Synergistic Effect of Calcium Carbonate and Biobased Particles for Rubber Reinforcement and Comparison to Silica Reinforced Rubber
Abstract
:1. Introduction
2. Experimental
2.1. Materials
2.2. Preparation of Composites
2.3. Physical Characterizations
2.4. Mechanical Properties
3. Results and Discussion
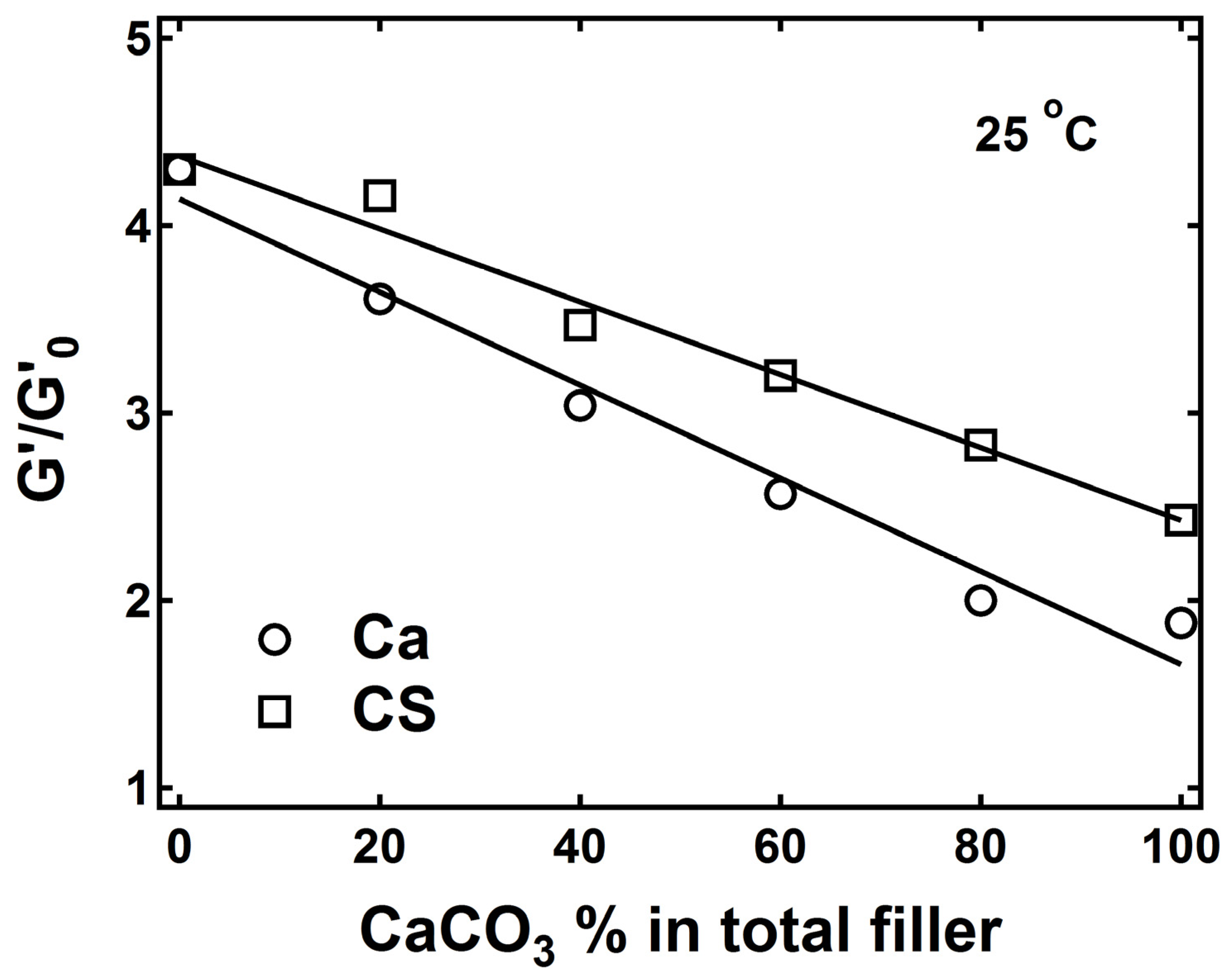
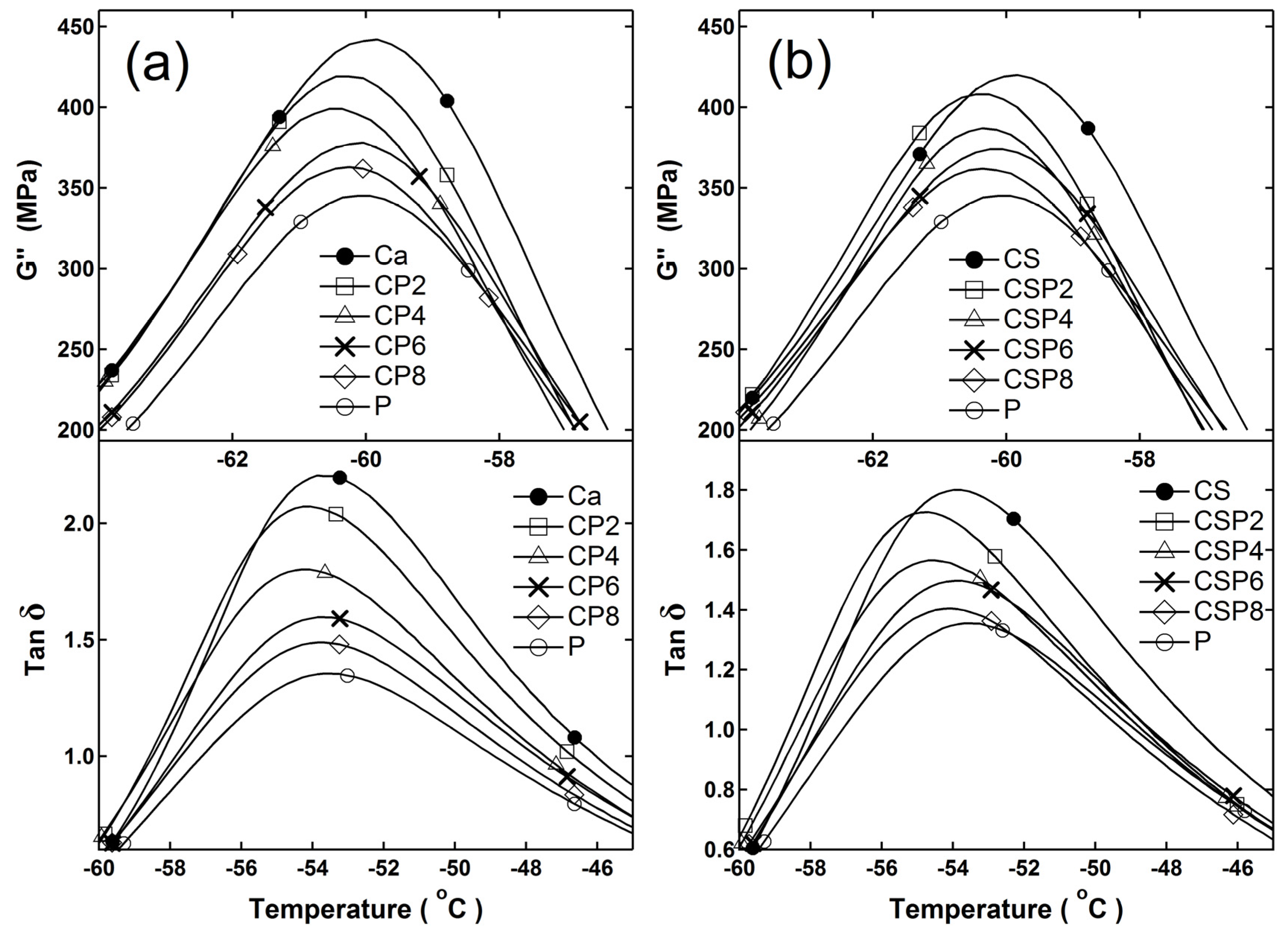
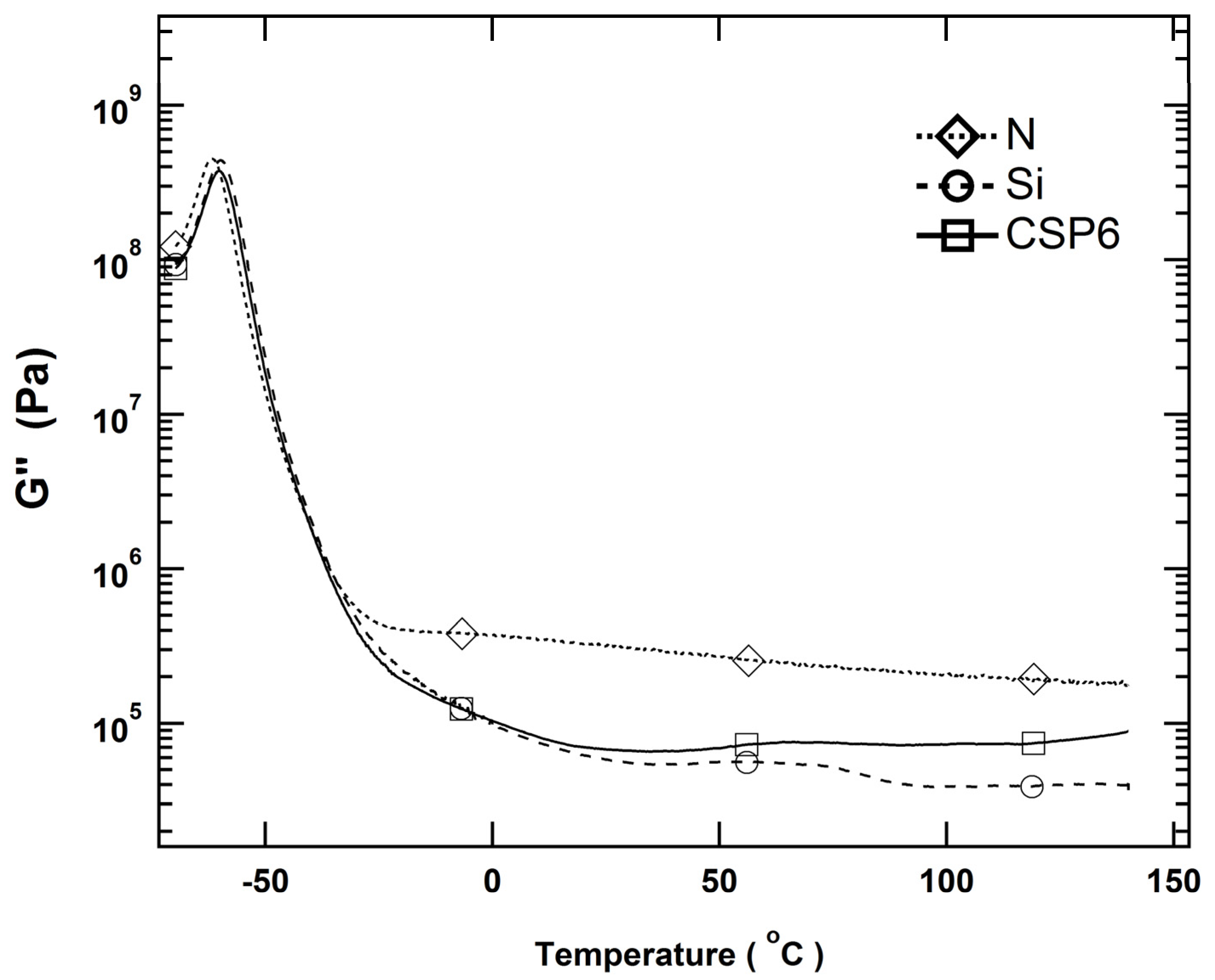
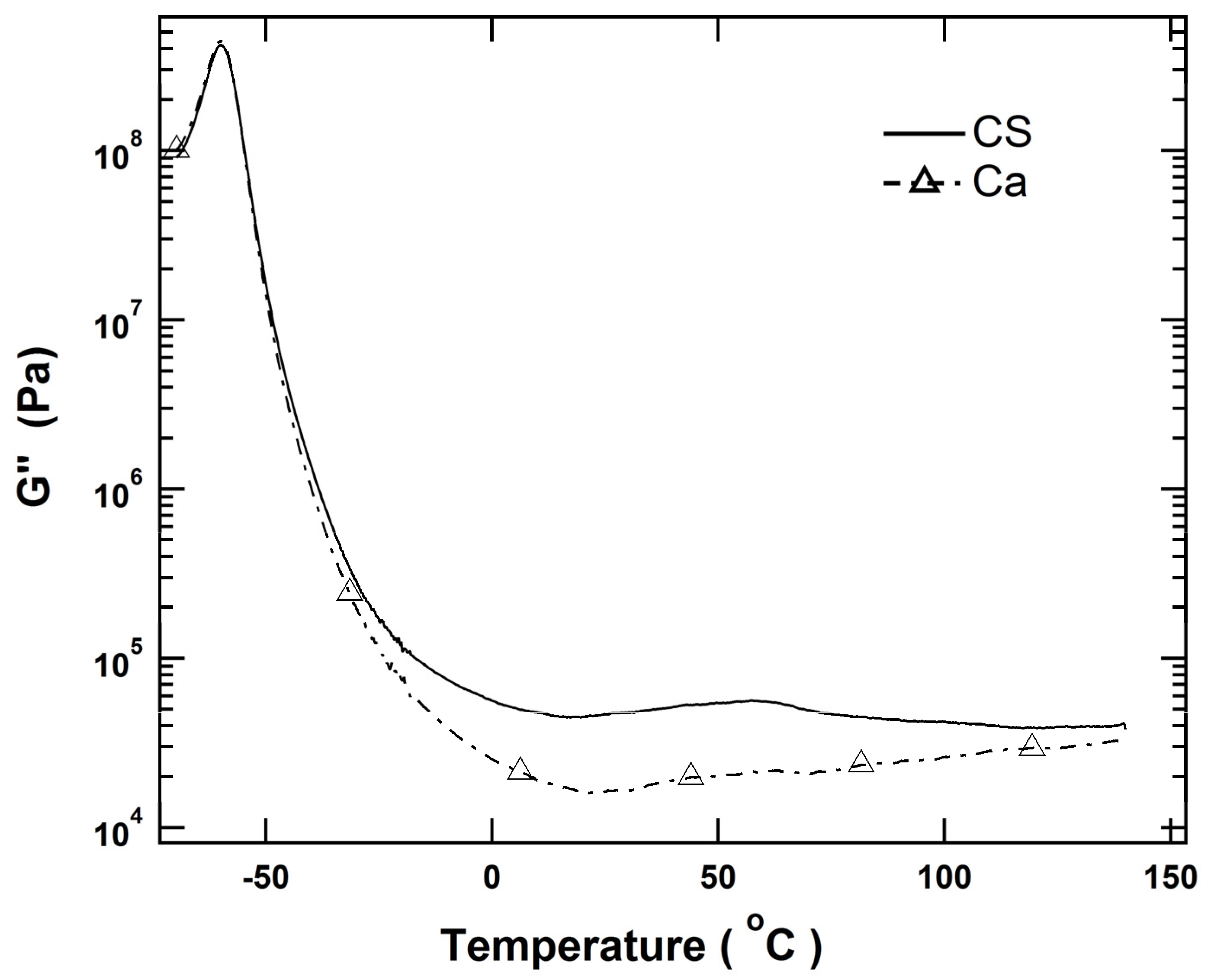
3.1. Immobilization of Polymer Chains by Crosslinks
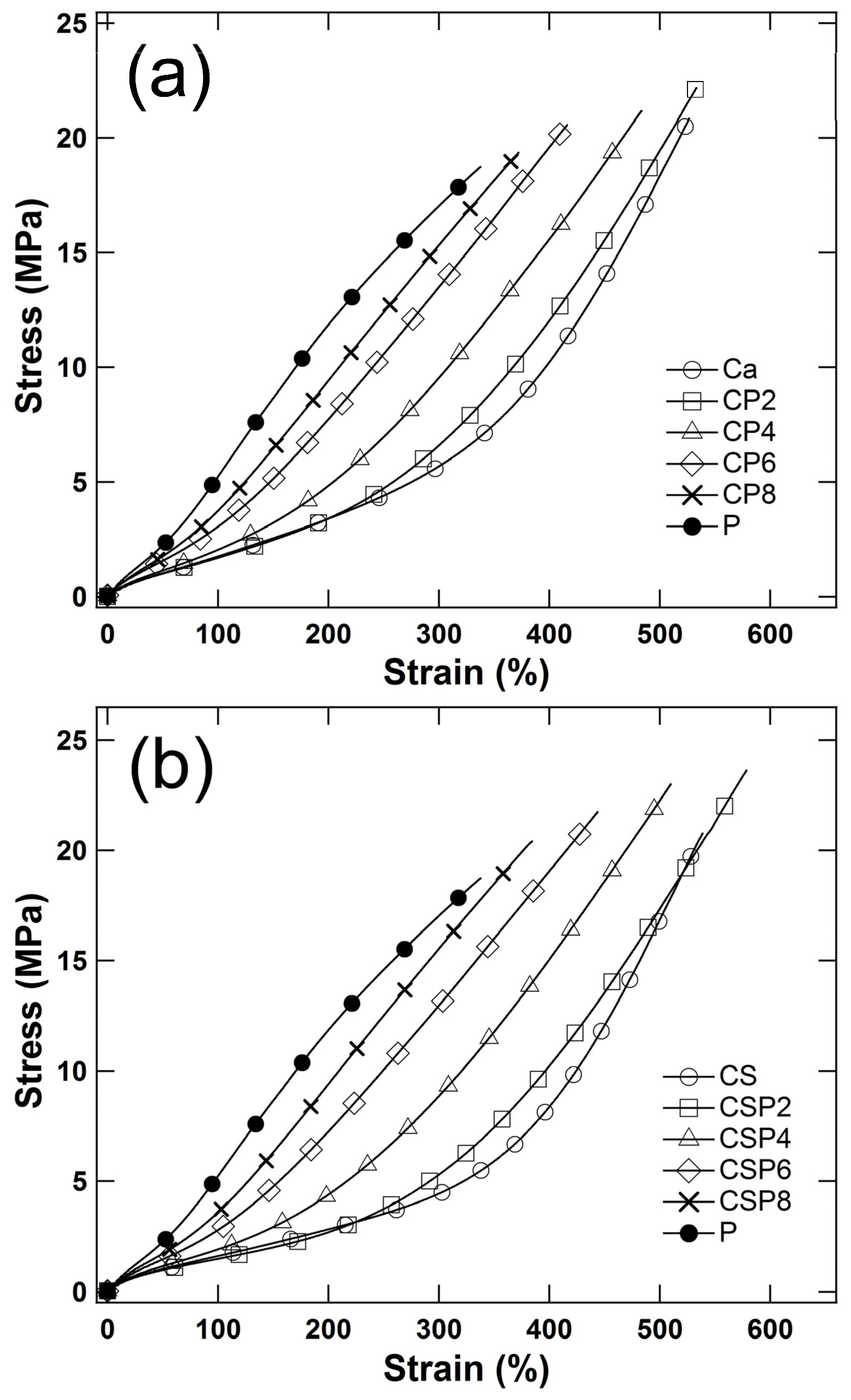
3.2. Mechanical Properties
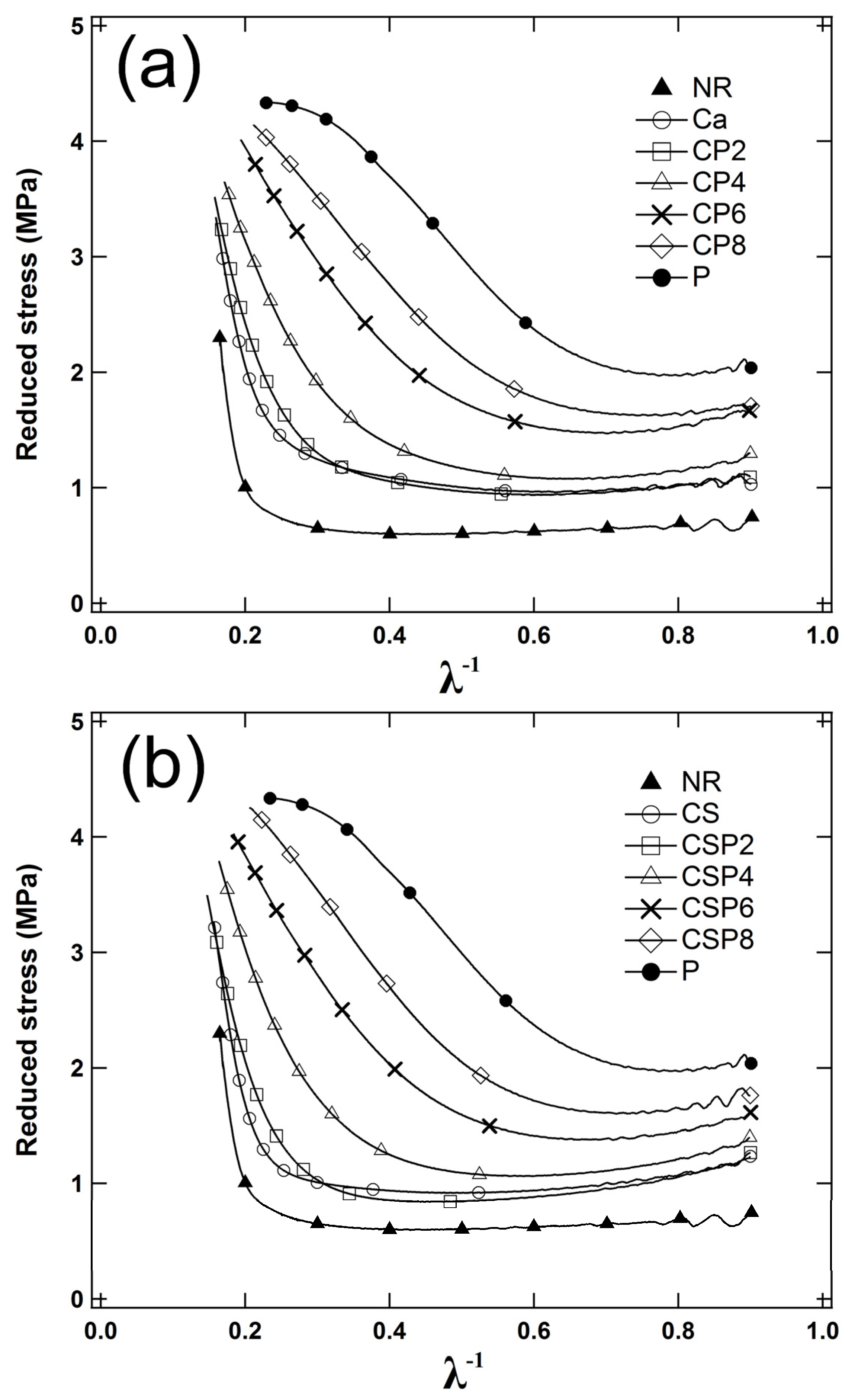
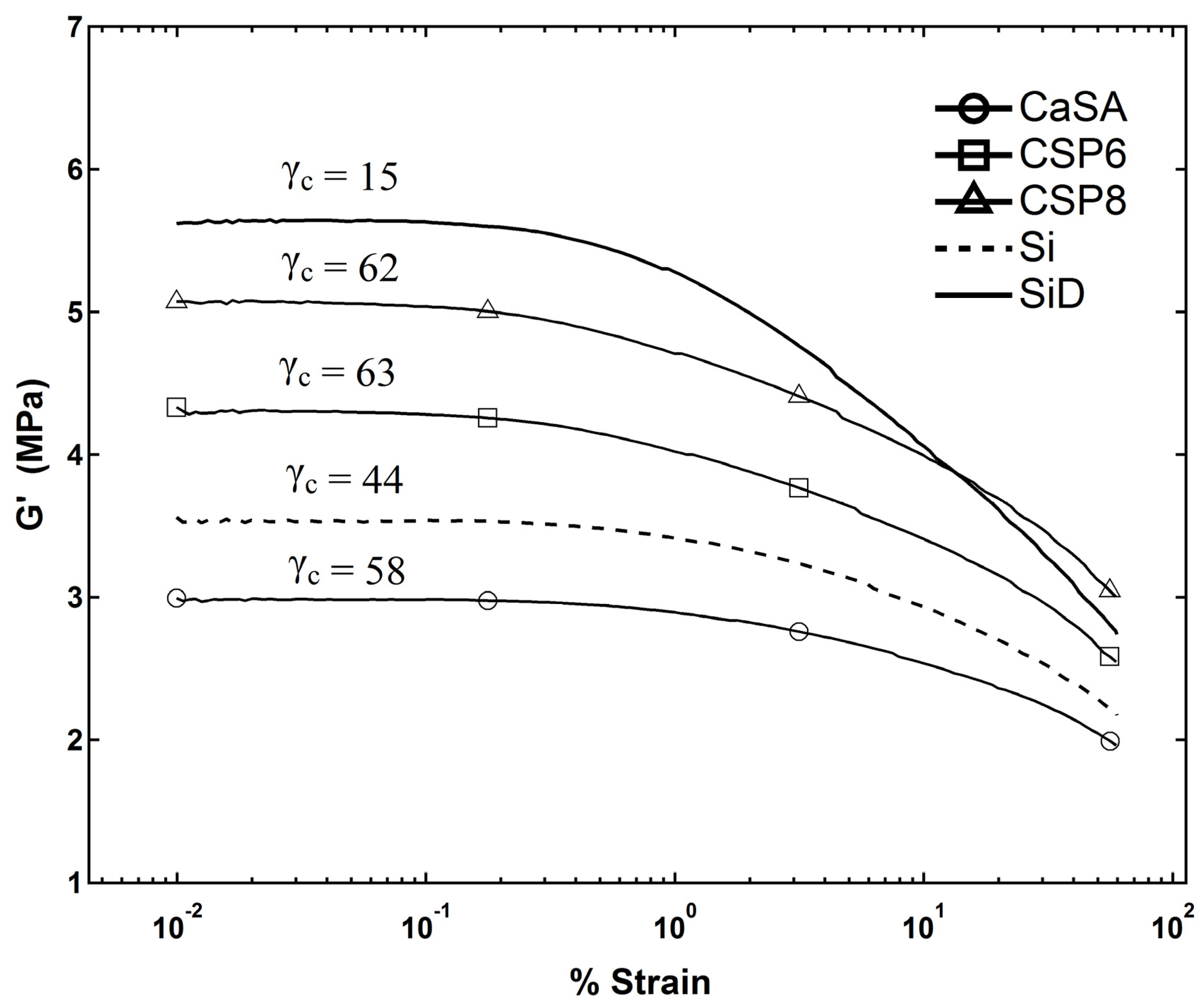
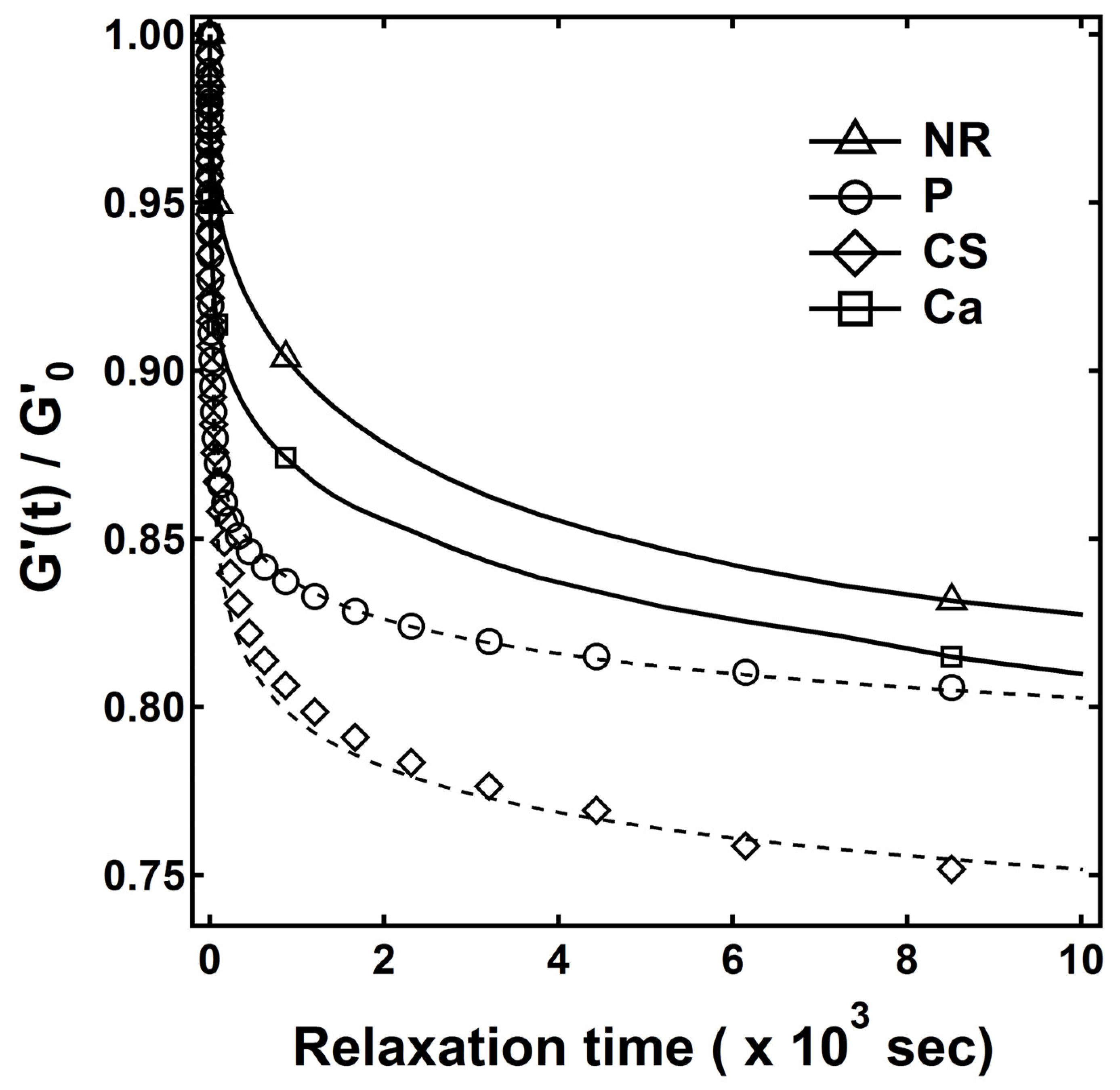
3.3. Relaxation and Filler-Polymer Interactions
4. Conclusions
Funding
Conflicts of Interest
References
- Jiang, L.; Lam, Y.C.; Tam, K.C.; Chua, T.H.; Sim, G.W.; Ang, L.S. Strengthening acrylonitrile-butadiene-styrene (ABS) with nano-sized and micro-sized calcium carbonate. Polymer 2005, 46, 243–252. [Google Scholar] [CrossRef]
- He, H.; Zhang, Z.; Wang, J.; Li, K. Compressive properties of nano-calcium carbonate/epoxy and its fiber composites. Compos. Part B 2013, 45, 919–924. [Google Scholar] [CrossRef]
- Chan, C.; Wu, J.; Li, J.; Cheung, Y. Polypropylene/calcium carbonate nanocomposites. Polymer 2002, 43, 2981–2992. [Google Scholar] [CrossRef]
- Nascimento, E.M.; Eiras, D.; Pessan, L.A. Effect of thermal treatment on impact resistance and mechanical properties of polypropylene/calcium carbonate nanocomposites. Compos. Part B 2016, 91, 228–234. [Google Scholar] [CrossRef]
