The Relationship between Structural Features of Lignocellulosic Materials and Ethanol Production Yield
Abstract
:1. Introduction
2. Materials and Methods
2.1. Raw Materials and Enzymes
2.2. Pretreatment Methods
2.2.1. Autohydrolysis
2.2.2. Dilute Sulfuric Acid
2.2.3. Concentrated Phosphoric Acid
2.2.4. Sodium Hydroxide
2.2.5. Sodium Carbonate
2.3. Enzymatic Hydrolysis
2.4. Fermentation
2.5. Solids Characterization
2.5.1. Biomass Composition
2.5.2. Accessible Surface Area
Nitrogen Adsorption (BET)
Water Retention Value (WRV)
2.5.3. Buffering Capacity
2.5.4. FTIR Spectra
2.5.5. X-ray Diffraction (XRD)
2.6. Statistical Analysis
3. Results and Discussion
3.1. Effects of Pretreatment on the Composition of lignocelluloses
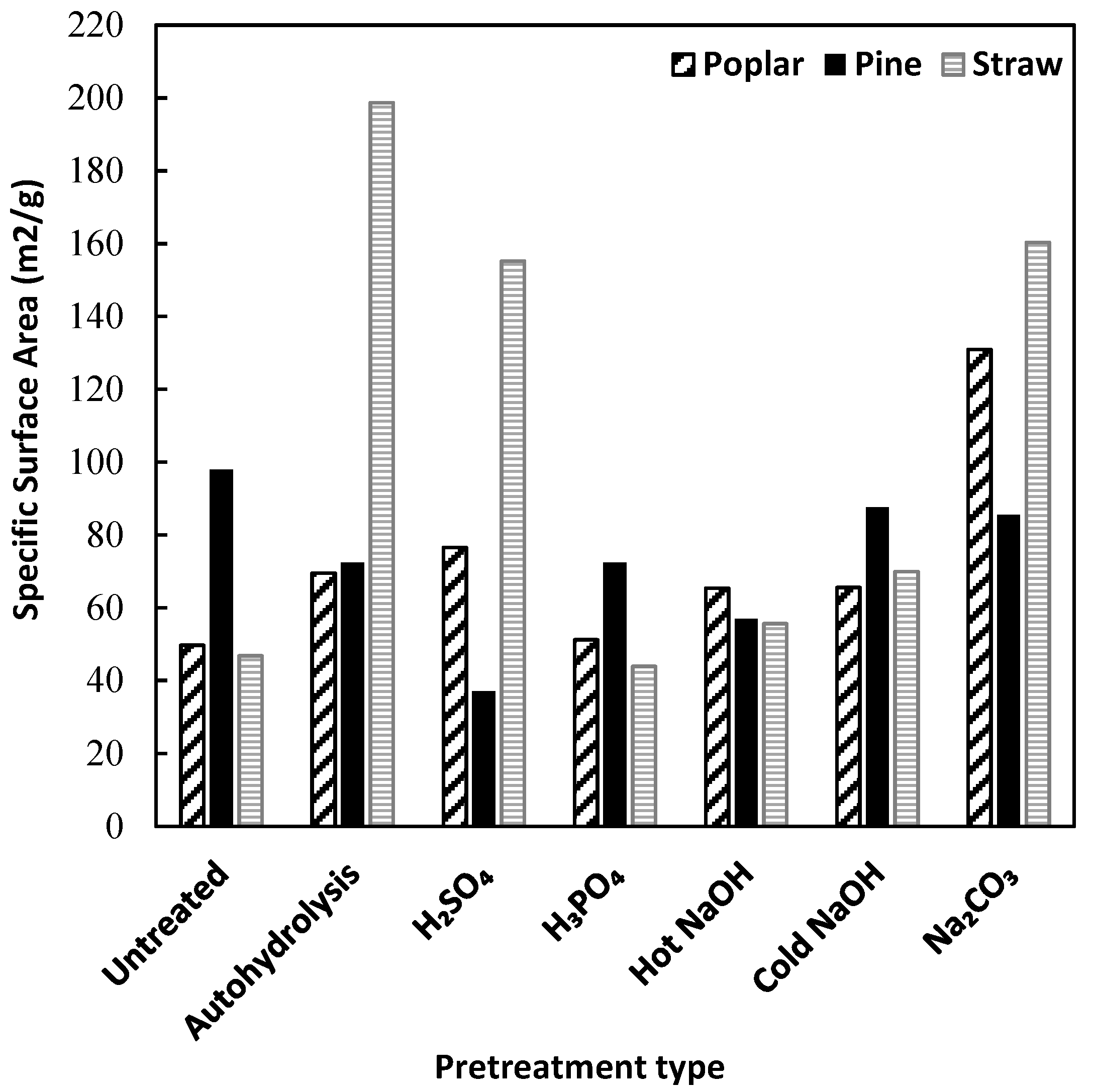
3.2. Accessible Surface Area
3.3. Buffering Capacity
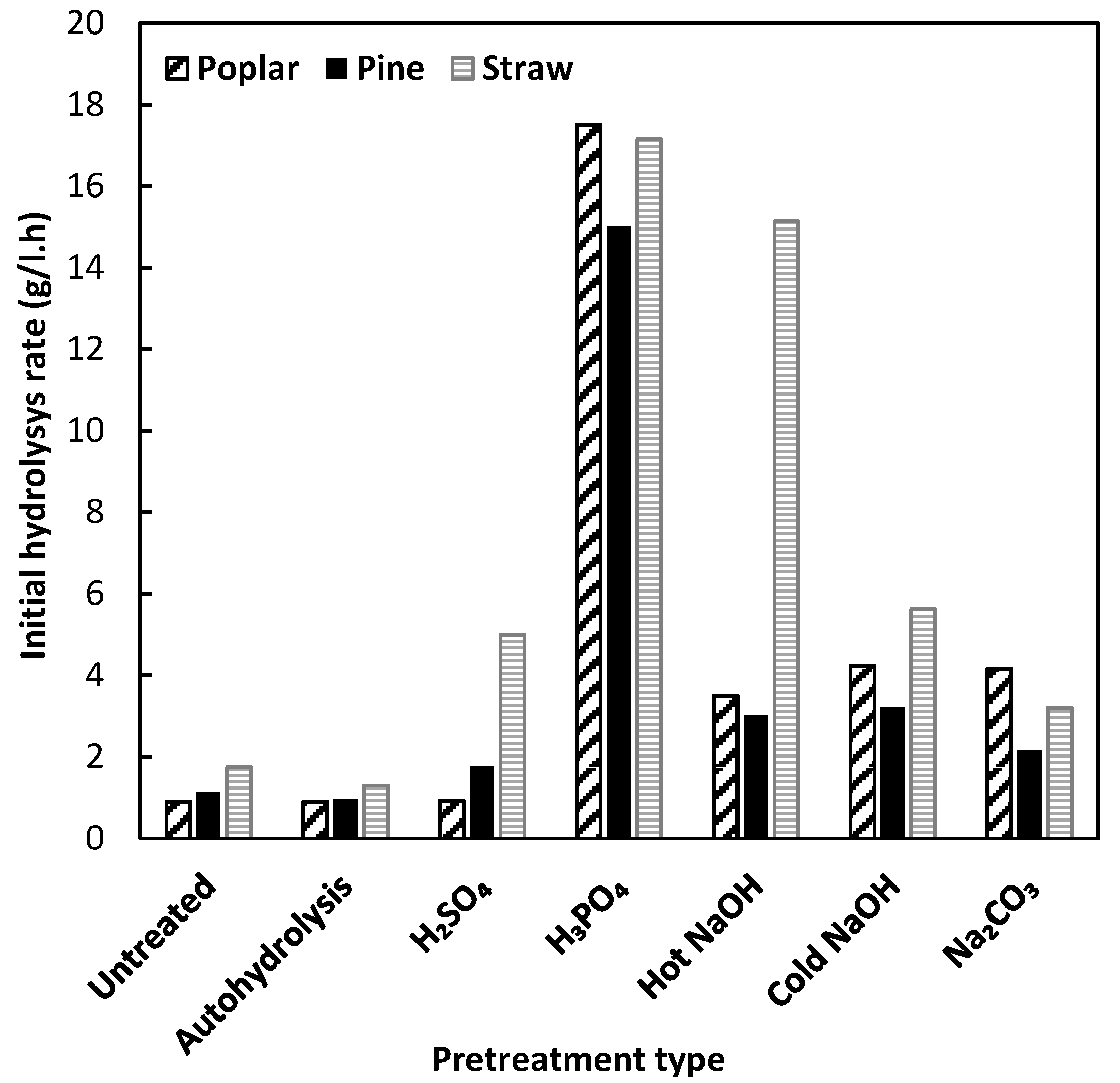
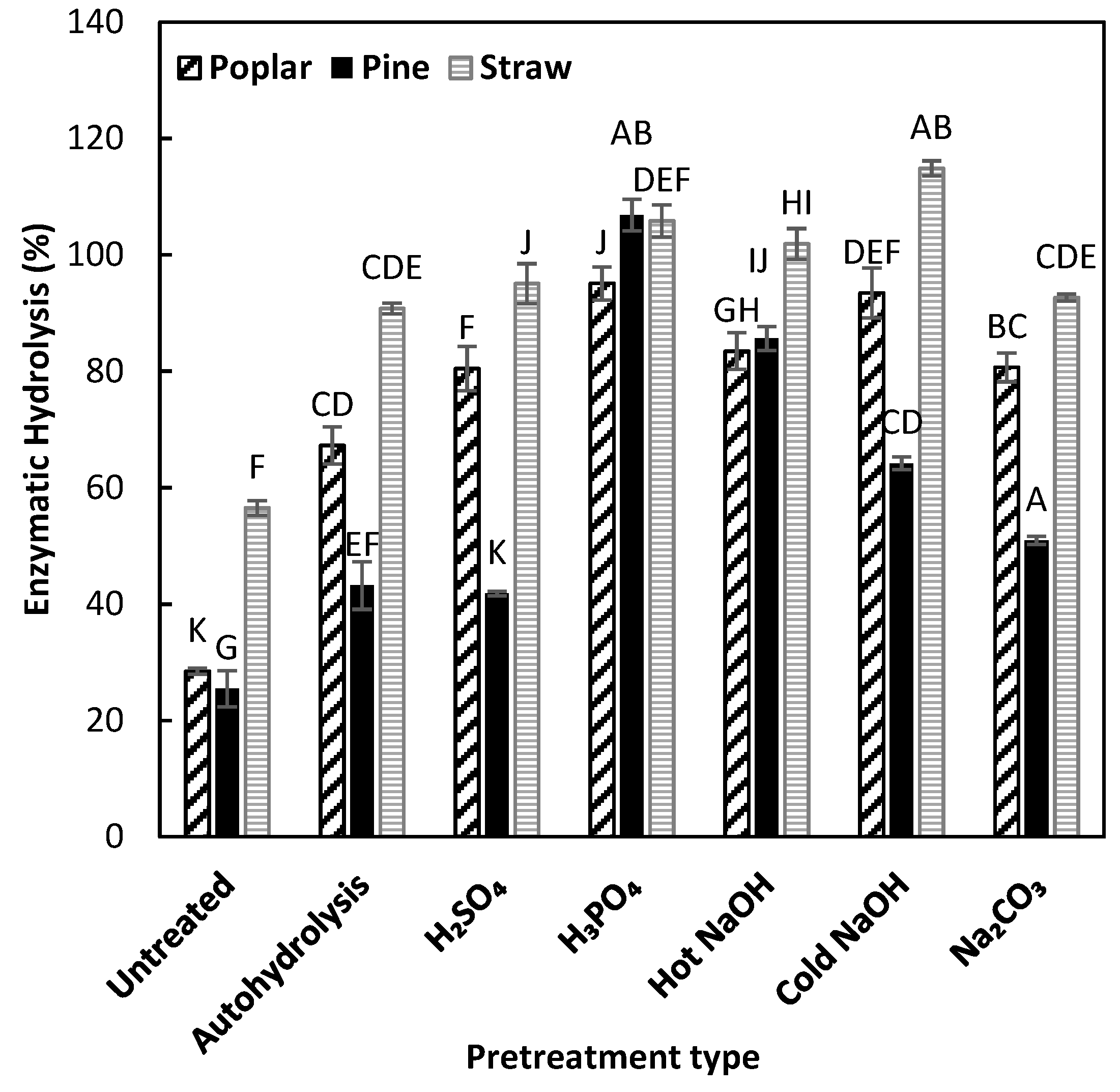
3.4. Enzymatic Hydrolysis
3.5. Simultaneous Enzymatic Hydrolysis and Fermentation
3.6. Ethanol Production Balance Based on Recovery
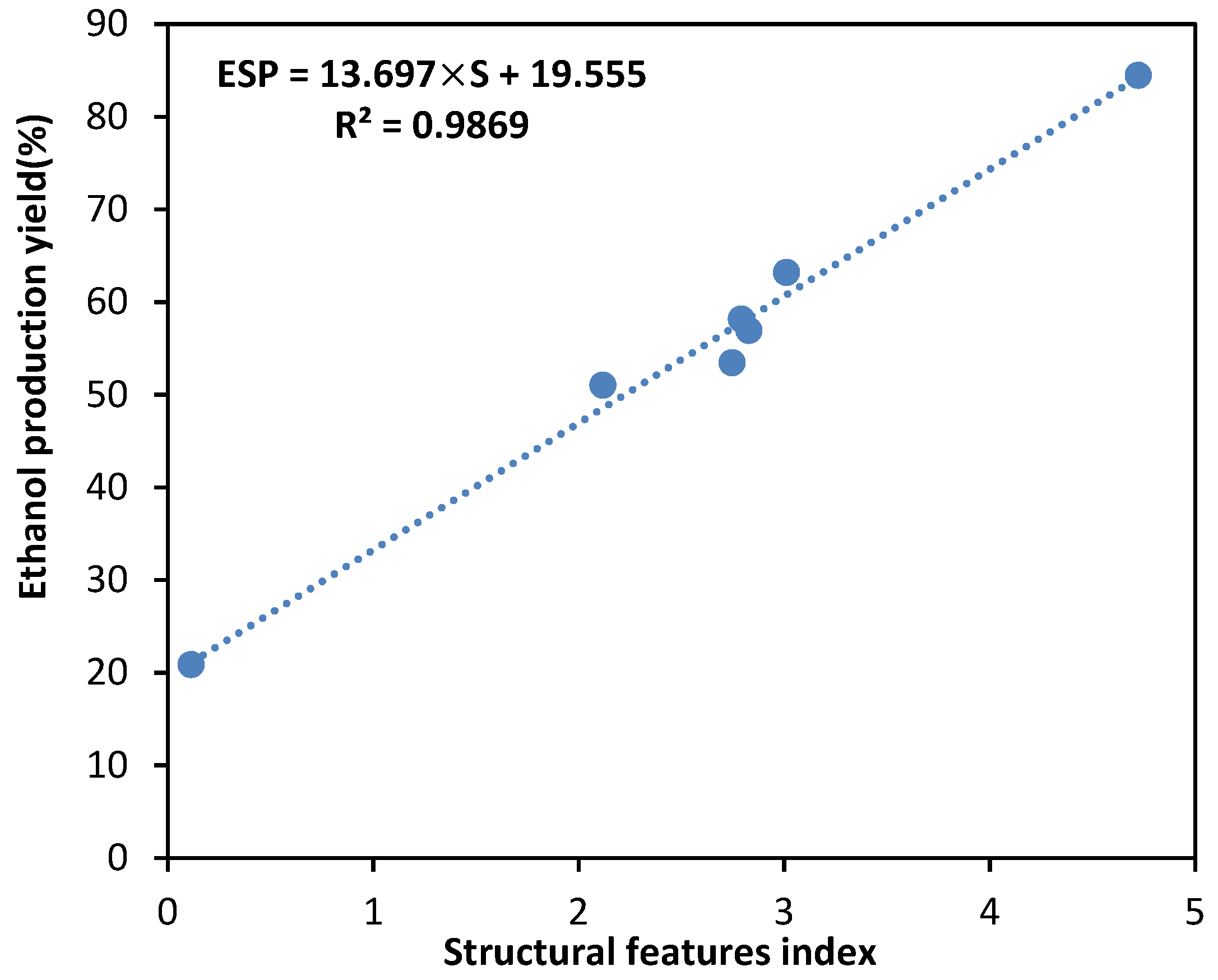
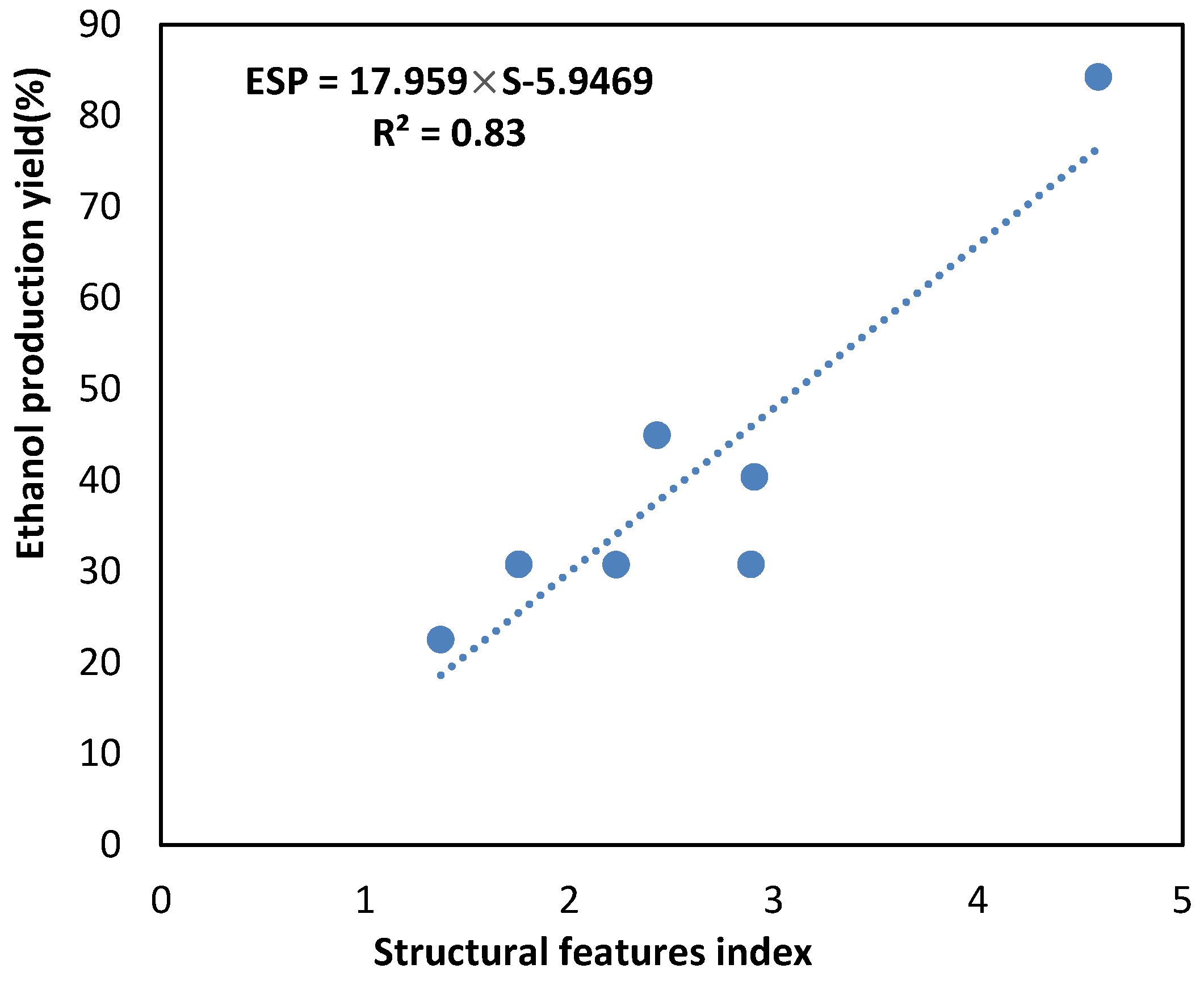
3.7. Prediction of Ethanol Production Yield Using Structural Features of Biomass
