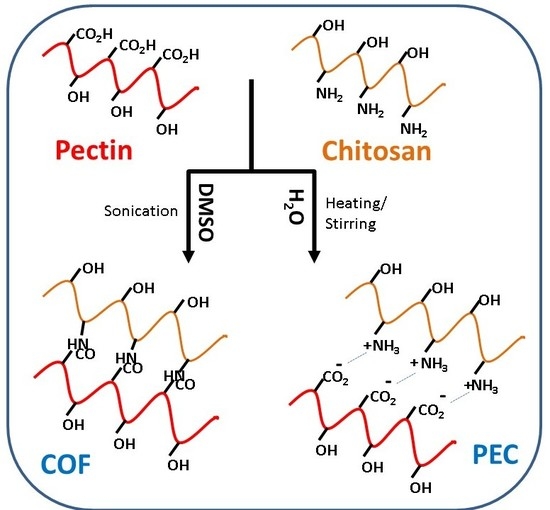
2.2. Synthesis of Pectin and Chitosan Composites
2.2.1. Pectin–Chitosan Polyelectrolyte Complexes in Water: PC15 W, PC11 W, PC51 W
2.2.2. Sonication-Assisted Synthesis of Pectin–Chitosan Composites in DMSO: PC15 S DMSO, PC11 S DMSO, and PC51 S DMSO
2.3. Characterization of Composite Materials
2.3.1. pH at the Point-of-Zero Charge (pHpzc)
2.3.2. FTIR Spectroscopy
2.3.3. Thermal Gravimetric Analysis (TGA)
2.3.4. Dye Adsorption Studies
Adsorption of Methylene Blue (MB)
Adsorption Isotherms
Surface Area Estimated from MB Adsorption
3. Results and Discussion
3.1. PZC Analysis
3.2. TGA Results of Pectin and the Pectin-Chitosan Composites
3.3. FTIR Spectral Results
3.4. Sorption Isotherm Results
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Guo, R.; Wilson, L.D. Synthetically engineered chitosan-based materials and their sorption properties with methylene blue in aqueous solution. J. Colloid Interface Sci. 2012, 388, 225–234. [Google Scholar] [CrossRef] [PubMed]
- Kumar, K.V.; Ramamurthi, V.; Sivanesan, S. Modeling the mechanism involved during the sorption of methylene blue onto fly ash. J. Colloid Interface Sci. 2005, 284, 14–21. [Google Scholar] [CrossRef] [PubMed]
- Forgacs, E.; Cserháti, T.; Oros, G. Removal of synthetic dyes from wastewaters: A review. Environ. Int. 2004, 30, 953–971. [Google Scholar] [CrossRef] [PubMed]
- Tünay, O.; Kabdasli, I.; Eremektar, G.; Orhon, D. Color removal from textile wastewaters. Water Sci. Technol. 1996, 34, 9–16. [Google Scholar]
- Zhang, L.; Zeng, Y.; Cheng, Z. Removal of heavy metal ions using chitosan and modified chitosan: A review. J. Mol. Liq. 2016, 214, 175–191. [Google Scholar] [CrossRef]
- Mahaninia, M.H.; Wilson, L.D. Cross-linked chitosan beads for phosphate removal from aqueous solution. J. Appl. Polym. Sci. 2015, 133, 42949. [Google Scholar] [CrossRef]
- Kumar, S.; Krishnakumar, B.; Sobral, A.J.; Koh, J. Bio-based (chitosan/PVA/ZnO) nanocomposites film: Thermally stable and photoluminescence material for removal of organic dye. Carbohydr. Polym. 2019, 205, 559–564. [Google Scholar] [CrossRef]
- Baroncini, E.A.; Yadav, S.K.; Palmese, G.R.; Stanzione, I.J.F. Recent advances in bio-based epoxy resins and bio-based epoxy curing agents. J. Appl. Polym. Sci. 2016, 133, 44103–44122. [Google Scholar] [CrossRef] [Green Version]
- Sabzevari, M.; Cree, D.E.; Wilson, L.D. Graphene Oxide–Chitosan Composite Material for Treatment of a Model Dye Effluent. ACS Omega 2018, 3, 13045–13054. [Google Scholar] [CrossRef] [Green Version]
- Birch, N.P.; Schiffman, J.D. Characterization of Self-Assembled Polyelectrolyte Complex Nanoparticles Formed from Chitosan and Pectin. Langmuir 2014, 30, 3441–3447. [Google Scholar] [CrossRef]
- Ventura, I.; Peled, H. Small-angle X-ray scatting study on pectin-chitosan mixed solutions and thermoreversible gels. Carbohydr. Polym. 2015, 123, 122–129. [Google Scholar] [CrossRef]
- Wu, L.; Wang, H.; Zhu, X.; Hou, Y.; Liu, W.; Yang, G.; Jiang., A. Pectin-chitosan complex: Preparation and application in colon-specific capsule. Int. J. Agric. Biol. Eng. 2015, 8, 151–160. [Google Scholar]
