Sensor and Embedded Control System for Liquid Crystal Implantable Eye Lens †
Abstract
:1. Introduction
2. Materials and Methods
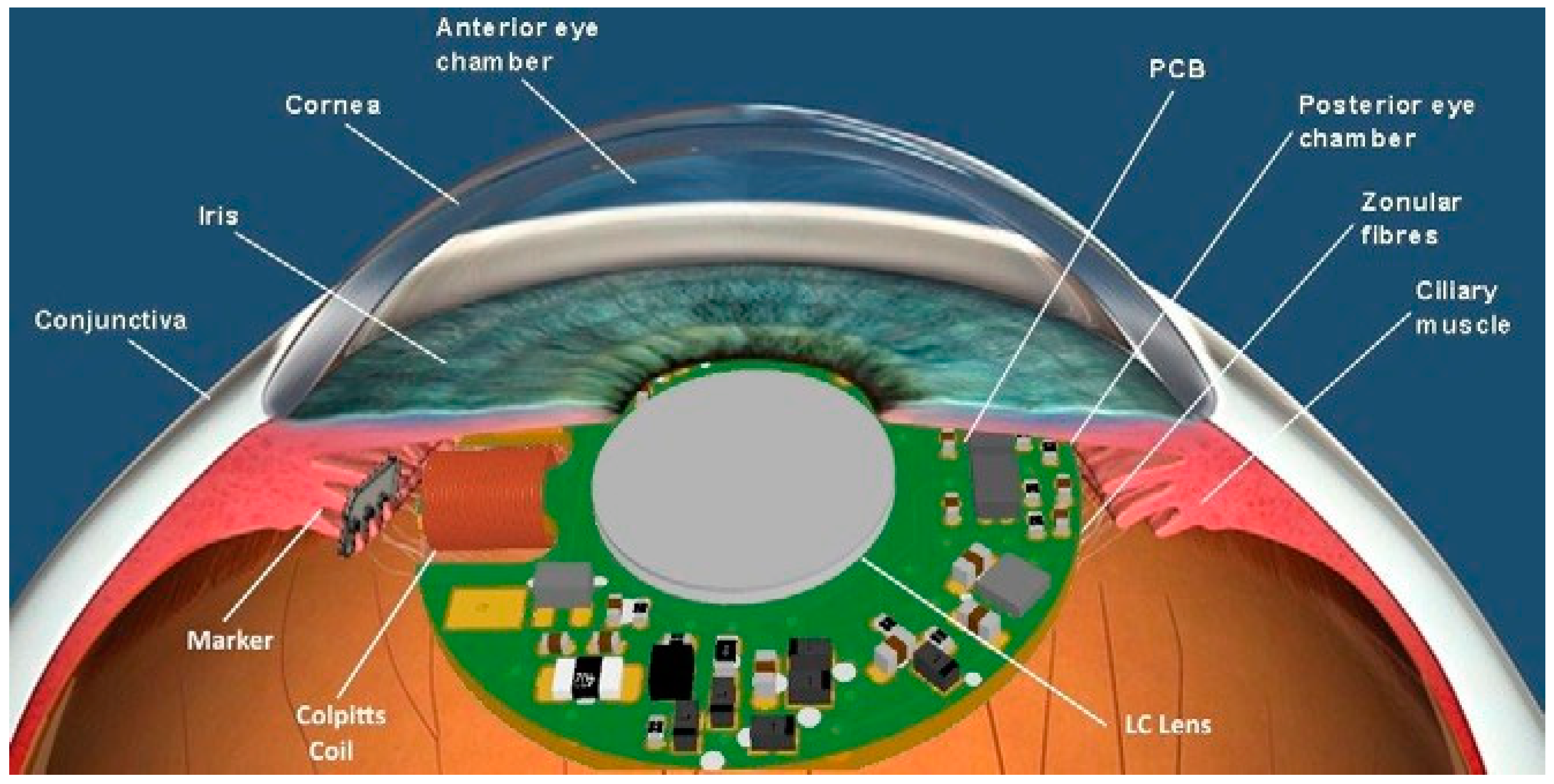
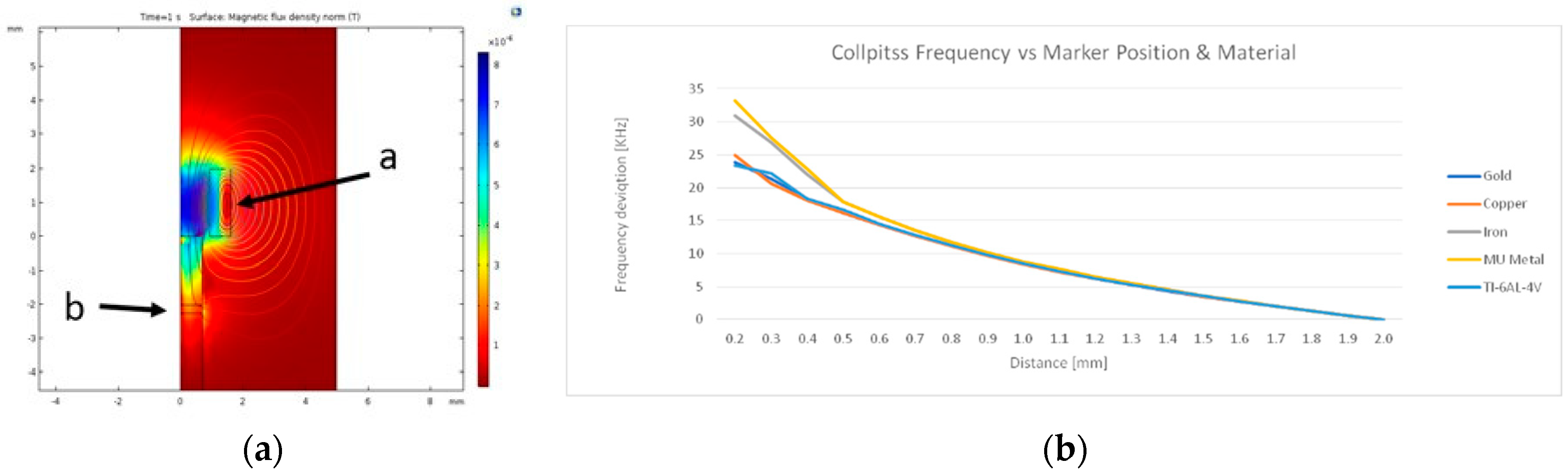
2.1. Sensor Design and Simulation
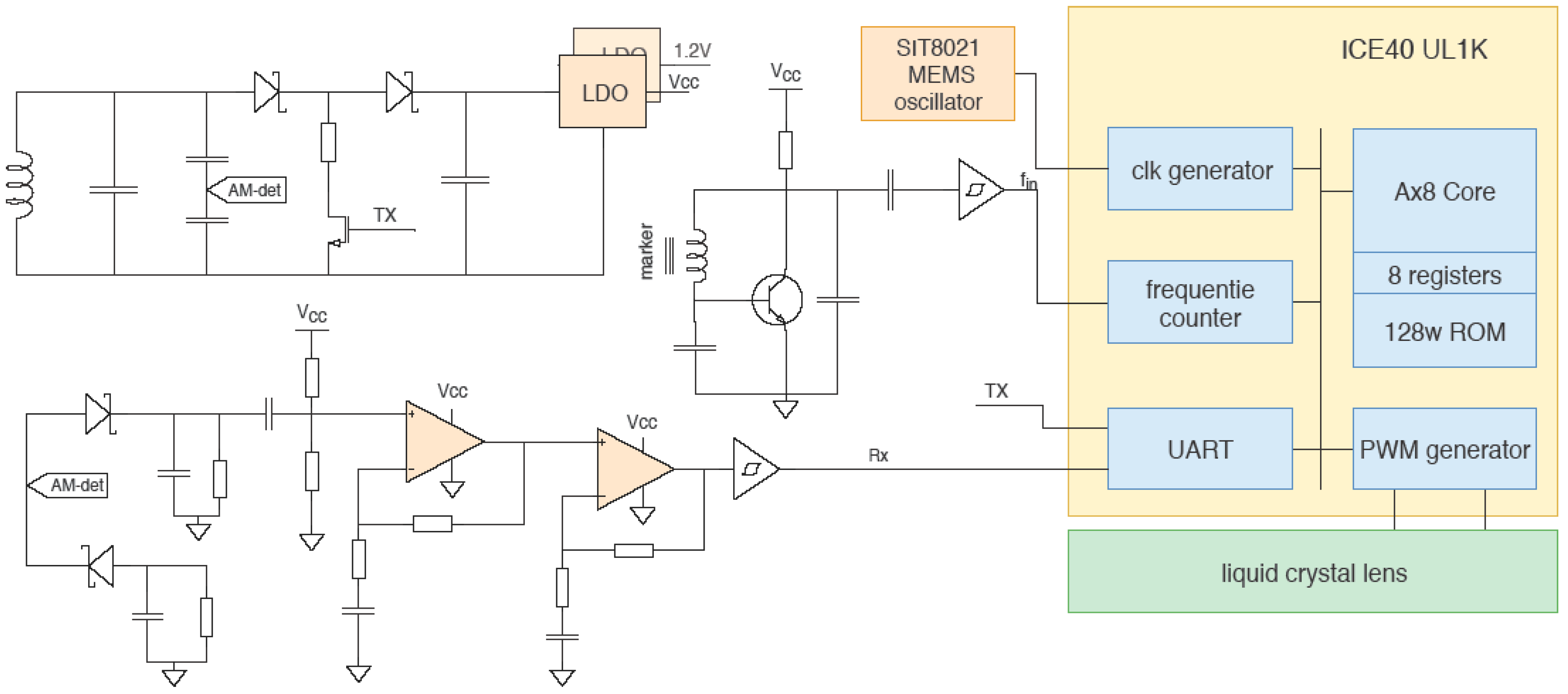
2.2. Embedded Control System
3. Results and Discussions
4. Conclusions
Acknowledgments
Conflicts of Interest
References
- Doornaert, D.; De Gresem, H.; Glorieux, C.; Puers, B.; Spileers, W.; Blankaert, J. Intraocular Electro-Optic Lens with Ciliary Muscle Controlled Accommodation. In Proceedings of the 35th Annual International Conference of the IEEE Engineering in Medicine and Biology Society—EMBC, Osaka, Japan, 3–7 July 2013; pp. 3190–3193. [Google Scholar]
