Abstract
In the past decade the significant progress in the cellular stress response was witnessed. Nevertheless, the development of the minimally-invasive and accurate sensing tools for the identification of the increasing number of potentially relevant species in clinical diagnostics, using smaller sample volumes is a major challenge. Herein, the potential of the electroplated nanomaterials towards biomedical sensing and diagnostics is summarized. The key factors affecting the surface functionality, dimensionality, S/N ratio and analytical response of the prepared chips are highlighted. Furthermore, the application of electroplated chips as a fast “read out” platform for profiling of clinical samples was demonstrated.
1. Introduction
The wide-ranging design, synthesis and integration of novel nanomaterials obtained in the last decade resulted in an increased research output in many application fields including analytical chemistry, lab-on-a-chip and biomedical sensing. The introduction of nanomaterials in the system leads to an enhanced recovery of bioanalytes in real samples, decrease of matrix interferences and increase of lifetime of nano-based sensing devices.
Numerous materials such as carbon nanotubes, silicon and metallic nanowires or fullerene-based substrates were applied to fabricate low cost devices without requiring sophisticated sample preparation and highly skilled personnel. The main requirement for nano-based materials is to exhibit high sorption surface capacity, chemical stability and excellent reproducibility of the analytical signal.
Meanwhile, addressing the real world challenge the development of rapid and inexpensive tools for analyzing of small markers in culture extracts, blood and serum samples electroplated chips were proposed and adapted for screening purposes in clinical diagnostics [1,2,3]. Electroplated nanostructures hold a great potential for biosensing due to the simple fabrication process driven by the adjustment of electrolyte composition, applied current and deposition time [3]. Thereby, a wide range of electroplated nanostructures deposited onto various surfaces/electrodes were applied with different rates of success for electrochemical sensing of important markers of human diseases and cellular stress, viz. ascorbic acid, glucose, galactose, lactase, dopamine, uric acid, H2O2, wide spectra of amino acids, acetaminophen, etc. [1,4,5]. An excellent potential of electroplated methodology towards screening was also approved and fully validated by numerous lab-on-chip mass spectrometry platforms, viz. laser desorption ionization mass spectrometry (LDI-MS) [6,7,8,9].
The aim of this study is to demonstrate the potential of electroplated chips as a powerful bioanalytical tool in biomedical sensing and diagnostics. Finally, the possible ways of implementing electroplated chips into the quick read out platform for minimally-invasive profiling of clinical samples are discussed.
2. Preparation of Electroplated Chips
The electroplating of the chips can be performed from various polyelectrolytes with a different concentration of metal salts, type of supplements and pH and by varying current density (Ik) from 0.01 to 0.5 A/dm2 and time of electrolysis (tel) from 10 sec to 30 min,Figure 1. Further increase of the applied current and deposition time should be prevented to avoid the formation of too thick layer of metallic coatings which would result in the lack of analytical merit (e.g., decay of signal response and increase of background intensity (i.e., S/N ratio)). Remarkably, electroplating using polyelectrolytes provides a co-deposition option. It means that besides the formation of metal nanoparticles/nanostructures with a tailored morphology that allows to attach polymers, enzymes and other compounds with functional groups, the surface chemistry of the electroplated chips can be readily fine-tuned following the requirements of analytical task [10].
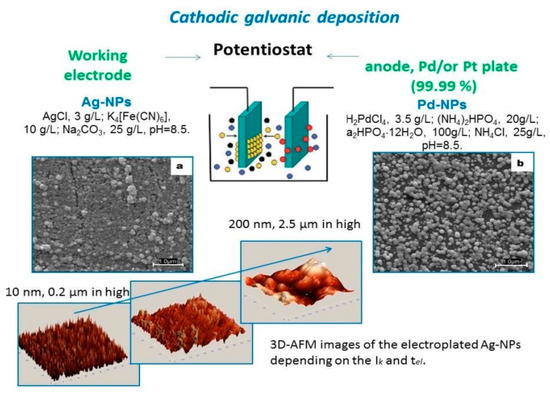
Figure 1.
The nanostructures are formed with a tuned morphology and functionality for further sensing applications (shown for nanoparticles of silver (Ag-NPs) and palladium (Pd-NPs) produced at Ik = 10 mA/dm2 and tel = 30 s).
The deposition process of galvanic electroplating allows high target-to-target synthesis with predictable size distribution of formed nanoparticles/nanostructures and does not require any stabilizers. Moreover, such preparation method is fully instrumentally controlled which would significantly improve chip-to-chip reproducibility and offer an efficient parallel tuning of surface morphology, chemistry and dimensionality towards the analysis of the specific bioanalyte.
3. Electroplated Chips Implemented for Rapid Biosensing Platforms with Quick read-out Capabilities
Previously, we have demonstrated the advantages of Lab-on-a-Chip (LOC) devices based on electroplating chips and combined with mass spectrometry (MS) measurements for the analysis of small biomarkers and lipids metabolites from complex matrices [2,9]. In the LOC-MS, analyte molecules are desorbed and ionized upon laser irradiation from nanosubstrates and subsequently analyzed by means of MS. This technique allows the detection of poorly ionized analytes, requires no chemical derivatization or auxiliary reagents, produces single-charged ions with little or no fragmentation and is tolerant to the presence of high salt environments and contaminated samples. The large surface area of nanomaterials provides high analyte loadings and homogenous samples distribution, which results in the improved detection limits, an increase of selectivity and high reproducibility of MS analysis.
More importantly, we have extended the application of LOC-MS to demonstrate how the electroplated chips can be implemented as a quick molecular weight “read out” platform for profiling of biomolecules from the clinical samples without prior phases separation, Figure 2.
