Dispersion and Localization Behavior of Modified MWCNTs in Immiscible Polymer Blends of Polystyrene and Polybutadiene and in Corresponding Nanostructured Block Copolymers
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of Functionalized Carbon Nanotubes
2.3. Thermogravimetric Analysis
2.4. Powder Conductivity
2.5. Preparation of Solution Cast Films
2.6. Characterization of CNT Dispersion and Composite Morphology
2.7. Electrical Conductivity Measurements of The Composites
2.8. Tensile Tests of BCP/CNT Composite Films
3. Results
3.1. Characterization of Functionalized CNTs
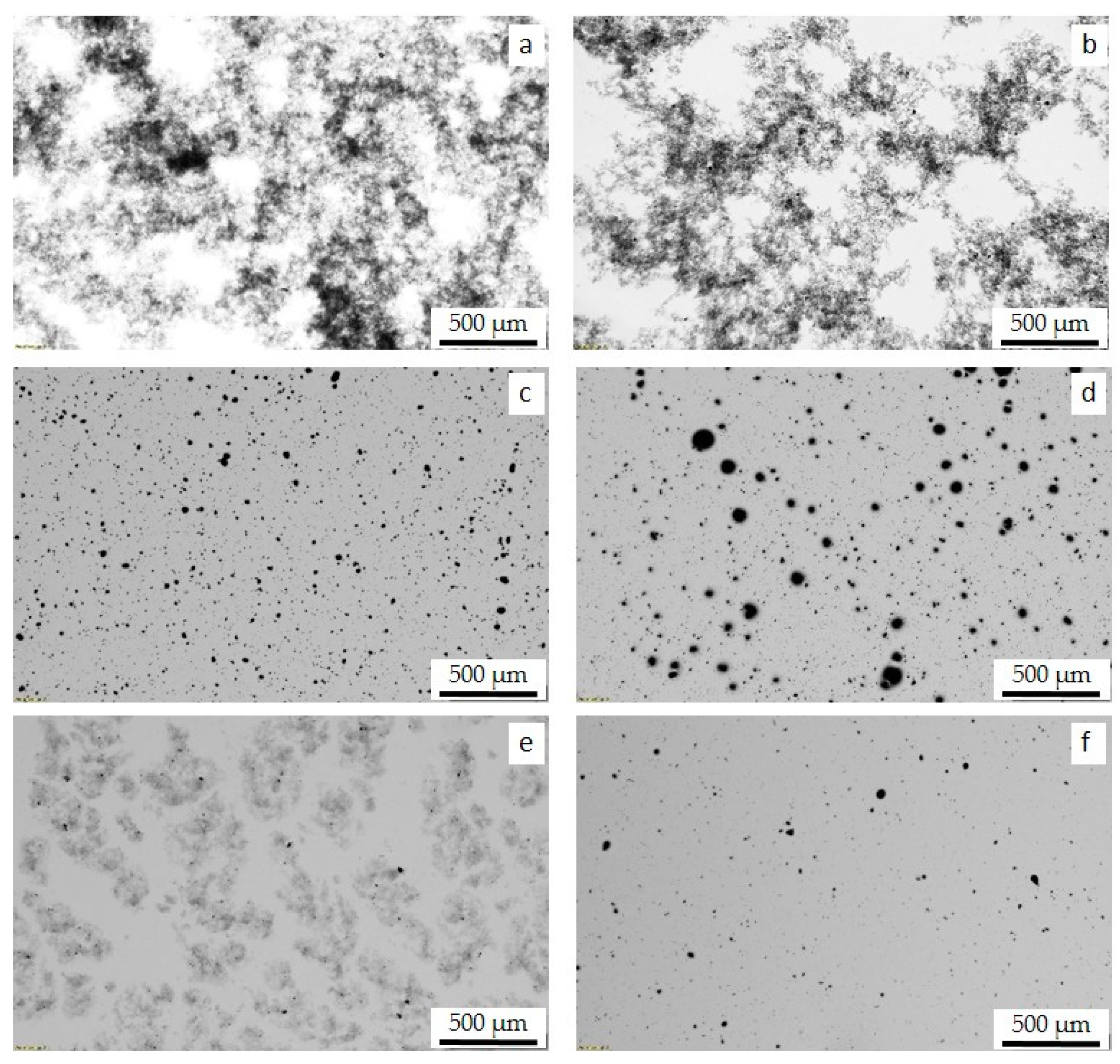
3.2. MWCNT Dispersion in PS and PB Homopolymers and in PS/PB Blends
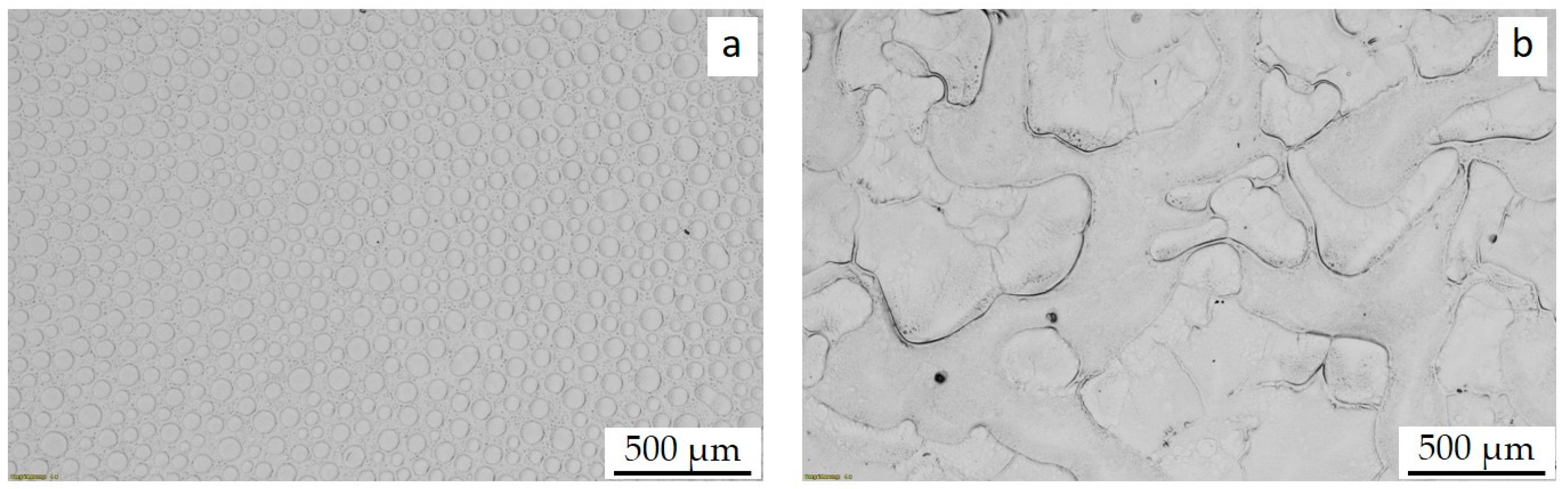
3.3. MWCNT Dispersion and Localization Behavior in PS/PB Blends
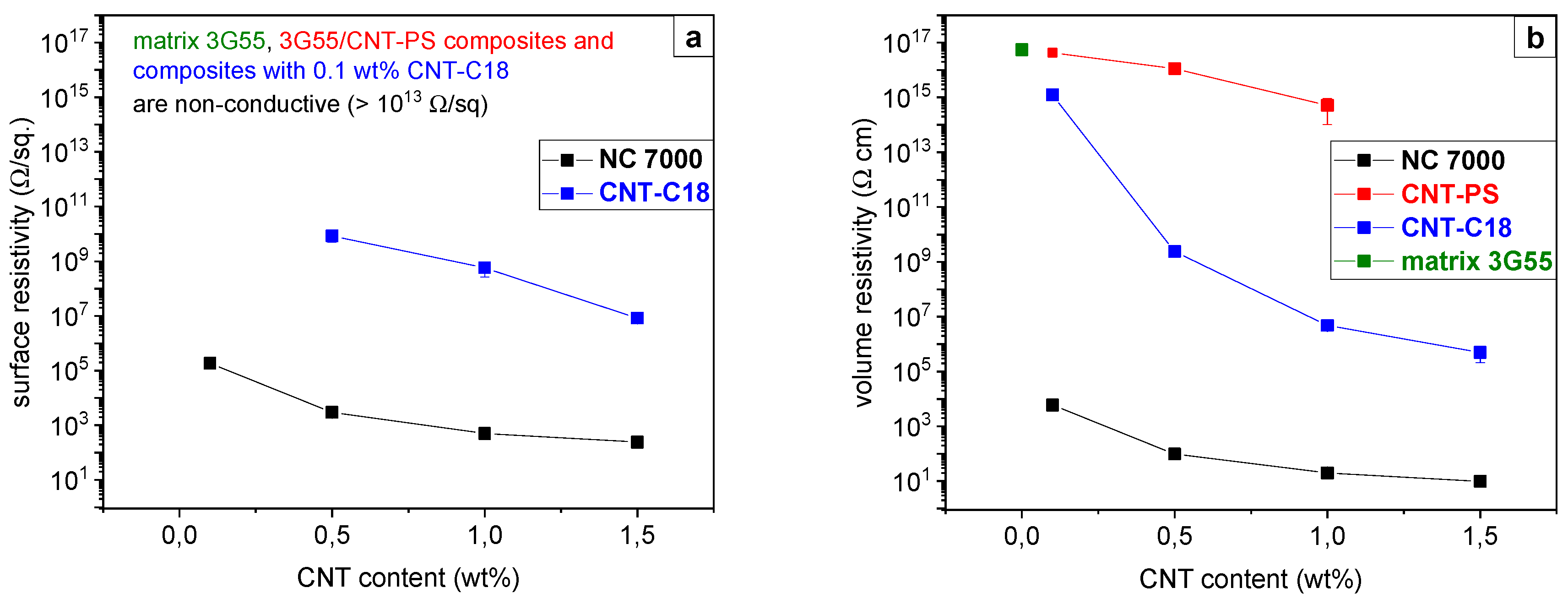
3.4. Electrical Surface Resistivity
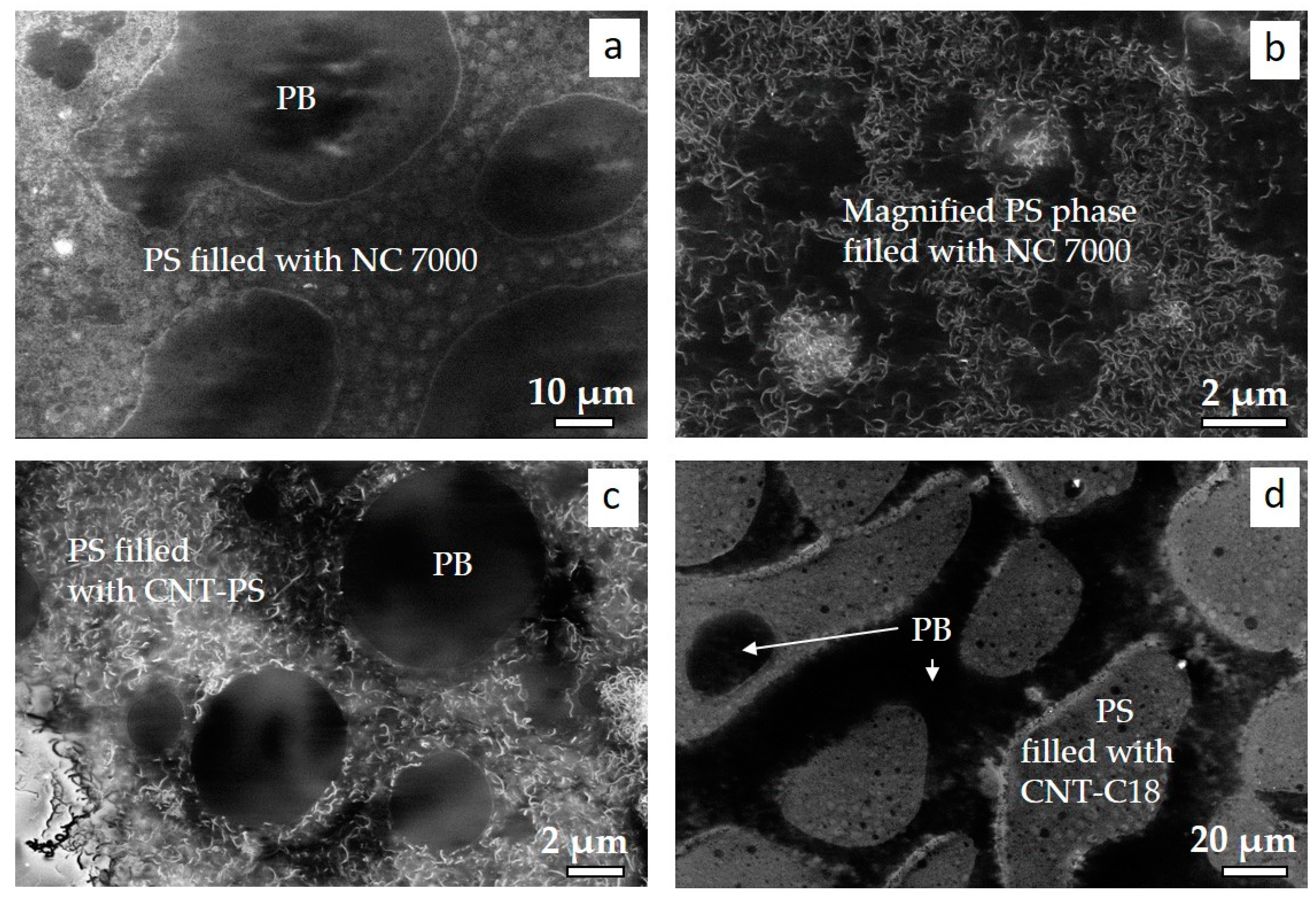
3.5. CNT Dispersion in BCP/MWCNT Composites
3.6. BCP Morphology and Nanocomposite Structure
3.7. Electrical Properties of BCP/MWCNT Composites
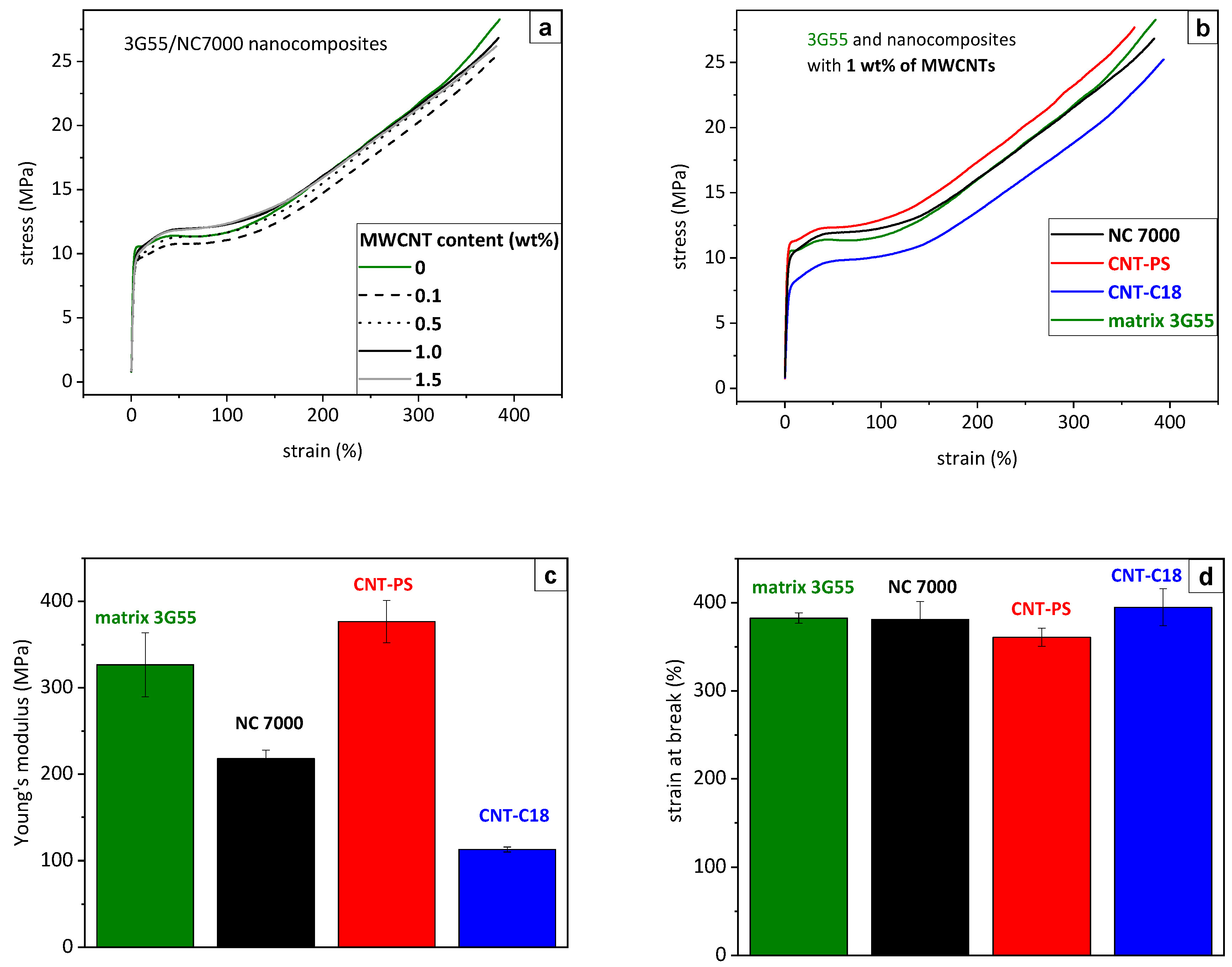
3.8. Stress–Strain Behavior of BCP/MWCNT Composites