- Udoetok, I.A.; Dimmick, R.M.; Wilson, L.D.; Headley, J.V. Adsorption properties of cross-linked cellulose-epichlorohydrin polymers in aqueous solution. Carbohydr. Polym. 2016, 136, 329–340. [Google Scholar] [CrossRef]
- Dolatkhah, A.; Wilson, L.D. Magnetite/Polymer Brush Nanocomposites with Switchable Uptake Behavior Toward Methylene Blue. ACS Appl. Mater. Interfaces 2016, 8, 5595–5607. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, M.H.; Wilson, L.D.; Headley, J.V. Design and characterization of novel β-cyclodextrin based copolymer materials. Carbohydr. Res. 2011, 346, 219–229. [Google Scholar] [CrossRef]
- Pelekani, C.; Snoeyink, V.L. Competitive adsorption between atrazine and methylene blue on activated carbon: The importance of pore size distribution. Carbon 2000, 38, 1423–1436. [Google Scholar] [CrossRef]
- Delmar, K.; Bianco-Peled, H. The dramatic effect of small pH changes on the properties of chitosan hydrogels crosslinked with genipin. Carbohydr. Polym. 2015, 127, 28–37. [Google Scholar] [CrossRef] [PubMed]
- Neufeld, L.; Bianco-Peled, H. Pectin–chitosan physical hydrogels as potential drug delivery vehicles. Int. J. Boil. Macromol. 2017, 101, 852–861. [Google Scholar] [CrossRef]
- Hubbe, M.A.; Beck, K.R.; O’Neal, W.G.; Sharma, Y.C. Cellulosic substrates for removal of pollutants from aqueous systems: A Review. 2. Dyes. BioResources 2012, 7, 5951–5962. [Google Scholar] [CrossRef]
- Zhao, X.J.; Zhou, Z. Synthesis and Applications of Pectin-based Nanomaterials. Curr. Nanosci. 2015, 12, 103–109. [Google Scholar] [CrossRef]
- Neto, J.D.O.M.; Milagres, J.L.; Pessoa, K.D.; Alvarenga, E.S.; Bellato, C.R. Preparation and evaluation of chitosan beads immobilized with Iron(III) for the removal of As(III) and As(V) from water. J. Braz. Chem. Soc. 2013, 24, 121–132. [Google Scholar] [CrossRef] [Green Version]
- Igberase, E.; Osifo, P.; Ofomaja, A. Chromium (VI) ion adsorption by grafted cross-linked chitosan beads in aqueous solution-A mathematical and statistical modelling study. Environ. Technol. 2017, 38, 1–30. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Sun, Y.; Ding, G.; Geng, Q.; Zhu, J.; Guo, M.; Duan, Y.; Wang, B.; Cao, Y. Synthesis, Characterization, and Application of Microbe-Triggered Controlled-Release Kasugamycin–Pectin Conjugate. J. Agric. Food Chem. 2015, 63, 4263–4268. [Google Scholar] [CrossRef]
- Pal, A.K.; Katiyar, V. Nanoamphiphilic Chitosan Dispersed Poly(lactic acid) Bionanocomposite Films with Improved Thermal, Mechanical, and Gas Barrier Properties. Biomacromolecules 2016, 17, 2603–2618. [Google Scholar] [CrossRef] [PubMed]
- Wade, L.G., Jr. Organic Chemistry, 6th ed.; Pearson Prentice Hall Inc.: Upper Saddle River, NJ, USA, 2006; Chapter 12. [Google Scholar]
- Chen, H.; Yang, W.; Chen, H.; Liu, L.; Gao, F.; Yang, X.; Jiang, Q.; Zhang, Q.; Wang, Y. Surface modification of Mitoxantrone-loaded PLGA nanospheres with chitosan. Colloids Surfaces B: Biointerfaces 2009, 73, 212–218. [Google Scholar] [CrossRef] [PubMed]