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Nomenclature
References
- Okolie, J.A.; Mukherjee, A.; Nanda, S.; Dalai, A.K.; Kozinski, J.A. Next-generation biofuels and platform biochemicals from lignocellulosic biomass. Int. J. Energy Res. 2021, 45, 14145–14169. [Google Scholar] [CrossRef]
- Sutrisno, J.; Dharma, S.; Silitonga, A.; Shamsuddin, A.; Sebayang, A. Experimental assessment of the performance and exhaust emissions characteristic of a diesel with Jatropa curcas-Ceiba pentandra mixture biodiesel/bioethanol blends. J. Mech. Eng. Res. Dev. 2020, 43, 396–412. [Google Scholar]
- Kim, E.J.; Kim, S.; Choi, H.-G.; Han, S.J. Co-production of biodiesel and bioethanol using psychrophilic microalga Chlamydomonas sp. KNM0029C isolated from Arctic sea ice. Biotechnol. Biofuels 2020, 13, 20. [Google Scholar] [CrossRef]
- Khorasani, R.; Khodaparasti, M.S.; Tavakoli, O. Hydrogen production from dairy wastewater using catalytic supercritical water gasification: Mechanism and reaction pathway. Int. J. Hydrog. Energy 2021, 46, 22368–22384. [Google Scholar] [CrossRef]