- Lin, Y.; Chen, H.; Chan, C.; Wu, J. The toughening mechanism of polypropylene/calcium carbonate nanocomposites. Polymer 2010, 51, 3277–3284. [Google Scholar] [CrossRef]
- Essabir, H.; Bensalah, M.O.; Rodrigue, D.; Bouhfid, R.; Qaiss, A.E.K. A comparison between bio- and mineral calcium carbonate on the properties of polypropylene composites. Constr. Build. Mater. 2017, 134, 549–555. [Google Scholar] [CrossRef]
- Zokaei, S.; Lesan khosh, M.R.; Bagheri, R. Study of scratch resistance in homo- and co-polypropylene filled with nanometric calcium carbonate. Mater. Sci. Eng. A 2007, 445–446, 526–536. [Google Scholar] [CrossRef]
- Rungruang, P.; Grady, B.P.; Supapho, P. Surface modified calcium carbonate particles by amicellar polymerization to be used as a filler for isotactic polypropylene. Colloids Surf. A 2006, 275, 114–125. [Google Scholar] [CrossRef]
- Cao, Z.; Daly, M.; Clemence, L.; Geever, L.M.; Major, I.; Higginbotham, C.L.; Devine, D.M. Chemical surface modification of calcium carbonate particles with stearic acid using different treating methods. Appl. Surf. Sci. 2016, 378, 320–329. [Google Scholar] [CrossRef]
- Bartczak, Z.; Argon, A.S.; Cohen, R.E.; Weinberg, M. Toughness mechanism in semi-crystalline polymer blend: II. High-density polyethylene toughened with calcium carbonate filler particles. Polymer 1999, 40, 2347–2365. [Google Scholar] [CrossRef]