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Khandpur, A.K.; Förster, S.; Bates, F.S.; Hamley, I.W.; Ryan, A.; Bras, W.; Almdal, K.; Mortensen, K. Polyisoprene-Polystyrene Diblock Copolymer Phase Diagram near the Order-Disorder Transition. Macromolecules 1995, 28, 8796–8806. [Google Scholar] [CrossRef]
- Bockstaller, M.R.; Mickiewicz, R.A.; Thomas, E.L. Block Copolymer Nanocomposites: Perspectives for Tailored Functional Materials. Adv. Mater. 2005, 17, 1331–1349. [Google Scholar] [CrossRef]
- Lu, L.; Zhou, Z.; Zhang, Y.; Wang, S.; Zhang, Y. Reinforcement of styrene–butadiene–styrene tri-block copolymer by multi-walled carbon nanotubes via melt mixing. Carbon 2007, 45, 2621–2627. [Google Scholar] [CrossRef]
- Pedroni, L.G.; Soto-Oviedo, M.A.; Rosolen, J.M.; Felisberti, M.I.; Nogueira, A.F. Conductivity and mechanical properties of composites based on MWCNTs and styrene-butadiene-styrene block™ copolymers. J. Appl. Polym. Sci. 2009, 112, 3241–3248. [Google Scholar] [CrossRef]
- Meier, J.G.; Crespo, C.; Pelegay, J.; Castell, P.; Sainz, R.; Maser, W.; Benito, A. Processing dependency of percolation threshold of MWCNTs in a thermoplastic elastomeric block copolymer. Polymer 2011, 52, 1788–1796. [Google Scholar] [CrossRef]
- Pedroni, L.; Araujo, J.; Felisberti, M.I.; Nogueira, A. Nanocomposites based on MWCNT and styrene–butadiene–styrene block copolymers: Effect of the preparation method on dispersion and polymer–filler interactions. Compos. Sci. Technol. 2012, 72, 1487–1492. [Google Scholar] [CrossRef]