- Ravindra, K.; Kaur-Sidhu, M.; Mor, S.; Chakma, J.; Pillarisetti, A. Impact of the COVID-19 pandemic on clean fuel programmes in India and ensuring sustainability for household energy needs. Environ. Int. 2021, 147, 106335. [Google Scholar] [CrossRef] [PubMed]
- Yusoff, M.N.A.M.; Zulkifli, N.W.M.; Sukiman, N.L.; Chyuan, O.H.; Hassan, M.H.; Hasnul, M.H.; Zulkifli, M.S.A.; Abbas, M.M.; Zakaria, M.Z. Sustainability of palm biodiesel in transportation: A review on biofuel standard, policy and international collaboration between Malaysia and Colombia. Bioenerg Res. 2021, 14, 43–60. [Google Scholar] [CrossRef]
- Wolfaardt, F.J.; Fernandes, L.G.L.; Oliveira, S.K.C.; Duret, X.; Görgens, J.F.; Lavoie, J.-M. Recovery approaches for sulfuric acid from the concentrated acid hydrolysis of lignocellulosic feedstocks: A mini-review. Energy Convers. Manag. X 2021, 10, 100074. [Google Scholar] [CrossRef]
- Hirani, A.H.; Javed, N.; Asif, M.; Basu, S.K.; Kumar, A. A review on first- and second-generation biofuel productions. In Biofuels: Greenhouse Gas Mitigation and Global Warming; Kumar, A., Ogita, S., Yau, Y.-Y., Eds.; Springer: New Delhi, India, 2018; pp. 141–154. [Google Scholar] [CrossRef]