- Hu, L.; Dong, P.; Zhen, G. Preparation of active CaCO3 nanoparticles and mechanical properties of the composite materials. Mater. Lett. 2009, 63, 373–375. [Google Scholar] [CrossRef]
- Zhao, L.; Zhang, Y.; Miao, Y.; Nie, L. Controlled synthesis, characterization and application of hydrophobic calcium carbonate nanoparticles in PVC. Powder Technol. 2016, 288, 184–190. [Google Scholar] [CrossRef]
- Wang, Q.; Zhang, X.; Dong, W.; Gui, H.; Gao, J.; Lai, J.; Liu, Y.; Huang, F.; Song, Z.; Qiao, J. Novel rigid poly(vinyl chloride) ternary nanocomposites containing ultrafine full-vulcanized powdered rubber and untreated nano-sized calcium carbonate. Mater. Lett. 2007, 61, 1174–1177. [Google Scholar] [CrossRef]
- Li, X.H.; Tjong, S.C.; Meng, Y.Z.; Zhu, Q.J. Fabrication and properties of poly(propylene carbonate) calcium carbonate composites. Polym. Sci. Part B Polym. Phys. 2003, 41, 1806–1813. [Google Scholar] [CrossRef]
- Osman, M.A.; Atallah, A.; Suter, U.W. Influence of excessive filler coating on the tensile properties of LDPE-calcium carbonate composites. Polymer 2004, 45, 1177–1183. [Google Scholar] [CrossRef]
- Saad, A.L.; Younan, A.F. Rheological, mechanical and electrical properties of natural rubber-white filler mixtures reinforced with nylon 6 short fibers. Polym. Degrad. Stab. 1995, 50, 133–140. [Google Scholar] [CrossRef]
- Poompradub, S.; Ikeda, Y.; Kokubo, Y.; Shiono, T. Cuttlebone as reinforcing filler for natural rubber. Eur. Polym. J. 2008, 44, 4157–4164. [Google Scholar] [CrossRef]
