1. Introduction
Human granulocytic anaplasmosis (HGA), caused by
Anaplasma phagocytophilum, and human monocytic ehrlichiosis (HME), caused by
Ehrlichia chaffeensis, often present as undifferentiated fever [
1,
2]. Not only are these infections difficult to recognize clinically, they are also difficult to diagnose. Further, they require specific treatment (doxycycline) that is not used for empiric treatment of acute febrile illness.As advanced diagnostic methods are employed to identify etiologic agents of undifferentiated fever, rickettsial infections are increasingly recognized causes of febrile illness in the tropics with substantive morbidity and mortality [
3]. Despite the increasing recognition of infections by
Rickettsia and
Orientia species in the Rickettsiaceae family, only limited study of human infection by Anaplasmataceae has been conducted in tropical regions [
4,
5,
6,
7,
8,
9,
10]. This sustained lack of progress occurs despite clear evidence of the presence of
Anaplasma,
Ehrlichia, and
Neorickettsia species in vectors and as agents of disease in wild and domestic animals. Limitations in diagnosis impair clinical diagnosis of these infections in humans and clinical and epidemiologic studies of these illnesses. Treatment remains empiric. Therefore, we developed a multiplex real-time PCR assay to rapidly detect and distinguish these pathogens in acutely ill patients.
2. Results
Clinical validation was accomplished with 13 and 39 blood samples for the singleplex and multiplex qPCR assays, respectively, from 42 patients with confirmed HGA. For HME, we used six and 21 blood samples from 23 patients with HME; 13 samples were provided as extracted DNA by J. Olano and J. McBride (University of Texas, Galveston, TX, USA). Confirmation included demonstration of morulae on peripheral blood smear, culture, alternative-target PCR, and/or a 4-fold rise in IgG titer in paired sera, and by 2 or more gold-standard methods in all but three [
11,
12]. For the singleplex assays, specificity was assessed by testing blood from 20 patients with confirmed typhus group rickettsiosis, eight with spotted fever group rickettsiosis, seven with scrub typhus, one convalescent from HGA, three convalescent from HME, 22 with malaria, and one with confirmed non-
Ehrlichia/Anaplasma bacteraemia. In addition, for HGA specificity, all six samples from patients with HME were tested, whereas for HME specificity, all 13 samples from patients with HGA samples were tested.
ACTB amplification from all samples was detected.
For the multiplex qPCR assay, specificity was assessed using 78 blood samples from patients with malaria (45 Plasmodium falciparum, 22 P. vivax, 11 Plasmodium spp.), two with Babesia spp., eight with bacteraemia (four S. aureus, one Pseudomonas fluorescens/putida, one Enterococcus non-faecalis, one E. coli, and one Enterococcus spp.), one convalescent from HME, and 33 from hospitalized patients with no identified infection.For HGA, eight samples from E. chaffeensis-infected patients were also used to assess specificity. Thirty-nine samples from patients with HGA were used to assess E. chaffeensis multiplex qPCR specificity.