- Chacón-Navarrete, H.; Martín, C.; Moreno-García, J. Yeast immobilization systems for second-generation ethanol production: Actual trends and future perspectives. Biofuel Bioprod. Biorefin 2021, 15, 1549–1565. [Google Scholar] [CrossRef]
- Hou, J.; Zhang, X.; Liu, S.; Zhang, S.; Zhang, Q. A critical review on bioethanol and biochar production from lignocellulosic biomass and their combined application in generation of high-value byproducts. Energy Technol. 2020, 8, 2000025. [Google Scholar] [CrossRef]
- Sandesh, K.; Ujwal, P. Trends and perspectives of liquid biofuel-process and industrial viability. Energy Convers. Manag. X 2021, 10, 100075. [Google Scholar] [CrossRef]
- Kumar, N.; Muley, P.D.; Boldor, D.; Coty, I.V.G.G.; Lynam, J.G. Pretreatment of waste biomass in deep eutectic solvents: Conductive heating versus microwave heating. Ind. Crops Prod. 2019, 142, 111865. [Google Scholar] [CrossRef]
- Selvakumari, I.A.; Jayamuthunagai, J.; Senthilkumar, K.; Bharathiraja, B. Biofuels production from diverse bioresources: Global scenario and future challenges. In Biofuels Production–Sustainability and Advances in Microbial Bioresources; Yadav, A.N., Rastegari, A.A., Yadav, N., Gaur, R., Eds.; Springer: Berlin/Heidelberg, Germany, 2020; pp. 163–184. [Google Scholar]