- Saeb, M.R.; Ramezani-Dakhel, H.; Ali Khonakdar, H.; Heinrich, G.; Wagenknecht, U. A comparative study on curing characteristics and thermomechanical properties of elastomeric nanocomposites: The effect of eggshell and calcium carbonate nanofillers. J. Appl. Polym. Sci. 2013, 127, 4241–4250. [Google Scholar] [CrossRef]
- Poh, B.T.; Ismail, H.; Tan, K.S. Effect of filler loading on tensile and tear properties of SMR L/ENR 25 and SMR L/SBR blends cured via a semi-efficient vulcanization system. Polym. Test. 2002, 21, 801–806. [Google Scholar] [CrossRef]
- Manroshan, S.; Baharin, A. Effect of nanosized calcium carbonate on the mechanical proeprties of latex films. J. Appl. Polym. Sci. 2005, 96, 1550–1556. [Google Scholar] [CrossRef]
- Deng, C.; Chen, M.; Ao, N.; Yan, D.; Zheng, Z. CaCO3/natural rubber latex nanometer composites and its properties. J. Appl. Polym. Sci. 2006, 101, 3442–3447. [Google Scholar] [CrossRef]
- Ghari, H.S.; Jalali-Arani, A. Nanocomposites based on natural rubber, organoclay and nano-calcium carbonate: Study on the structure, cure behavior, static and dynamic-mechanical properties. Appl. Clay Sci. 2016, 119, 348–357. [Google Scholar] [CrossRef]
- Fang, Q.; Song, B.; Tee, T.; Sin, L.T.; Hui, D.; Bee, S. Investigation of dynamic characteristics of nano-sized calcium carbonate added in natural rubber vulcanizate. Compos. Part B 2014, 60, 561–567. [Google Scholar] [CrossRef]
- Moonchai, S.; Moonchai, D. Modeling and optimization of rebound resilience and hardness of defatted rice bran/calcium carbonate-filled NR vulcanisates. Polym. Test. 2013, 32, 1472–1478. [Google Scholar] [CrossRef]