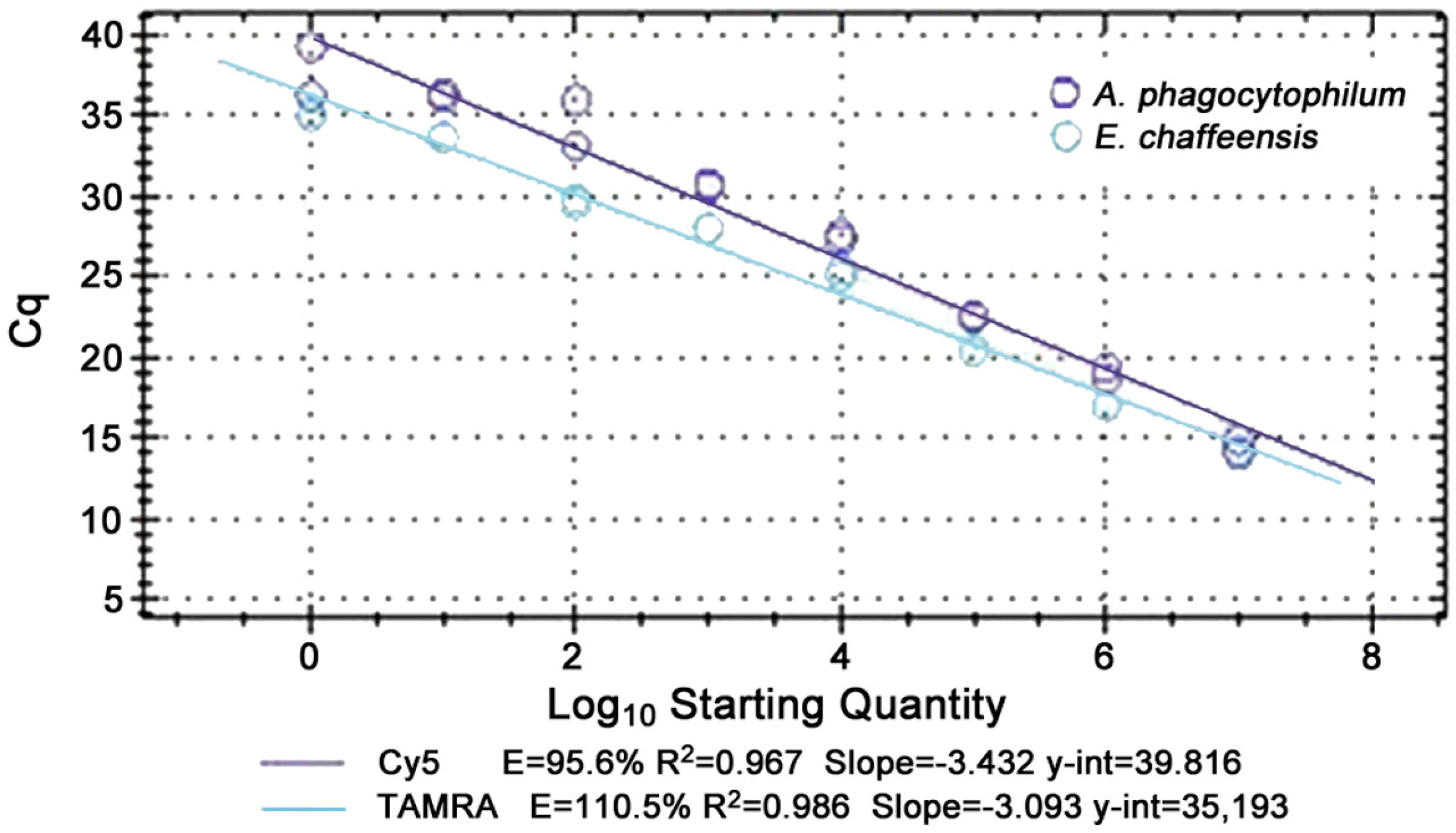
Analytical sensitivity was as low as one copy (<1 organism/μL blood) for
A. phagocytophilum and
E. chaffeensis, and analytical specificity was 100% for both singleplex and multiplex assays. The multiplex reaction was linear from 10
7 to 10
0 copies for all targets with replicate runs and efficiencies of 85 to 115% were achieved with R
2 values usually above 0.95 (
Figure 1). Analysis of receiver operator characteristic (ROC) curves for the multiplex qPCR assay identified mean relative fluorescence units (RFU) of negative controls plus 10% of high RFU-low RFU per plate as the optimal cut-off for
A. phagocytophilum and 3% as best for
E. chaffeensis.
The clinical sensitivity of the singleplex qPCR for A. phagocytophilum was 92% (12/13 A. phagocytophilum) and specificity 100%. For the E. chaffeensis singleplex qPCR, sensitivity was 83% (5/6 E. chaffeensis) and specificity was 100%. The multiplex clinical sensitivity and specificity for A. phagocytophilum were 100% (39/39) and 100% (130/130 with other infections or no identified infection), respectively. The multiplex’s clinical sensitivity and specificity for E. chaffeensis was 95% (20/21) and specificity 99% (160/161 with other infections or no identified infection). The assays could be performed in <3 h. The single false positive result at these cutoffs was found when testing for E. chaffeensis in a sample from a patient with HGA. Of the 39 samples from patients with A. phagocytophilum tested, the median bacterial load was 1723/μL (IQR 436 to 8885; min 0.04, max 290,857) and for the 21 from those with E. chaffeensis, the median bacterial load was 1929/μL (IQR 21 to 12,204; min 2, max 131,835).