- Périé, T.; Brosse, A.-C.; Tencé-Girault, S.; Leibler, L. Mechanical and electrical properties of multi walled carbon nanotube/ABC block terpolymer composites. Carbon 2012, 50, 2918–2928. [Google Scholar] [CrossRef]
- Costa, P.; Silva, J.; Ansón-Casaos, A.; Martínez, M.T.; Abad, M.-J.; Viana, J.C.; Lanceros-Mendez, S. Effect of carbon nanotube type and functionalization on the electrical, thermal, mechanical and electromechanical properties of carbon nanotube/styrene–butadiene–styrene composites for large strain sensor applications. Compos. Part B Eng. 2014, 61, 136–146. [Google Scholar] [CrossRef] [Green Version]
- Staudinger, U.; Satapathy, B.K. Influence of Carbon Nanotubes on the Morphology and Properties of Styrene-Butadiene Based Block Copolymers. Macromol. Symp. 2017, 373, 1700030. [Google Scholar] [CrossRef]
- Staudinger, U.; Satapathy, B.K.; Jehnichen, D. Nanofiller Dispersion, Morphology, Mechanical Behavior, and Electrical Properties of Nanostructured Styrene-Butadiene-Based Triblock Copolymer/CNT Composites. Polymer 2019, 11, 1831. [Google Scholar] [CrossRef] [Green Version]
