The Synergistic Effects of Sio2 Nanoparticles and Organic Photostabilizers for Enhanced Weathering Resistance of Acrylic Polyurethane Coating
Abstract
:1. Introduction
2. Experimental
2.1. Materials
2.2. Preparation of Coatings
2.3. Accelerated Weathering Test
2.4. Characterizations
2.4.1. Field Emission Scanning Electron Microscopy (FESEM) and Transmission Electron Microscopy (TEM) Analysis
2.4.2. Infrared (IR) Analysis
2.4.3. Ultraviolet-Visible (UV-Vis) Analysis
2.4.4. Weight Loss Measurements
2.4.5. Gloss Loss Measurements
2.4.6. Determination of Abrasion Resistance
3. Results and Discussion
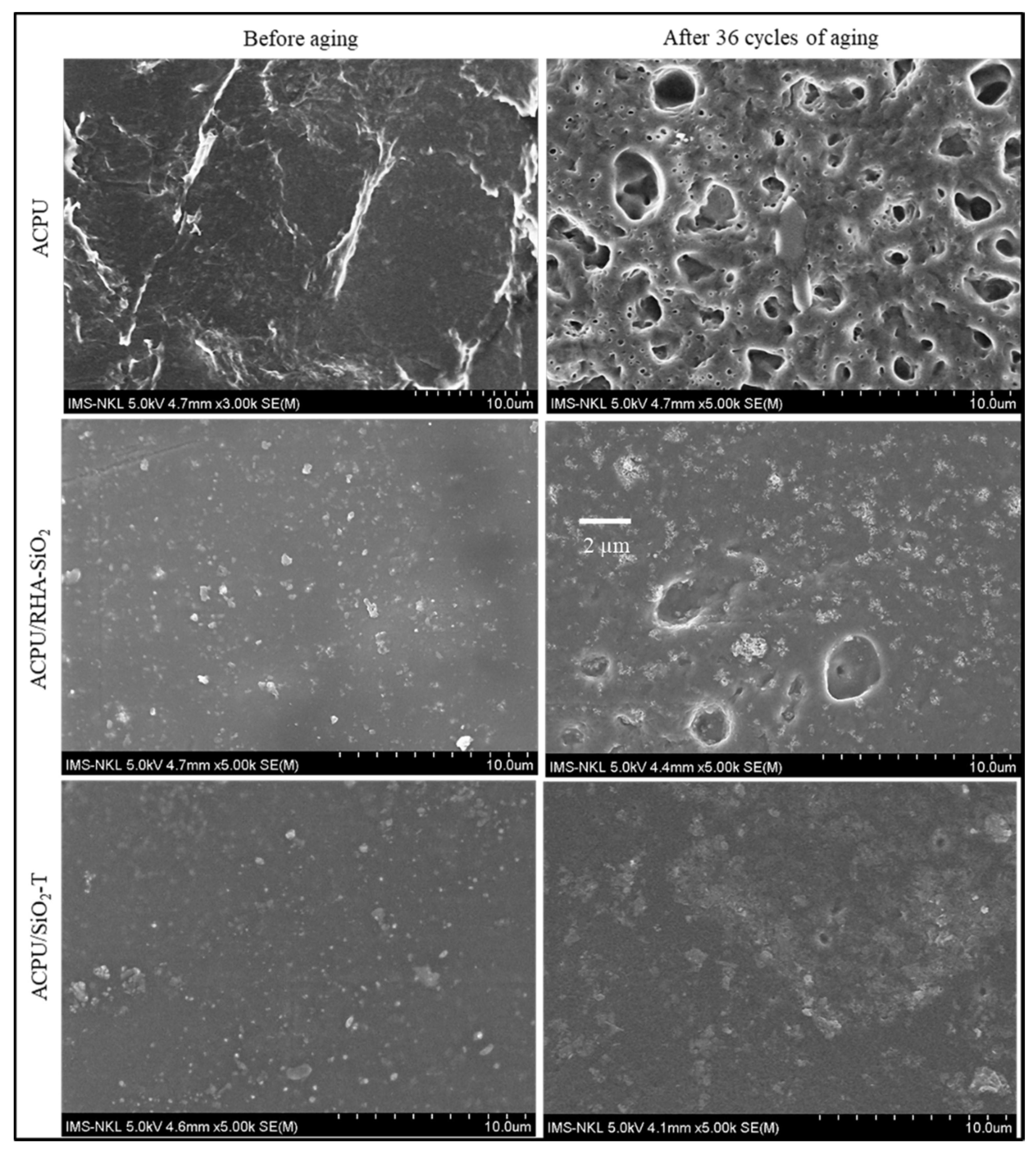
3.1. Morphology Study of Coating
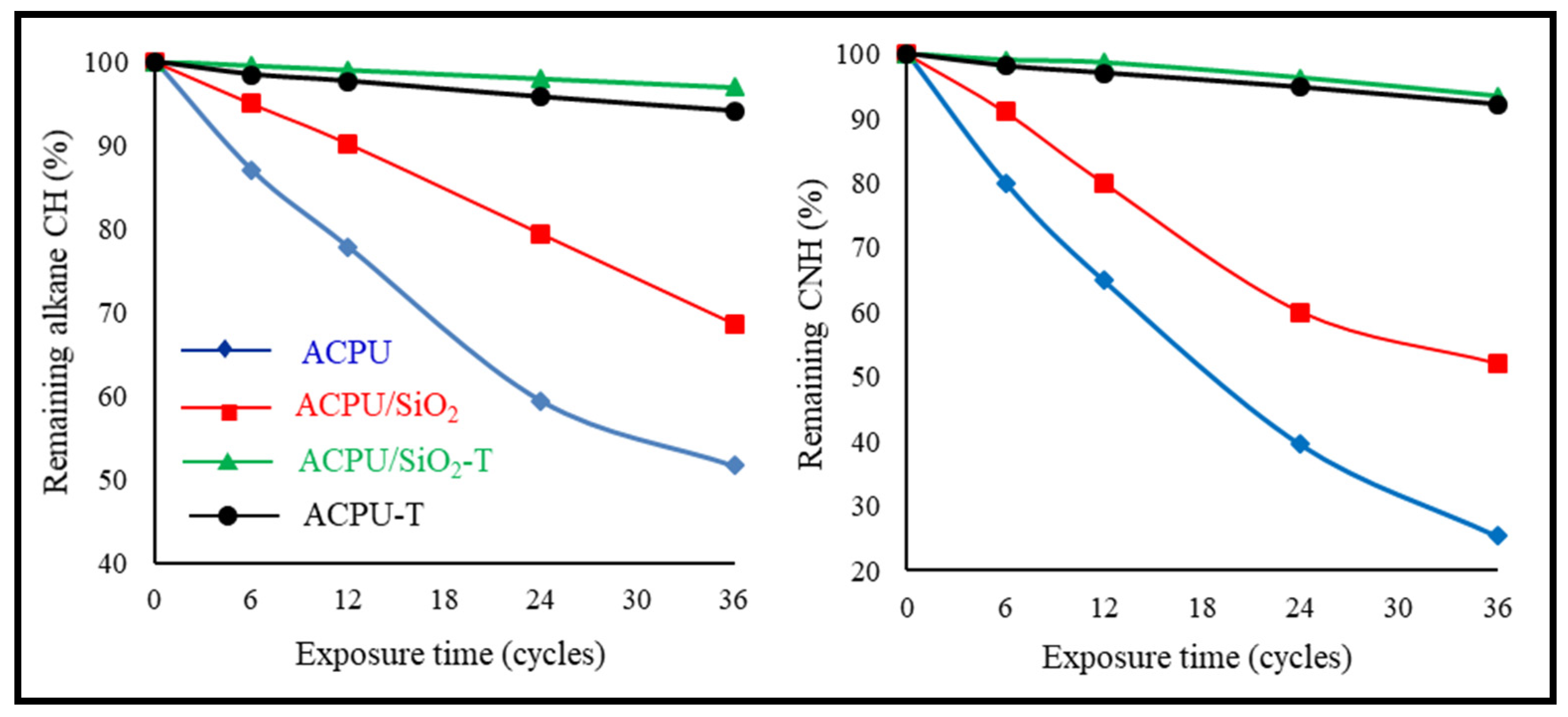