3. Discussion
HGA and HME are globally-distributed, life-threatening tick-borne rickettsial diseases [
13] that present as undifferentiated fever but are not treated by typical empiric regimens for acute febrile illness. Clinically suspected HGA and HME with supportive serologic and occasional molecular evidence have been reported in humans in Europe, North and South America, Africa, and the Far East, including China and Korea. We documented
Ehrlichia chaffeensis as a cause of acute febrile illness in Nicaragua [
6]. To define a confirmed case of HGA or HME, the European Society of Clinical Microbiology and Infectious Diseases and the Centers for Disease Control and Prevention require 1) a 4-fold increase in IgG titer by indirect immunofluorescence assay (IFA) between paired (acute and convalescent) sera, since testing acute phase sera alone is neither sensitive nor specific, or 2) direct detection of the pathogen by PCR or cell culture [
14,
15]. Diagnosis by IFA using paired sera is infrequently accomplished and is inherently retrospective, subjective, and time-consuming. Screening convalescent sera using a peptide-based ELISA and confirming positives by IFA [
6] improves throughput and objectivity to better support large clinical studies but still cannot confirm diagnoses in real-time, as is required for patient care. Further, serologic cross-reactions commonly (3–30%) preclude species-level diagnosis [
16]. Culture is slow and not widely available.Although other molecular assays for Anaplasmataceae have been reported [
12,
17,
18,
19], we uniquely describe extensive clinical validation with samples from patients in whom the targeted pathogens were confirmed by two or more gold-standard methods. Further, we used the same conditions we used for detection of other clinical mimics [
20,
21,
22,
23] in the tropics to allow adaptation of the assay to detect other geographically-relevant causes of undifferentiated fever, as shown here by high specificity when samples from patients with
E. chaffeensis and
A. phagocytophilum were tested for spotted fever or typhus rickettsioses, scrub typhus, malaria, and babesiosis, as well as other bacteraemias. Our real-time quantitative qPCR assay could support needed epidemiologic assessments of the global distribution of
A. phagocytophilum and
E. chaffeensis and earlier diagnosis and treatment, both required to reduce morbidity and mortality from these diseases worldwide.
4. Materials and Methods
We designed a singleplex 5′ nuclease quantitative PCR (qPCR) assay that targets
A. phagocytophilum multicopy (10
2 copies per genome)
msp2. We adapted the assay to simultaneously detect and distinguish
E. chaffeensis using the same conditions we have used for other targets, including
Rickettsia,
Orientia,
Borrelia, and malaria [
20,
21]. AlleleID
® v 6.1 software (Premier Biosoft International, Palo Alto, CA, USA) was used to design primers and probes using standardized parameters (18–25 base-pair primer length; 59 ± 5 °C primer Tm; 100 mM monovalent ions; 5.0 mM free Mg
+2; and cycling at 95 °C for 3 min, 40 cycles of 95 °C for 10 s, and 55 °C for 30 s) to allow efficient multiplexing with interchangeable component target ‘cassettes’. Targets included a 118 base-pair conserved region on the 5′ end of
A. phagocytophilum msp2 and a 79 base-pair region nearer to the 3′ end of
E. chaffeensis single copy
vlpt (
Table 1). Human beta-actin (
ACTB) was used as an internal amplification control. All primers and probes were synthesized by Integrated DNA Technologies (IDT, Coralville, IA, USA). To determine analytical sensitivity, DNA from cultured
A. phagocytophilum and
E. chaffeensis was amplified, cloned, and plasmid quantity determined to develop a standard curve (10
7, 10
5, 10
3, 10
1 and 10
0 copies/μL).