- Park, I.; Lee, W.; Kim, J.; Park, M.; Lee, H. Selective sequestering of multi-walled carbon nanotubes in self-assembled block copolymer. Sens. Actuators B Chem. 2007, 126, 301–305. [Google Scholar] [CrossRef]
- Peponi, L.; Tercjak, A.; Gutierrez, J.; Cardinali, M.; Mondragon, I.; Valentini, L.; Kenny, J.M. Mapping of carbon nanotubes in the polystyrene domains of a polystyrene-b-polyisoprene-b-polystyrene block copolymer matrix using electrostatic force microscopy. Carbon 2010, 48, 2590–2595. [Google Scholar] [CrossRef]
- Liu, Y.-T.; Zhang, Z.-L.; Zhao, W.; Xie, X.-M.; Ye, X. Selective self-assembly of surface-functionalized carbon nanotubes in block copolymer template. Carbon 2009, 47, 1883–1885. [Google Scholar] [CrossRef]
- Wode, F.; Tzounis, L.; Kirsten, M.; Constantinou, M.; Georgopanos, P.; Rangou, S.; Zafeiropoulos, N.; Avgeropoulos, A.; Stamm, M. Selective localization of multi-wall carbon nanotubes in homopolymer blends and a diblock copolymer. Rheological orientation studies of the final nanocomposites. Polymer 2012, 53, 4438–4447. [Google Scholar] [CrossRef]
- Albuerne, J.; Fierro, A.B.-D.; Abetz, C.; Fierro, D.; Abetz, V. Block Copolymer Nanocomposites Based on Multiwall Carbon Nanotubes: Effect of the Functionalization of Multiwall Carbon Nanotubes on the Morphology of the Block Copolymer. Adv. Eng. Mater. 2011, 13, 803–810. [Google Scholar] [CrossRef]