3.2. IR Spectra Study
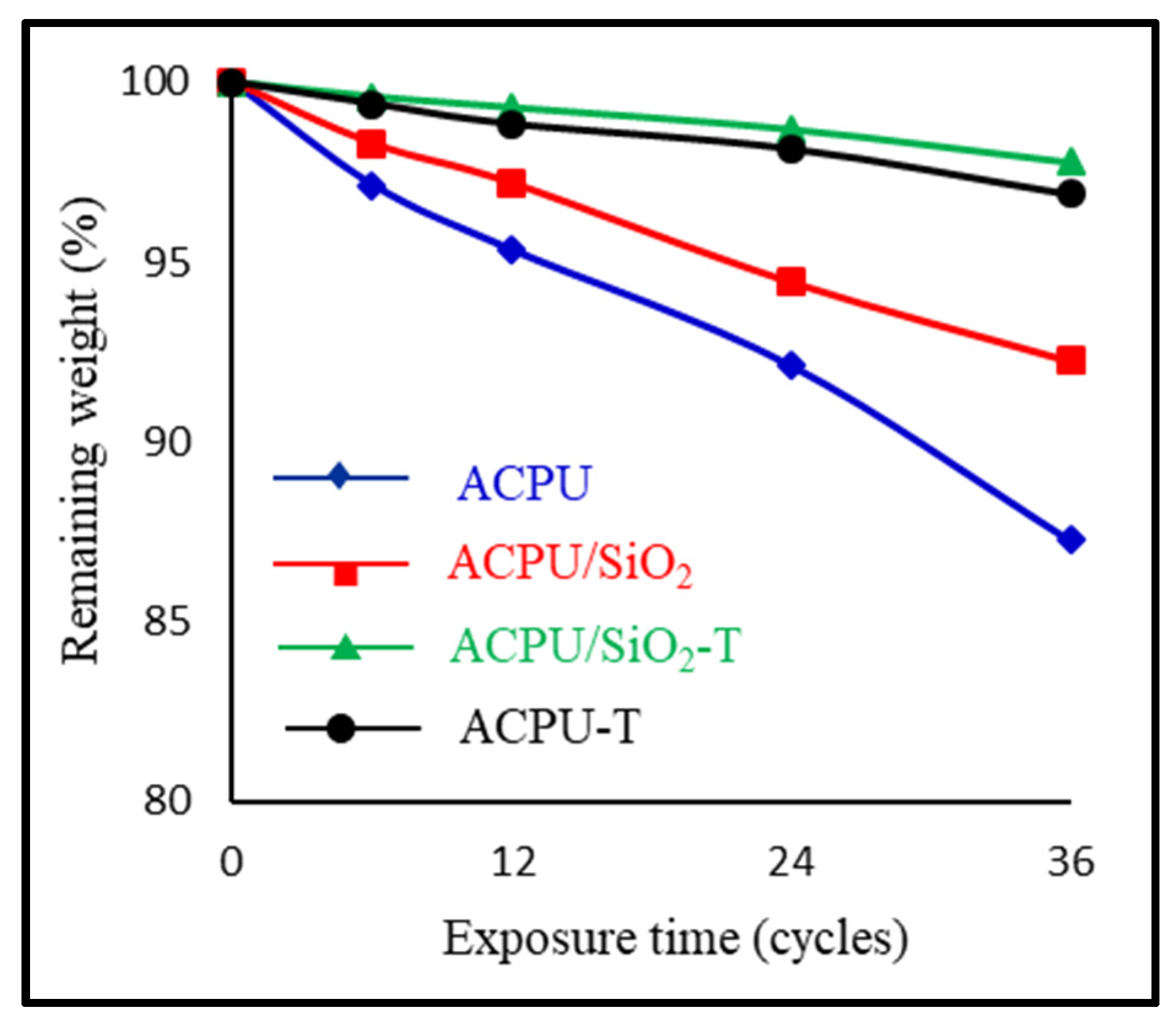
3.3. Weight Loss Study
3.4. Coating Gloss Loss Study
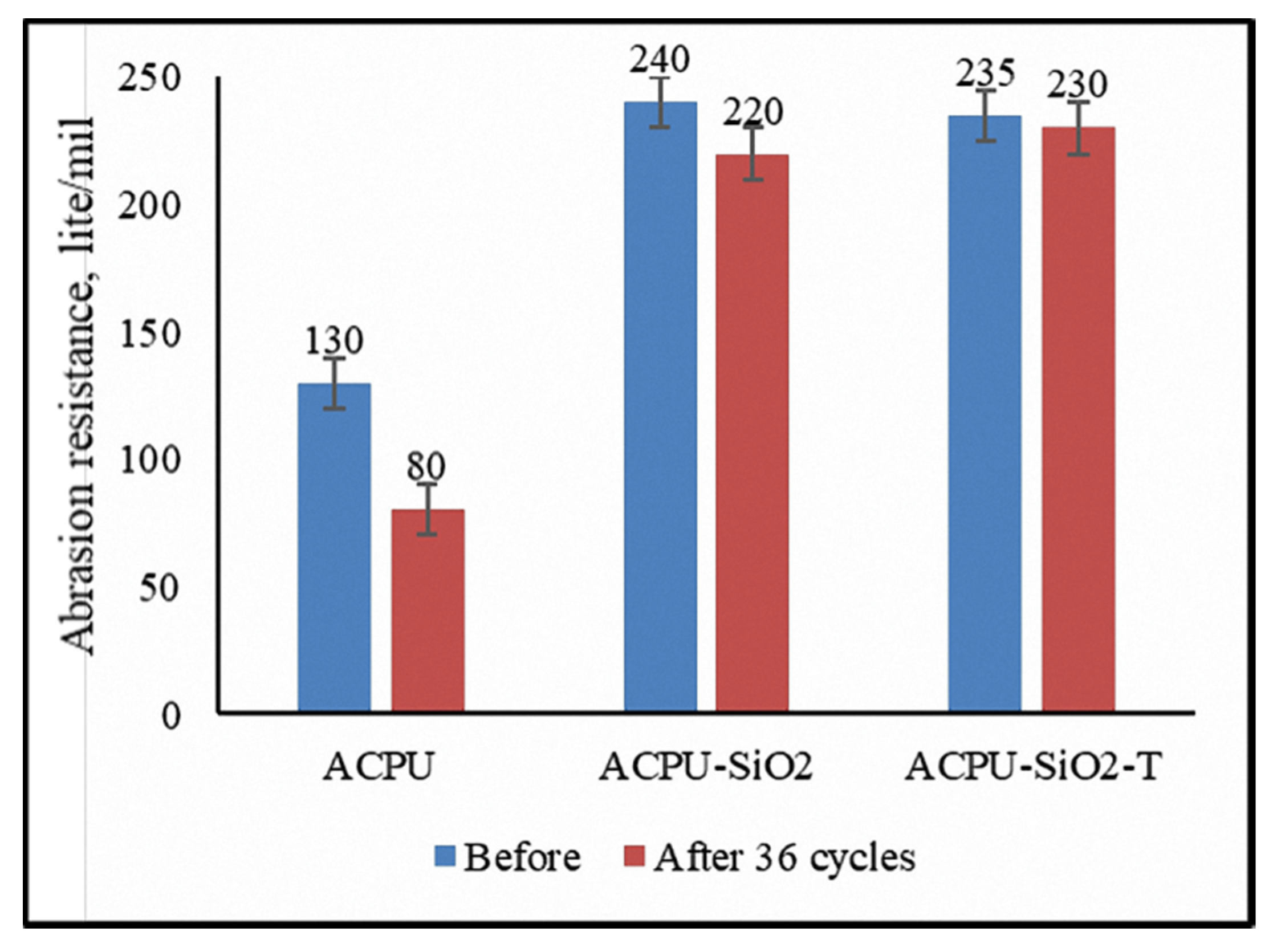
3.5. Coating Abrasion Study
4. Conclusions
- The obtained data indicated that incorporation of nano-SiO2 in the acrylic polyurethane matrix not only improved its mechanical property but also enhanced its weathering resistance.
- After 36 testing cycles, the remaining content of alkane CH groups was 51.6%, 68.6%, and 97.1%, in the ACPU, ACPU/SiO2, and ACPU/SiO2-T coatings, respectively. In case of CNH groups, their remained content was 25.4%, 52.1%, 93.5% in the ACPU, ACPU/SiO2, and ACPU/SiO2-T coatings, respectively.