The IRBs of Johns Hopkins Medicine, the Duluth Clinic/St. Mary’s Hospital (Duluth, MN, USA), St. Luke’s Hospital (Duluth, MN, USA), New York Medical College (Valhalla, NY, USA), the New York State Department of Health (Albany, NY, USA) and the University of Texas Medical Branch (Galveston, TX, USA) reviewed and either approved the research or declared it exempt.
DNA was prepared from 200 µL of EDTA anti-coagulated blood (Qiagen DNeasy Blood and Tissue kit, Qiagen Inc., Valencia, CA, USA or the Purelink Pro96 Genomic DNA Purification kit, Invitrogen, #K182104A) and resuspended in 200 µL of water. Multiplex PCR was performed with the BioRadiCycler’s iQ5 Multicolor Real-Time PCR Detection System for 96 well plates or the C1000 Thermal Cycler’s CFX™384 Real-Time PCR Detection System (BioRad) for 384 well plates. For the iQ5, each reaction included 2 µL of DNA, 12.5 µL of IQ Multiplex Powermix (BioRad), and primers and probes at a final concentration of 10nM each. For the CFX, each reaction included 5 µL of DNA, 5 µL of IQ Multiplex Powermix (BioRad), and primers and probes at a final concentration of 200 µM each. Each run included duplicates of template, plasmid standards, positive and negative DNA controls, and no template controls. Standard two-step qPCR was performed with initial denaturation at 95 °C for 3 min, 40 cycles of denaturation at 95 °C for 10 s, and annealing/extension at 55 °C for 30 s.
Results were automatically analyzed using a threshold for each probe–fluor pair and BioRad’s baseline-subtract-curvefit algorithm to normalize each run and manually inspected for quality with baselines corrected when necessary. We required positives to be positive in duplicate. Endpoint analysis was accomplished using the BioRad IQ5 or CFX384 software. RFU were averaged over the last 5 cycles and tolerance calculated as a percentage of the difference between the highest and the lowest average RFU for the plate. Potential cut-offs were determined by adding a tolerance to the negative controls’ average RFU and receiver operator characteristic (ROC) curves were constructed to choose optimal cut-offs. Starting quantity means were determined for duplicate positive samples to quantify bacterial load. Final bacterial loads were adjusted to account for 100 copies of msp2 in the A. phagocytophilum genome and for a single vlpt copy in the E. chaffeensis genome.
Acknowledgments
We thank Meg Lichay for conducting the assays, Peggy Coulter for work on the singleplex assay, and Johan S. Bakken, Jere McBride, and Juan Olano for providing clinical samples for validation. M.E.R. was supported by a Johns Hopkins Center for Global Health Junior Faculty Grant, a Clinician Scientist Career Development Award from Johns Hopkins School of Medicine, and the National Institute of Allergy and Infectious Diseases, National Institutes of Health (K23AIO83931). The work was supported in part by NIAID R01 AI44102, R01AI41213, and R21AI080911 grants to J.S.D. The opinions expressed herein are those of the author(s) and are not necessarily representative of those of the Uniformed Services University of the Health Sciences (USUHS), the Department of Defense (DOD); or, the United States Army, Navy, or Air Force.
Author Contributions
The work was jointly conceived, interpreted and written by M.E.R. and J.S.D. The analytical approach, specific reagents, analysis of multiplex results was done by J.S.D. and M.E.R. conducted the final analysis and drafts of the manuscript.
Conflicts of Interest
The authors report no conflicts of interest.