- Hasanabadi, N.; Nazockdast, H.; Gajewska, B.; Balog, S.; Gunkel, I.; Bruns, N.; Lattuada, M. Structural Behavior of Cylindrical Polystyrene-block-Poly(ethylene-butylene)-block-Polystyrene (SEBS) Triblock Copolymer Containing MWCNTs: On the Influence of Nanoparticle Surface Modification. Macromol. Chem. Phys. 2017, 218, 1700231. [Google Scholar] [CrossRef] [Green Version]
- Garate, H.; Fascio, M.L.; Mondragon, I.; D’Accorso, N.B.; Goyanes, S. Surfactant-aided dispersion of polystyrene-functionalized carbon nanotubes in a nanostructured poly(styrene-b-isoprene-b-styrene) block copolymer. Polymer 2011, 52, 2214–2220. [Google Scholar] [CrossRef]
- Meincke, O.; Kaempfer, D.; Weickmann, H.; Friedrich, C.; Vathauer, M.; Warth, H. Mechanical properties and electrical conductivity of carbon-nanotube filled polyamide-6 and its blends with acrylonitrile/butadiene/styrene. Polymer 2004, 45, 739–748. [Google Scholar] [CrossRef]
- Göldel, A.; Kasaliwal, G.; Pötschke, P. Selective Localization and Migration of Multiwalled Carbon Nanotubes in Blends of Polycarbonate and Poly(styrene-acrylonitrile). Macromol. Rapid Commun. 2009, 30, 423–429. [Google Scholar] [CrossRef]