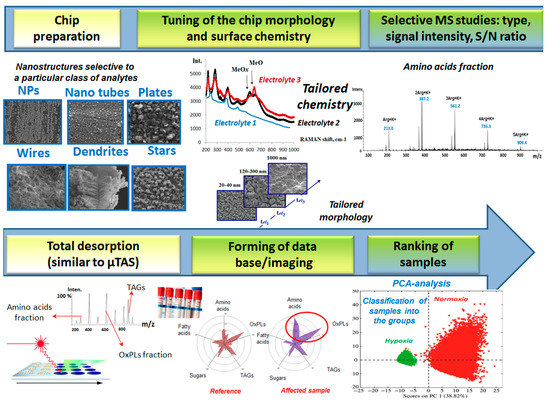
Figure 2.
Schematic workflow for biosamples screening using read-out platform based on electroplated chips.
Remarkably, the nature of the ionization/desorption process and the type of generated ion species strongly depends on the functional groups present on the chip surface. Therefore, selective MS studies for particular class/fraction of bioanalytes are crucial. On the next step, total desorption/ionization of the provided clinical samples, in the manner similar to µTAS approach, is requested. This approach allows fractionation of loaded/spotted samples (max of 5–6 µL in volume) directly onto the electroplated chips followed by an accurate MS analysis. Finally, by means of chemometrics the total cellular biochemical profiles between reference/known and affected samples (cellular stress) can be compared and therefore, the unknown probes can be readily ranked with respect to the biological response.
The application of the platform is for the purposes of monitoring and quantifying of the markers of cellular oxidative stress, human diseases, etc. Thus, different surface chemistry of the electroplated chips allows screening of the biosample profiles without prior complex pre-treatment or phase separation.
4. Conclusions
Following numerous research, it has been established that electroplated chips were utilized in series of novel biosensing platforms with quick read-out and enhanced capabilities towards specific bioanalyte detection. The electroplated chips hold a great potential towards high sensitivity, specificity, good LOD and LLOQ, advanced long-term stability, reproducibility of the signal and low cost. Therefore, the electroplated chips can be applied for the development of the useful biosensing tools, viz. screening of clinical samples and, thus, significantly contribute and improve the therapeutic strategies.
Acknowledgments
The authors would like to acknowledge Dr. Claudia Fink-Straube (Chair of Chem Anal., INM—Leibniz Institute for New Materials, Germany) for continuing support of bioanalytical research at INM.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Wang, S.; Zhang, W.; Zhong, X.; Chai, Y.; Yuan, R. Simultaneous determination of dopamine, ascorbic acid and uric acid using a multi-walled carbon nanotube and reduced graphene oxide hybrid functionalized by PAMAM and Au nanoparticles. Anal. Methods 2015, 7, 1471–1477. [Google Scholar] [CrossRef]
- Silina, Y.E.; Meier, F.; Nebolsin, V.A.; Koch, M.; Volmer, D.A. Novel galvanic nanostructures of Ag and Pd for efficient laser desorption/ionization of low molecular weight compounds. J. Am. Soc. Mass Spectrom. 2014, 25, 841–850. [Google Scholar] [CrossRef] [PubMed]
- Bosch-Navarro, C.; Rourke, J.P.; Wilson, N.R. Controlled electrochemical and electroless deposition of noble metal nanoparticles on graphene. RSC Adv. 2016, 6, 73790–73796. [Google Scholar] [CrossRef]
- Dhara, K.; Stanley, J.; Ramachandran, T.; Nair, B.G. Pt-CuO nanoparticles decorated reduced graphene oxide for the fabrication of highly sensitive non-enzymatic disposable glucose sensor. Sens. Actuators B Chem. 2014, 195, 197–205. [Google Scholar] [CrossRef]
- Angeli, M.C.; Cattarinuzzi, E.; Gastaldi, D.; Vena, P.; Magagnin, L. Characterization of a Flexible Glucose Amperometric Sensor Obtained through Electroless Deposited NiP Electrodes. ECS Trans. 2017, 77, 911–918. [Google Scholar] [CrossRef]
- Tsao, C.W.; Yang, Z.J. High Sensitivity and High Detection Specificity of Gold-Nanoparticle-Grafted Nanostructured Silicon Mass Spectrometry for Glucose Analysis. ACS Appl. Mater. Interfaces 2015, 7, 22630–22637. [Google Scholar] [CrossRef] [PubMed]
- Kawasaki, H.; Yao, T.; Suganuma, T.; Okumura, K.; Iwaki, Y.; Yonezawa, T.; Kikuchi, T.; Arakawa, R. Platinum nanoflowers on scratched silicon by galvanic displacement for an effective SALDI substrate. Chem. A Eur. J. 2010, 16, 10832–10843. [Google Scholar] [CrossRef] [PubMed]
- Kawasaki, H.; Yonezawa, T.; Watanabe, T.; Arakawa, R. Platinum nanoflowers for surface-assisted laser desorption/ionization mass spectrometry of biomolecules. J. Phys. Chem. C 2007, 111, 16278–16283. [Google Scholar] [CrossRef]
- Silina, Y.E.; Herbeck-Engel, P.; Koch, M. A study of enhanced ion formation from metal-semiconductor complexes in atmospheric pressure laser desorption/ionization mass spectrometry. J. Mass Spectrom. 2017, 52, 43–53. [Google Scholar] [CrossRef]
- Sung, W.J.; Bae, Y.H. A Glucose Oxidase Electrode Based on Electropolymerized Conducting Polymer with Polyanion-Enzyme Conjugated Dopant. Anal. Chem. 2000, 72, 2177–2181. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).