References
- Ismail, N.; McBride, J.W. Tick-borne emerging infections: Ehrlichiosis and anaplasmosis. Clin. Lab. Med. 2017, 37, 317–340. [Google Scholar] [CrossRef] [PubMed]
- Bakken, J.S.; Dumler, J.S. Human granulocytic anaplasmosis. Infect. Dis. Clin. North Am. 2015, 29, 341–355. [Google Scholar] [CrossRef] [PubMed]
- Alirol, E.; Horie, N.S.; Barbe, B.; Lejon, V.; Verdonck, K.; Gillet, P.; Jacobs, J.; Buscher, P.; Kanal, B.; Bhattarai, N.R.; et al. Diagnosis of persistent fever in the tropics: Set of standard operating procedures used in the NIDIAG febrile syndrome study. PLoS Negl. Trop. Dis. 2016, 10, e0004749. [Google Scholar] [CrossRef] [PubMed]
- Prabhu, M.; Nicholson, W.L.; Roche, A.J.; Kersh, G.J.; Fitzpatrick, K.A.; Oliver, L.D.; Massung, R.F.; Morrissey, A.B.; Bartlett, J.A.; Onyango, J.J.; et al. Q fever, spotted fever group, and typhus group rickettsioses among hospitalized febrile patients in northern Tanzania. Clin. Infect. Dis. 2011, 53, e8–e15. [Google Scholar] [CrossRef] [PubMed]
- Reller, M.E.; Chikeka, I.; Miles, J.J.; Dumler, J.S.; Woods, C.W.; Mayorga, O.; Matute, A.J. First identification and description of rickettsioses and Q fever as causes of acute febrile illness in Nicaragua. PLoS Negl. Trop. Dis. 2016, 10, e0005185. [Google Scholar] [CrossRef] [PubMed]
- Chikeka, I.; Matute, A.J.; Dumler, J.S.; Woods, C.W.; Mayorga, O.; Reller, M.E. Use of peptide-based enzyme-linked immunosorbent assay followed by immunofluorescence assay to document Ehrlichia chaffeensis as a cause of febrile illness in Nicaragua. J. Clin. Microbiol. 2016, 54, 1581–1585. [Google Scholar] [CrossRef] [PubMed]
- Reller, M.E.; Bodinayake, C.; Nagahawatte, A.; Devasiri, V.; Kodikara-Arachichi, W.; Strouse, J.J.; Flom, J.E.; Ostbye, T.; Woods, C.W.; Dumler, J.S. Unsuspected rickettsioses among patients with acute febrile illness, Sri Lanka, 2007. Emerg. Infect. Dis. 2012, 18, 825–829. [Google Scholar] [CrossRef] [PubMed]
- Blacksell, S.D.; Kantipong, P.; Watthanaworawit, W.; Turner, C.; Tanganuchitcharnchai, A.; Jintawon, S.; Laongnuanutit, A.; Nosten, F.H.; Day, N.P.; Paris, D.H.; et al. Underrecognized arthropod-borne and zoonotic pathogens in northern and northwestern Thailand: Serological evidence and opportunities for awareness. Vector Borne Zoonotic Dis. 2015, 15, 285–290. [Google Scholar] [CrossRef] [PubMed]
- Koh, F.X.; Kho, K.L.; Kisomi, M.G.; Wong, L.P.; Bulgiba, A.; Tan, P.E.; Lim, Y.A.L.; Nizam, Q.N.H.; Panchadcharam, C.; Tay, S.T. Ehrlichia and Anaplasma infections: Serological evidence and tick surveillance in peninsular Malaysia. J. Med. Entomol. 2017. [Google Scholar] [CrossRef] [PubMed]
- Bezerra, M.C.F.; Melo, A.L.T.; Taques, I.; Aguiar, D.M.; Pacheco, R.C.; Slhessarenko, R.D. Seropositivity for Rickettsia spp. and Ehrlichia spp. in the human population of Mato Grosso, central-western Brazil. Rev. Soc. Bras. Med. Trop. 2017, 50, 399–403. [Google Scholar] [CrossRef] [PubMed]
- Olano, J.P.; Masters, E.; Hogrefe, W.; Walker, D.H. Human monocytotropic ehrlichiosis, Missouri. Emerg. Infect. Dis. 2003, 9, 1579–1586. [Google Scholar] [CrossRef] [PubMed]
- Doyle, C.K.; Labruna, M.B.; Breitschwerdt, E.B.; Tang, Y.W.; Corstvet, R.E.; Hegarty, B.C.; Bloch, K.C.; Li, P.; Walker, D.H.; McBride, J.W. Detection of medically important Ehrlichia by quantitative multicolor Taqman real-time polymerase chain reaction of the dsb gene. J. Mol. Diagn. 2005, 7, 504–510. [Google Scholar] [CrossRef]