- After 36 aging cycles, the gloss remaining values of ACPU, ACPU/SiO2, and ACPU/SiO2-T coatings, were 83.3%, 92.5%, and 98.5 %, respectively.
- After 36 aging cycles, the abrasion resistance of the ACPU coating reduced strongly (down to 38.46%), from 130 to 80 lite/mil. However, for ACPU/SiO2 coating, it reduced slightly from 240 to 220 lite/mil (8.33%). In the case of ACPU/SiO2-T coating its reduction was only 2.12% (from 235 to 230 lite/mil) after aging test.
Author Contributions
Funding
Conflicts of Interest
References
- Nguyen, T.N.L.; Do, T.V.; Nguyen, T.V.; Hung, D.P.; Trinh, V.T.; Mac, V.P.; Nguyen, A.H.; Le, T.L.; Nguyen, T.A.; Vo, T.K.A.; et al. Antimicrobial activity of acrylic polyurethane/Fe3O4-Ag nanocomposite coating. Prog. Org. Coat. 2019, 132, 15–20. [Google Scholar] [CrossRef]
- Maganty, S.; Roma, M.P.C.; Meschter, S.J.; Starkey, D.; Gomez, M.; Edwards, D.G.; Elsken, A.E.K.; Cho, J. Enhanced mechanical properties of polyurethane composite coatings through nanosilica addition. Prog. Org. Coat. 2016, 90, 243–251. [Google Scholar] [CrossRef] [Green Version]
- Nguyen, T.V.; Le, X.H.; Dao, P.H.; Decker, C.; Nguyen, T.P. Stability of acrylic polyurethane coatings under accelerated aging tests and natural outdoor exposure: The critical role of the used photo-stabilizers. Prog. Org. Coat. 2018, 124, 137–146. [Google Scholar] [CrossRef]
- Chen, X.D.; Wang, Z.; Liao, Z.F.; Mai, Y.L.; Zhang, M.Q. Roles of anatase and rutile TiO2 nanoparticles in photooxidation of polyurethane. Polym. Test. 2007, 26, 202–208. [Google Scholar] [CrossRef]
- Nguyen, T.V.; Nguyen, T.P.; Nguyen, T.D.; Aidani, R.; Trinh, V.T.; Decker, C. Accelerated degradation of water borne acrylic nanocomposites used outdoor protective coatings. Polym. Degrad. Stab. 2016, 128, 65–76. [Google Scholar] [CrossRef]
- Nguyen, T.V.; Dao, P.H.; Duong, K.L.; Duong, Q.H.; Vu, Q.T.; Nguyen, A.H.; Mac, V.P.; Le, T.L. Effect of R-TiO2 and ZnO nanoparticles on the UV-shielding efficiency of water-borne acrylic coating. Prog. Org. Coat. 2017, 110, 114–121. [Google Scholar] [CrossRef]
- Nguyen, T.V.; Nguyen, T.A.; Dao, P.H.; Mac, V.P.; Nguyen, A.H.; Do, M.T.; Nguyen, T.H. Effect of rutile titania dioxide nanoparticles on the mechanical property, thermal stability, weathering resistance and antibacterial property of styrene acrylic polyurethane coating. Adv. Nat. Sci. Nanosci. Nanotechnol. 2016, 7, 045015–045024. [Google Scholar] [CrossRef] [Green Version]