- Alam, P.; Fagerlund, P.; Hagerstrand, P.; Toyryla, J.; Amini, S.; Tadayon, M.; Miserez, A.; Kumar, V.; Pahlevan, M.; Toivakka, M. L-Lysine template CaCO3 precipitated to flax develops flowery crystal structures that improve the mechanical properties of natural fiber reinforced composites. Compos. Part A 2015, 75, 84–88. [Google Scholar] [CrossRef]
- Ismail, H.; Mathialagan, M. Comparative study on the effect of partial replacement of silica or calcium carbonate by bentonite on the properties of EPDM composites. Polym. Test. 2012, 31, 199–208. [Google Scholar] [CrossRef]
- Wang, Q.; Song, Q.; Qiao, J.; Zhang, X.; Zhang, L.; Song, Z. Good dispersion of hydrophilic nanoscale calcium carbonate particles in nitrile butadiene rubber matrix. Polymer 2011, 52, 3496–3502. [Google Scholar] [CrossRef]
- Konar, S.K.; Gu, R.; Sain, M. Preparation and characterization of baked nitrile latex foam reinforced with biomass. Ind. Crop. Prod. 2013, 42, 261–267. [Google Scholar] [CrossRef]
- Jin, F.; Park, S. Thermo-mechanical behaviors of butadiene rubber reinforced with nano-sized calcium carbonate. Mater. Sci. Eng. A 2008, 478, 406–408. [Google Scholar] [CrossRef]
- Badley, R.A.; Atkinson, D.; Hauser, H.; Oldani, D.; Green, J.P.; Stubbs, J.M. The structure, physical and chemical properties of the soy bean protein glycinin. Biochim. Biophys. Acta. 1975, 412, 214–228. [Google Scholar] [CrossRef]
- Jong, L. Influence of protein hydrolysis on the mechanical properties of natural rubber composites reinforced with soy protein particles. Ind. Crop. Prod. 2015, 65, 102–109. [Google Scholar] [CrossRef]