- Fu, C.; Li, Z.; Jia, C.; Zhang, W.; Zhang, Y.; Yi, C.; Xie, S. Recent advances on bio-based isobutanol separation. Energy Convers. Manag. X 2020, 10, 100059. [Google Scholar] [CrossRef]
- Ou, L.; Dou, C.; Yu, J.H.; Kim, H.; Park, Y.C.; Park, S.; Kelley, S.; Lee, E.Y. Techno-economic analysis of sugar production from lignocellulosic biomass with utilization of hemicellulose and lignin for high-value co-products. Biofuel Bioprod. Biorefin 2021, 15, 404–415. [Google Scholar] [CrossRef]
- Krutul, D.; Antczak, A.; Radomski, A.; Drożdżek, M.; Kłosińska, T.; Zawadzki, J. The chemical composition of poplar wood in relation to the species and age of trees. For. Wood Technol. 2019, 107, 131–138. [Google Scholar]
- Amarasekara, A.S.; Wiredu, B.; Grady, T.L.; Obregon, R.G.; Margetić, D. Solid acid catalyzed aldol dimerization of levulinic acid for the preparation of C10 renewable fuel and chemical feedstocks. Catal. Commun. 2019, 124, 6–11. [Google Scholar] [CrossRef]
- Shi, F.; Wang, Y.; Davaritouchaee, M.; Yao, Y.; Kang, K. Directional structure modification of poplar biomass-inspired high efficacy of enzymatic hydrolysis by sequential dilute acid–alkali treatment. ACS Omega 2020, 5, 24780–24789. [Google Scholar] [CrossRef]