- Seentrakoon, B.; Junhasavasdikul, B.; Chavasiri, W. Enhanced UV-protection and antibacterial properties of natural rubber/rutile-TiO2 nanocomposites. Polym. Degrad. Stab. 2013, 98, 566–578. [Google Scholar] [CrossRef]
- Nguyen, T.V.; Tri, P.N.; Azizi, S.; Dang, T.C.; Hoang, D.M.; Hoang, T.H.; Nguyen, T.L.; Bui, T.T.L.; Dang, V.H.; Nguyen, N.L.; et al. The role of organic and inorganic UV-absorbents on photopolymerization and mechanical properties of acrylate-urethane coating. J. Mater. Today Commun. 2020, 22, 100780. [Google Scholar] [CrossRef]
- Tri, P.N.; Nguyen, T.A.; Nguyen, T.H.; Carriere, P. Antibacterial behavior of hybrid nanoparticles—Chapter 7. In Noble Metal-Metal Oxide Hybrid Nanoparticles: Fundamentals and Applications; Mohapatra, S., Nguyen, T.A., Nguyen-Tri, P., Eds.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 141–155. [Google Scholar] [CrossRef]
- Ngo, T.D.; Le, T.M.H.; Nguyen, T.H.; Nguyen, T.V.; Nguyen, T.A.; Le, T.L.; Nguyen, T.T.; Tran, T.T.V.; Le, T.B.T. Antibacterial nanocomposites based on Fe3O4-Ag hybrid nanoparticles and natural rubber-polyethylene blends. Int. J. Polym. Sci. 2016, 2016. [Google Scholar] [CrossRef] [Green Version]
- Nguyen, T.V.; Do, T.V.; Ha, M.H.; Le, H.K.; Le, T.T.; Nguyen, T.N.L.; Dam, X.T.; Lu, L.T.; Tran, D.L.; Vu, Q.T.; et al. Crosslinking process, mechanical and antibacterial properties of UV-curable acrylate/Fe3O4-Ag nanocomposite coating. J. Prog. Org. Coat. 2020, 139, 105325. [Google Scholar] [CrossRef]
- Nguyen, T.H.; Nguyen, T.A.; Nguyen, T.V.; Le, V.K.; Dinh, T.M.T.; Thai, H.; Shi, X. Effect of Electrochemical Injection of Corrosion Inhibitor (EICI) on the corrosion of steel rebar in chloride contanminated mortar. Int. J. Corros. 2015, 2015. [Google Scholar] [CrossRef] [Green Version]
- Nguyen, T.V.; Dao, P.H.; Nguyen, T.A.; Dang, V.H.; Ha, M.N.; Nguyen, T.T.T.; Vu, Q.T.; Nguyen, N.L.; Dang, C.T.; Tri, P.N.; et al. Photocatalytic degradation and heat reflectance recovery of water-borne acrylic polymer/ZnO nanocomposite coating. J. Compos. Sci. 2020. [Google Scholar] [CrossRef]
- Wu, G.; Liu, D.; Chen, J.; Liu, G.; Kong, Z. Preparation and properties of super hydrophobic films from siloxane-modified two-component waterborne polyurethane and hydrophobic nano SiO2. Prog. Org. Coat. 2019, 127, 80–87. [Google Scholar] [CrossRef]