- Bose, S.; Bhattacharyya, A.R.; Khare, R.A.; Kulkarni, A.R.; Patro, T.U.; Sivaraman, P. Tuning the dispersion of multiwall carbon nanotubes in co-continuous polymer blends: A generic approach. Nanotechnology 2008, 19, 335704. [Google Scholar] [CrossRef]
- Bose, S.; Bhattacharyya, A.R.; Kulkarni, A.R.; Pötschke, P. Electrical, rheological and morphological studies in co-continuous blends of polyamide 6 and acrylonitrile–butadiene–styrene with multiwall carbon nanotubes prepared by melt blending. Compos. Sci. Technol. 2009, 69, 365–372. [Google Scholar] [CrossRef]
- Gültner, M.; Göldel, A.; Pötschke, P. Tuning the localization of functionalized MWCNTs in SAN/PC blends by a reactive component. Compos. Sci. Technol. 2011, 72, 41–48. [Google Scholar] [CrossRef]
- Knoll, K.; Nießner, N. Styrolux+ and Styroflex+-From Transparent High Impact Polystyrene to New Thermoplastic Elastomers: Syntheses, Applications and Blends with other Styrene based Polymers. Macromol. Symp. 1998, 132, 231–243. [Google Scholar] [CrossRef]
- Saalwächter, K.; Thomann, Y.; Hasenhindl, A.; Schneider, H. Direct Observation of Interphase Composition in Block Copolymers. Macromolecules 2008, 41, 9187–9191. [Google Scholar] [CrossRef] [Green Version]
- NANOCYL® NC7000TM Technical Data Sheet, 12th July 2016, V08. Available online: https:www.nanocyl.com (accessed on 30 March 2020).