- Schild, N.; Wolf, B.A. Polymer-Solvent Interaction Parameters. In Polymer Handbook, 4th ed.; Brandrup, J., Immergut, E.H., Grulke, E.A., Eds.; John Wiley & Sons: New York, NY, USA, 1999; pp. 250–262. [Google Scholar]
- Flory, P.J. Principles of Polymer Chemistry; Cornell University Press: New York, NY, USA, 1953. [Google Scholar]
- Van der Hoff, B.M.E.; Buckler, E.J. Transient changes in topology and energy on extension of polybutadiene networks. J. Macromol. Sci. Chem. 1967, 1, 747–788. [Google Scholar] [CrossRef]
- Trabelsi, P.A.; Albouy, P.-A.; Rault, J. Stress-induced crystallization around a cracked tip in natural rubber. Macromolecules 2002, 35, 10054–10061. [Google Scholar] [CrossRef]
- Trabelsi, P.A.; Albouy, P.-A.; Rault, J. Effective local deformation in stretched filled rubber. Macromolecules 2003, 36, 9093–9099. [Google Scholar] [CrossRef]
- Jong, L. Improved mechanical properties of silica reinforced rubber with natural polymer. Polym. Test. 2019, 79, 106009. [Google Scholar] [CrossRef]
- Vilgis, T.A.; Heinrich, G.; Kluppel, M. Reinforcement of Polymer Nano-Composites: Theory, Experiments and Applications; Cambridge University Press: Cambridge, UK, 2009; pp. 174–182. [Google Scholar]
- Chasset, R.; Thirion, P. Physics of Non-Crystalline Solids. In Proceedings of the International Conference, Delft, Netherlands, July 1964; Prins, J.A., Ed.; North-Holland Publishing Co.: Amsterdam, The Netherlands, 1965; pp. 345–359. [Google Scholar]