- Jouyandeh, M.; Rahmati, N.; Movahedifar, E.; Hadavand, B.S.; Karami, Z.; Ghaffari, M.; Taheri, P.; Bakhshandeh, E.; Vahabi, H.; Ganjali, M.R.; et al. Properties of nano-Fe3O4 incorporated epoxy coatings from cure index perspective. Prog. Org. Coat. 2019, 133, 220–228. [Google Scholar] [CrossRef]
- Allahverdi, A.; Ehsani, M.; Janpour, H.; Ahmadi, S. The effect of nanosilica on mechanical, thermal and morphological properties of epoxy coating. Prog. Org. Coat. 2012, 75, 543–548. [Google Scholar] [CrossRef]
- Yari, H.; Moradian, S.; Tahmasebi, N. The weathering performance of acrylic melamine automotive clearcoats containing hydrophobic nanosilica. J. Coat. Technol. Res. 2013, 11, 351–360. [Google Scholar] [CrossRef]
- Dao, P.H.; Nguyen, T.V.; Dang, M.H.; Nguyen, T.L.; Trinh, V.T.; Mac, V.P.; Nguyen, A.H.; Duong, T.M. Effect of silica nanoparticles on properties of coatings based on acrylic emulsion resin. Vietnam J. Sci. Technol. 2018, 56, 117–125. [Google Scholar]
- Nguyen, T.A.; Nguyen, T.H.; Nguyen, T.V.; Thai, H.; Shi, X. Effect of nanoparticles on the thermal and mechanical properties of epoxy coatings. J. Nanosci. Nanotechnol. 2016, 16, 9874–9881. [Google Scholar] [CrossRef]
- Shi, X.; Nguyen, T.A.; Suo, Z.; Liu, Y.; Avci, R. Effect of nanoparticles on the anticorrosion and mechanical properties of epoxy coating. Surf. Coat. Technol. 2009, 204, 237–245. [Google Scholar] [CrossRef]
- Junaidi, M.U.M.; Azaman, S.A.H.; Ahmad, N.N.R.; Leo, C.P.; Lim, G.W.; Chan, D.J.C.; Yee, H.M. Superhydrophobic coating of silica with photoluminescence properties synthesized from rice husk ash. Prog. Org. Coat. 2017, 111, 29–37. [Google Scholar] [CrossRef]
- Kumar, A.M.; Latthe, S.S.; Sudhagar, P.; Obot, I.B.; Gasem, Z.M. In-situ synthesis of hydrophobic SiO2-PMMA composite for surface protective coatings: Experimental and quantum chemical analysis. Polymer 2015, 77, 79–86. [Google Scholar] [CrossRef]
- Ammar, S.; Ramesh, K.; Ma, I.A.W.; Farah, Z.; Arof, A.K. Studies on SiO2-hybrid polymeric nanocomposite coatings with superior corrosion protection and hydrophobicity. Surf. Coat. Technol. 2017, 324, 536–545. [Google Scholar] [CrossRef]
- Chen, H.; Zhang, X.; Zhang, P.; Zhang, Z. Facile approach in fabricating superhydrophobic SiO2/polymer nanocomposite coating. Appl. Surf. Sci. 2012, 261, 628–632. [Google Scholar] [CrossRef]