- Demarger-André, S.; Domard, A. Chitosan carboxylic acid salts in solution and in the solid state. Carbohydr. Polym. 1994, 23, 211–219. [Google Scholar] [CrossRef]
- Marudova, M.; MacDougall, A.J.; Ring, S.G. Pectin–chitosan interactions and gel formation. Carbohydr. Res. 2004, 339, 1933–1939. [Google Scholar] [CrossRef]
- Chetouani, A.; Follain, N.; Marais, S.; Rihouey, C.; Elkolli, M.; Bounekhel, M.; Benachour, D.; Le Cerf, D. Physicochemical properties and biological activities of novel blend films using oxidized pectin/chitosan. Int. J. Boil. Macromol. 2017, 97, 348–356. [Google Scholar] [CrossRef]
- Rampino, A.; Borgogna, M.; Bellich, B.; Blasi, P.; Virgilio, F.; Cesàro, A. Chitosan-pectin hybrid nanoparticles prepared by coating and blending techniques. Eur. J. Pharm. Sci. 2016, 84, 37–45. [Google Scholar] [CrossRef]
- Li, F.T.; Yang, H.; Zhao, Y.; Xu, R. Novel modified pectin for heavy metal adsorption. Chin. Chem. Lett. 2007, 18, 325–328. [Google Scholar] [CrossRef]
- Bernabe, P.; Peniche, C.; Argüelles-Monal, W. Swelling behavior of chitosan/pectin polyelectrolyte complex membranes. Effect of thermal cross-linking. Polym. Bull. 2005, 55, 367–375. [Google Scholar] [CrossRef]
- Ogutu, F.O.; Mu, T.; Elahi, R.; Zhang, M.; Sun, H.-N. Ultrasonic Modification of Selected Polysaccharides-Review. J. Food Process. Technol. 2015, 6, 1000446. [Google Scholar] [CrossRef] [Green Version]
- Kong, D.; Wilson, L.D. Synthesis and characterization of cellulose-goethite composites and their adsorption properties with roxarsone. Carbohydr. Polym. 2017, 169, 282–294. [Google Scholar] [CrossRef] [PubMed]
- Yu, L.-L.; Jiang, L.-N.; Wang, S.-Y.; Sun, M.-M.; Li, D.-Q.; Du, G.-M. Pectin microgel particles as high adsorption rate material for methylene blue: Performance, equilibrium, kinetic, mechanism and regeneration studies. Int. J. Boil. Macromol. 2018, 112, 383–389. [Google Scholar] [CrossRef] [PubMed]





| Sample Name | Ks (M−1) | Qm (mmol/g) | ns | Adjusted R2 | SA (m2/g) 1 |
|---|---|---|---|---|---|
| Pectin | 0.50 ± 0.015 | 2.2 ± 0.012 | 0.99 | 0.99 | 170 |
| PC51 W | 0.54 ± 0.10 | 1.2 ± 0.089 | 1.3 | 0.96 | 92 |
| PC11 W | 0.54 ± 0.081 | 0.96 ± 0.051 | 1.0 | 0.97 | 75 |
| PC15 W | 0.69 ± 0.093 | 0.26 ± 0.011 | 1.1 | 0.96 | 20 |
| PC51 S DMSO | 0.36 ± 0.071 | 1.9 ± 0.10 | 1.1 | 0.97 | 146 |
| PC11 S DMSO | 0.45 ± 0.023 | 1.6 ± 0.011 | 1.0 | 0.97 | 123 |
| PC15 S DMSO | 0.50 ± 0.0.53 | 0.78 ± 0.028 | 0.84 | 0.97 | 60 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kong, D.; Wilson, L.D. Uptake of Methylene Blue from Aqueous Solution by Pectin–Chitosan Binary Composites. J. Compos. Sci. 2020, 4, 95. https://doi.org/10.3390/jcs4030095
Kong D, Wilson LD. Uptake of Methylene Blue from Aqueous Solution by Pectin–Chitosan Binary Composites. Journal of Composites Science. 2020; 4(3):95. https://doi.org/10.3390/jcs4030095
Chicago/Turabian StyleKong, Dexu, and Lee D. Wilson. 2020. "Uptake of Methylene Blue from Aqueous Solution by Pectin–Chitosan Binary Composites" Journal of Composites Science 4, no. 3: 95. https://doi.org/10.3390/jcs4030095