- Nevita, T.; Sharma, G.; Pandey, P. Differences in rice rhizosphere bacterial community structure by application of lignocellulolytic plant-probiotic bacteria with rapid composting traits. Ecol. Eng. 2018, 120, 209–221. [Google Scholar] [CrossRef]
- Davaritouchaee, M.; Chen, S.; Mancini, R.J. Delignification and enzyme-diffusion kinetics of radical systems treating wheat straw. Ind. Eng. Chem. Res. 2020, 59, 20656–20666. [Google Scholar] [CrossRef]
- Li, M.; Pu, Y.; Ragauskas, A.J. Current understanding of the correlation of lignin structure with biomass recalcitrance. Front. Chem. 2016, 4, 45. [Google Scholar] [CrossRef] [Green Version]
- Martín, C. Pretreatment of crop residues for bioconversion. Agronomy 2021, 11, 924. [Google Scholar] [CrossRef]
- Kumar, B.; Bhardwaj, N.; Agrawal, K.; Chaturvedi, V.; Verma, P. Current perspective on pretreatment technologies using lignocellulosic biomass: An emerging biorefinery concept. Fuel Process. Technol. 2020, 199, 106244. [Google Scholar] [CrossRef]
- Amiri, H.; Karimi, K. Autohydrolysis: A promising pretreatment for the improvement of acetone, butanol, and ethanol production from woody materials. Chem. Eng. Sci. 2015, 137, 722–729. [Google Scholar] [CrossRef]
- Chen, H. Lignocellulose biorefinery engineering: An overview. In Lignocellulose Biorefinery Engineering; Chen, H., Ed.; Woodhead Publishing: Cambridge, UK, 2015; pp. 1–17. [Google Scholar]
- Domínguez, E.; Nóvoa, T.; Pablo, G.; Garrote, G.; Romaní, A. Sequential two-stage autohydrolysis biorefinery for the production of bioethanol from fast-growing Paulownia biomass. Energy Convers. Manag. 2020, 226, 113517. [Google Scholar] [CrossRef]
- Conde-Mejia, C.; Jimenez-Gutierrez, A.; El-Halwagi, M. A comparison of pretreatment methods for bioethanol production from lignocellulosic materials. Process Saf. Environ. Prot. 2012, 90, 189–202. [Google Scholar] [CrossRef]
- Obeng, A.K.; Premjet, D.; Premjet, S. Fermentable sugar production from the peels of two durian (Durio zibethinus Murr.) cultivars by phosphoric acid pretreatment. Resources 2018, 7, 60. [Google Scholar] [CrossRef] [Green Version]
- Syaichurrozi, I.; Villta, P.K.; Nabilah, N.; Rusdi, R. Effect of sulfuric acid pretreatment on biogas production from Salvinia molesta. J. Environ. Chem. Eng. 2019, 7, 102857. [Google Scholar] [CrossRef]