- Yuen, L.; Trattler, W.; Wachler, B.S.B. Two cases of Z syndrome with Crystalens after uneventful cataract surgery. J. Cataract. Refract. Surg. 2008, 34, 1986–1989. [Google Scholar] [CrossRef] [PubMed]
- Doornaert, D.; Glorieux, C.; Puers, R.; De Gresem, H.; Spileers, W.; Blankaert, J. Physiological constraints for an intraocular inductive distance sensor. In Proceedings of the 36th Annual International Conference of the IEEE Engineering in Medicine and Biology Society—EMBC, Chicago, IL, USA, 26–30 August 2014; pp. 646–649. [Google Scholar]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pelgrims, P.; Glorieux, C.; Blanckaert, J.; Puers, R. Sensor and Embedded Control System for Liquid Crystal Implantable Eye Lens. Proceedings 2018, 2, 936. https://doi.org/10.3390/proceedings2130936
Pelgrims P, Glorieux C, Blanckaert J, Puers R. Sensor and Embedded Control System for Liquid Crystal Implantable Eye Lens. Proceedings. 2018; 2(13):936. https://doi.org/10.3390/proceedings2130936
Chicago/Turabian StylePelgrims, Patrick, Christ Glorieux, Johan Blanckaert, and Robert Puers. 2018. "Sensor and Embedded Control System for Liquid Crystal Implantable Eye Lens" Proceedings 2, no. 13: 936. https://doi.org/10.3390/proceedings2130936