- Postma, A.; Davis, T.P.; Evans, R.; Li, G.; Moad, G.; O’Shea, M.S. Synthesis of Well-Defined Polystyrene with Primary Amine End Groups through the Use of Phthalimido-Functional RAFT Agents. Macromolecules 2006, 39, 5293–5306. [Google Scholar] [CrossRef]
- Krause, B.; Boldt, R.; Häußler, L.; Pötschke, P. Ultralow percolation threshold in polyamide 6.6/MWCNT composites. Compos. Sci. Technol. 2015, 114, 119–125. [Google Scholar] [CrossRef]
- Price, G.; Smith, P.F. Ultrasonic degradation of polymer solutions. 1. Polystyrene revisited. Polym. Int. 1991, 24, 159–164. [Google Scholar] [CrossRef]
- Price, G.; Smith, P.F. Ultrasonic degradation of polymer solutions: 2. The effect of temperature, ultrasound intensity and dissolved gases on polystyrene in toluene. Polymer 1993, 34, 4111–4117. [Google Scholar] [CrossRef]
- Chubarova, E.V.; Melenevskaya, E.Y.; Shamanin, V.V. Chain Degradation under Low-Intensity Sonication of Polymer Solutions in the Presence of Filler: Mechanism of Ultrasonic Degradation of Flexible Chain Macromolecules. J. Macromol. Sci. Part B 2012, 52, 873–896. [Google Scholar] [CrossRef]
- Ameli, A.; Arjmand, M.; Pötschke, P.; Krause, B.; Sundararaj, U. Effects of synthesis catalyst and temperature on broadband dielectric properties of nitrogen-doped carbon nanotube/polyvinylidene fluoride nanocomposites. Carbon 2016, 106, 260–278. [Google Scholar] [CrossRef]
- Arjmand, M.; Chizari, K.; Krause, B.; Pötschke, P.; Sundararaj, U. Effect of synthesis catalyst on structure of nitrogen-doped carbon nanotubes and electrical conductivity and electromagnetic interference shielding of their polymeric nanocomposites. Carbon 2016, 98, 358–372. [Google Scholar] [CrossRef]
- Krause, B.; Mende, M.; Pötschke, P.; Petzold, G. Dispersability and particle size distribution of CNTs in an aqueous surfactant dispersion as a function of ultrasonic treatment time. Carbon 2010, 48, 2746–2754. [Google Scholar] [CrossRef]
- Staudinger, U.; Krause, B.; Steinbach, C.; Pötschke, P.; Voit, B. Dispersability of multiwalled carbon nanotubes in polycarbonate-chloroform solutions. Polymer 2014, 55, 6335–6344. [Google Scholar] [CrossRef]
- Alig, I.; Pötschke, P.; Lellinger, D.; Skipa, T.; Pegel, S.; Kasaliwal, G.R.; Villmow, T. Establishment, morphology and properties of carbon nanotube networks in polymer melts. Polymer 2012, 53, 4–28. [Google Scholar] [CrossRef]
- Joseph, S.; Rutkowska, M.; Jastrzębska, M.; Janik, H.; Haponiuk, J.T.; Thomas, S. Polystyrene/polybutadiene blends: An analysis of the phase-inversion region and cophase continuity and a comparison with theoretical predictions. J. Appl. Polym. Sci. 2003, 89, 3700. [Google Scholar] [CrossRef]
- Martins, J.A.; Cruz, V.S.; Paiva, M.C. Flow Activation Volume in Composites of Polystyrene and Multiwall Carbon Nanotubes with and without Functionalization. Macromolecules 2011, 44, 9804–9813. [Google Scholar] [CrossRef]