- Curro, J.; Pincus, P.A. Theoretical Basis for Viscoelastic Relaxation of Elastomers in the Long-Time Limit. Macromolecules 1983, 16, 559–562. [Google Scholar] [CrossRef]





| Ca | CP2 | CP4 | CP6 | CP8 | P | CS | CSP2 | CSP4 | CSP6 | CSP8 | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| NR | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 |
| Ca | 50 | 40 | 30 | 20 | 10 | 0 | 0 | 0 | 0 | 0 | 0 |
| CS | 0 | 0 | 0 | 0 | 0 | 0 | 50 | 40 | 30 | 20 | 10 |
| P | 0 | 10 | 20 | 30 | 40 | 50 | 0 | 10 | 20 | 30 | 40 |
| Tensile Strength (MPa) | Elongation at Break (%) | Young’s Modulus (MPa) | 100% Modulus (MPa) | 300% Modulus (MPa) | |
|---|---|---|---|---|---|
| NR | 12.1 ± 1.5 | 487 ± 18 | 1.46 ± 0.09 | 1.06 ± 0.01 | 2.94 ± 0.03 |
| Ca | 20.4 ± 0.8 | 525 ± 15 | 2.65 ± 0.09 | 1.73 ± 0.10 | 5.62 ± 0.27 |
| CP2 | 23.0 ± 1.1 | 541 ± 9 | 2.70 ± 0.12 | 1.72 ± 0.05 | 6.67 ± 0.23 |
| CP4 | 21.5 ± 0.5 | 477 ± 12 | 3.16 ± 0.18 | 2.14 ± 0.07 | 9.95 ± 0.29 |
| CP6 | 20.6 ± 0.3 | 423 ± 13 | 4.13 ± 0.15 | 2.99 ± 0.15 | 13.27 ± 0.43 |
| CP8 | 19.3 ± 0.3 | 376 ± 8 | 4.55 ± 0.17 | 3.71 ± 0.11 | 15.12 ± 0.29 |
| P | 18.4 ± 0.8 | 333 ± 17 | 5.52 ± 0.06 | 5.14 ± 0.11 | 16.95 ± 0.16 |
| CS | 20.3 ± 1.1 | 530 ± 14 | 3.05 ± 0.08 | 1.62 ± 0.04 | 4.54 ± 0.17 |
| CSP2 | 23.3 ± 0.8 | 573 ± 14 | 3.05 ± 0.13 | 1.47 ± 0.03 | 5.27 ± 0.21 |
| CSP4 | 23.1 ± 0.5 | 516 ± 4 | 3.47 ± 0.07 | 1.89 ± 0.06 | 8.81 ± 0.28 |
| CSP6 | 22.3 ± 1.1 | 453 ± 14 | 4.16 ± 0.29 | 2.75 ± 0.15 | 12.89 ± 0.54 |