- Bakken, J.S.; Dumler, J.S. Clinical diagnosis and treatment of human granulocytotropic anaplasmosis. Ann. N. Y. Acad. Sci. 2006, 1078, 236–247. [Google Scholar] [CrossRef] [PubMed]
- Chapman, A.S.; Bakken, J.S.; Folk, S.M.; Paddock, C.D.; Bloch, K.C.; Krusell, A.; Sexton, D.J.; Buckingham, S.C.; Marshall, G.S.; Storch, G.A.; et al. Diagnosis and management of tickborne rickettsial diseases: Rocky Mountain spotted fever, ehrlichioses, and anaplasmosis—United States: A practical guide for physicians and other health-care and public health professionals. MMWR Recomm. Rep. 2006, 55, 1–27. [Google Scholar] [PubMed]
- Brouqui, P.; Bacellar, F.; Baranton, G.; Birtles, R.J.; Bjoersdorff, A.; Blanco, J.R.; Caruso, G.; Cinco, M.; Fournier, P.E.; Francavilla, E.; et al. Guidelines for the diagnosis of tick-borne bacterial diseases in Europe. Clin. Microbiol. Infect. 2004, 10, 1108–1132. [Google Scholar] [CrossRef] [PubMed]
- Centers for Disease Control. Anaplasmosis and ehrlichiosis—Maine, 2008. MMWR Morb. Mortal. Wkly. Rep. 2009, 58, 1033–1036. [Google Scholar]
- Dong, T.; Qu, Z.; Zhang, L. Detection of A. phagocytophilum and E. chaffeensis in patient and mouse blood and ticks by a duplex real-time PCR assay. PLoS ONE 2013, 8, e74796. [Google Scholar] [CrossRef] [PubMed]
- Pritt, B.S.; Sloan, L.M.; Johnson, D.K.; Munderloh, U.G.; Paskewitz, S.M.; McElroy, K.M.; McFadden, J.D.; Binnicker, M.J.; Neitzel, D.F.; Liu, G.; et al. Emergence of a new pathogenic Ehrlichia species, Wisconsin and Minnesota, 2009. N. Engl. J. Med. 2011, 365, 422–429. [Google Scholar] [CrossRef] [PubMed]
- Bell, C.A.; Patel, R. A real-time combined polymerase chain reaction assay for the rapid detection and differentiation of Anaplasma phagocytophilum, Ehrlichia chaffeensis, and Ehrlichia ewingii. Diagn. Microbiol. Infect. Dis. 2005, 53, 301–306. [Google Scholar] [CrossRef] [PubMed]
- Reller, M.E.; Clemens, E.G.; Schachterle, S.E.; Mtove, G.A.; Sullivan, D.J.; Dumler, J.S. Multiplex 5′ nuclease-quantitative PCR for diagnosis of relapsing fever in a large Tanzanian cohort. J. Clin. Microbiol. 2011, 49, 3245–3249. [Google Scholar] [CrossRef] [PubMed]
- Prakash, J.A.J.; Reller, M.E.; Barat, N.; Dumler, J.S. Assessment of a quantitative multiplex 5′ nuclease real-time PCR for spotted fever and typhus group rickettsioses and Orientia tsutsugamushi. Clin. Microbiol. Infect. 2009, 15, 292–293. [Google Scholar] [CrossRef] [PubMed]
- Schachterle, S.E.; Mtove, G.; Levens, J.P.; Clemens, E.G.; Shi, L.; Raj, A.; Munoz, B.; Reller, M.E.; West, S.; Dumler, J.S.; et al. Prevalence and density-related concordance of three diagnostic tests for malaria in a region of Tanzania with hypoendemic malaria. J. Clin. Microbiol. 2011, 49, 3885–3891. [Google Scholar] [CrossRef] [PubMed]
- Reller, M.E.; Chen, W.H.; Dalton, J.; Lichay, M.A.; Dumler, J.S. Multiplex 5′ nuclease quantitative real-time PCR for clinical diagnosis of malaria and species-level identification and epidemiologic evaluation of malaria-causing parasites, including Plasmodium knowlesi. J. Clin. Microbiol. 2013, 51, 2931–2938. [Google Scholar] [CrossRef] [PubMed]
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).