- Adhikari, R.; Buschnakowski, M.; Henning, S.; Goerlitz, S.; Huy, T.A.; Lebek, W.; Godehardt, R.; Michler, G.H.; Lach, R.; Geiger, K.; et al. Double Yielding in a Styrene/Butadiene Star Block Copolymer. Macromol. Rapid Commun. 2004, 25, 653–658. [Google Scholar] [CrossRef]
- Satapathy, B.; Lach, R.; Weidisch, R.; Schneider, K.; Janke, A.; Knoll, K. Morphology and crack toughness behaviour of nanostructured block copolymer/homopolymer blends. Eng. Fract. Mech. 2006, 73, 2399–2412. [Google Scholar] [CrossRef]
- Thomann, Y.; Thomann, R.; Hasenhindl, A.; Mülhaupt, R.; Heck, B.; Knoll, K.; Steininger, H.; Saalwächter, K. Gradient Interfaces in SBS and SBS/PS Blends and Their Influence on Morphology Development and Material Properties. Macromolecules 2009, 42, 5684–5699. [Google Scholar] [CrossRef]
- Sakurai, S.; Sakamoto, J.; Shibayama, M.; Nomura, S. Effects of microdomain structures on the molecular orientation of poly(styrene-block-butadiene-block-styrene) triblock copolymer. Macromolecules 1993, 26, 3351–3356. [Google Scholar] [CrossRef]
- Alexandridis, P.; Spontak, R.J. Solvent-regulated ordering in block copolymers. Curr. Opin. Colloid Interface Sci. 1999, 4, 130–139. [Google Scholar] [CrossRef]
- Adhikari, R.; Huy, T.A.; Buschnakowski, M.; Michler, G.H.; Knoll, K. Asymmetric PS-block-(PS-co-PB)-block-PS block copolymers: Morphology formation and deformation behaviour. New J. Phys. 2004, 6, 28. [Google Scholar] [CrossRef]







| CNT Sample | Powder Conductivity (S/cm) at 30 MPa | Standard Deviation (S/cm) | Powder Density (g/cm³) |
|---|---|---|---|
| NC 7000 | 17.5 | 0.3 | 0.90 |
| CNT-C18 | 6.5 | 0.2 | 0.72 |
| CNT-PS | 1.3 | 0.1 | 0.67 |
| Polymer Matrix | Surface Resistivity (Ohm/sq.) | |
|---|---|---|
| Solvent Toluene | Solvent Chloroform | |
| PS | 2.6 × 104 ± 1.7 × 104 | 8.5 × 106 ± 3.1 × 107 |
| PB | non-conductive (out of measuring range) | 9.8 × 1011 ± 1.8 × 1012 |
| PS/PB (60/40) | 2 × 106 ± 6.2 × 106 | 3 × 106 ± 8.3 × 106 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Staudinger, U.; Jakisch, L.; Hilbig, L. Dispersion and Localization Behavior of Modified MWCNTs in Immiscible Polymer Blends of Polystyrene and Polybutadiene and in Corresponding Nanostructured Block Copolymers. J. Compos. Sci. 2020, 4, 40. https://doi.org/10.3390/jcs4020040
Staudinger U, Jakisch L, Hilbig L. Dispersion and Localization Behavior of Modified MWCNTs in Immiscible Polymer Blends of Polystyrene and Polybutadiene and in Corresponding Nanostructured Block Copolymers. Journal of Composites Science. 2020; 4(2):40. https://doi.org/10.3390/jcs4020040
Chicago/Turabian StyleStaudinger, Ulrike, Lothar Jakisch, and Luise Hilbig. 2020. "Dispersion and Localization Behavior of Modified MWCNTs in Immiscible Polymer Blends of Polystyrene and Polybutadiene and in Corresponding Nanostructured Block Copolymers" Journal of Composites Science 4, no. 2: 40. https://doi.org/10.3390/jcs4020040