| CSP8 | 20.9 ± 0.6 | 394 ± 11 | 4.79 ± 0.16 | 3.69 ± 0.06 | 15.60 ± 0.18 |
| Si | 23.9 ± 0.6 | 467 ± 12 | 3.73 ± 0.07 | 2.40 ± 0.06 | 12.23 ± 0.25 |
| SiD | 21.9 ± 0.7 | 400 ± 7 | 5.12 ± 0.18 | 3.34 ± 0.13 | 14.74 ± 0.24 |
| Hardness (Shore A) | Young’s Modulus (MPa) | Tear Strength (N/mm) | |
|---|---|---|---|
| CSP6 | 67 | 4.16 ± 0.29 | 9.0 ± 1.3 |
| CSP8 | 68 | 4.79 ± 0.16 | 8.3 ± 3.2 |
| Si | 67 | 3.73 ± 0.07 | 15.7 ± 2.4 |
| SiD | 71 | 5.12 ± 0.18 | 12.2 ± 3.2 |
| P | 0.4912 | 0.4 | 0.045 |
| CS | 0.4345 | 40 | 0.057 |
| CP2 | 0.4967 | 0.4 | 0.0375 |
| CP4 | 0.4958 | 0.4 | 0.040 |
| CP6 | 0.4954 | 0.4 | 0.041 |
| CP8 | 0.4927 | 0.4 | 0.0465 |
| CSP2 | 0.4853 | 0.8 | 0.066 |
| CSP4 | 0.4867 | 0.8 | 0.052 |
| CSP6 | 0.4871 | 0.8 | 0.047 |
| CSP8 | 0.4858 | 0.8 | 0.048 |
© 2020 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jong, L. Synergistic Effect of Calcium Carbonate and Biobased Particles for Rubber Reinforcement and Comparison to Silica Reinforced Rubber. J. Compos. Sci. 2020, 4, 113. https://doi.org/10.3390/jcs4030113
Jong L. Synergistic Effect of Calcium Carbonate and Biobased Particles for Rubber Reinforcement and Comparison to Silica Reinforced Rubber. Journal of Composites Science. 2020; 4(3):113. https://doi.org/10.3390/jcs4030113
Chicago/Turabian StyleJong, Lei. 2020. "Synergistic Effect of Calcium Carbonate and Biobased Particles for Rubber Reinforcement and Comparison to Silica Reinforced Rubber" Journal of Composites Science 4, no. 3: 113. https://doi.org/10.3390/jcs4030113