- Lin, B.; Zhou, S. Poly(ethylene glycol)-grafted silica nanoparticles for highly hydrophilic acrylic-based polyurethane coatings. Prog. Org. Coat. 2017, 106, 145–154. [Google Scholar] [CrossRef]
- Le, T.T.; Nguyen, T.V.; Nguyen, T.A.; Nguyen, T.H.; Hoang, T.; Tran, D.L.; Dinh, D.A.; Nguyen, T.M.; Lu, T. Thermal, mechanical and antibacterial properties of water-based acrylic polymer/SiO2-Ag nanocomposite coating. J. Mater. Chem. Phys. 2019, 232, 362–366. [Google Scholar] [CrossRef]
- Mekuria, T.D.; Zhang, C.; Liu, Y.; Diaa, E.D.F.; Zhou, Y. Surface modification of nano-silica by diisocyanates and their application in polyimide matrix for enhanced mechanical, thermal and water proof properties. Mater. Chem. Phys. 2019, 225, 358–364. [Google Scholar] [CrossRef]
- Bui, T.M.A.; Nguyen, T.V.; Nguyen, T.M.; Hoang, H.; Nguyen, T.T.; Lai, T.H.; Tran, T.N.; Nguyen, V.H.; Hoang, V.H.; Le, T.L.; et al. Investigation of crosslinking, mechanical properties and weathering stability of acrylic polyurethane nanocomposite coating reinforced by SiO2 nanoparticles issued from rice husk ash. J. Mater. Chem. Phys. 2020, 241, 122445. [Google Scholar] [CrossRef]
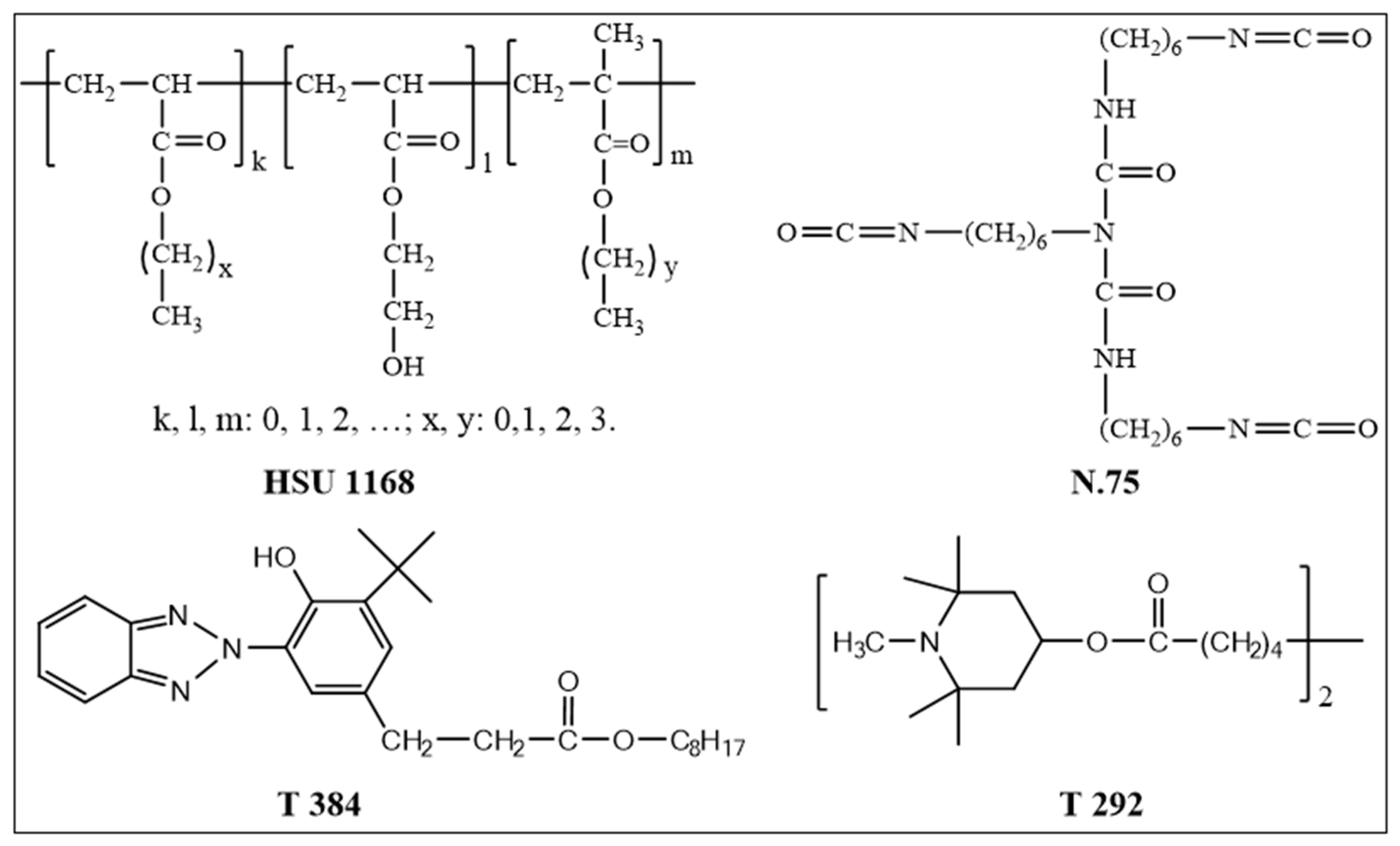




| No | Materials | Coating Samples | ||
|---|---|---|---|---|
| ACPU | ACPU/SiO2 | ACPU/SiO2-T | ||
| 1 | HSU 1168 | 60.40 | 60.40 | 60.40 |
| 2 | SiO2 | 0 | 1.33 | 1.33 |
| 3 | T 384 | 0 | 0 | 1.33 |
| 4 | T 292 | 0 | 0 | 0.66 |
| 5 | Toluene | 18.10 | 18.76 | 18.76 |
| 6 | Butyl acetate | 18.10 | 18.76 | 18.76 |
| 7 | N-75 | 36.20 | 36.20 | 36.20 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nguyen, T.V.; Nguyen, T.A.; Nguyen, T.H. The Synergistic Effects of Sio2 Nanoparticles and Organic Photostabilizers for Enhanced Weathering Resistance of Acrylic Polyurethane Coating. J. Compos. Sci. 2020, 4, 23. https://doi.org/10.3390/jcs4010023
Nguyen TV, Nguyen TA, Nguyen TH. The Synergistic Effects of Sio2 Nanoparticles and Organic Photostabilizers for Enhanced Weathering Resistance of Acrylic Polyurethane Coating. Journal of Composites Science. 2020; 4(1):23. https://doi.org/10.3390/jcs4010023
Chicago/Turabian StyleNguyen, Thien Vuong, Tuan Anh Nguyen, and Thi Hau Nguyen. 2020. "The Synergistic Effects of Sio2 Nanoparticles and Organic Photostabilizers for Enhanced Weathering Resistance of Acrylic Polyurethane Coating" Journal of Composites Science 4, no. 1: 23. https://doi.org/10.3390/jcs4010023