- Sarbishei, S.; Goshadrou, A.; Hatamipour, M.S. Mild sodium hydroxide pretreatment of tobacco product waste to enable efficient bioethanol production by separate hydrolysis and fermentation. Biomass Convers. Biorefin 2020, 11, 2963–2973. [Google Scholar] [CrossRef]
- Molaverdi, M.; Karimi, K.; Mirmohamadsadeghi, S. Improvement of dry simultaneous saccharification and fermentation of rice straw to high concentration ethanol by sodium carbonate pretreatment. Energy 2019, 167, 654–660. [Google Scholar] [CrossRef]
- Deshpande, M.; Scheicher, R.H.; Ahuja, R.; Pandey, R. Binding strength of sodium ions in cellulose for different water contents. J. Phys. Chem. B 2008, 112, 8985–8989. [Google Scholar] [CrossRef]
- Noori, M.S.; Karimi, K. Detailed study of efficient ethanol production from elmwood by alkali pretreatment. Biochem. Eng. J. 2016, 105, 197–204. [Google Scholar] [CrossRef]
- Shi, S.; Guan, W.; Blersch, D.; Li, J. Improving the enzymatic digestibility of alkaline-pretreated lignocellulosic biomass using polyDADMAC. Ind. Crops Prod. 2021, 162, 113244. [Google Scholar] [CrossRef]
- Adney, B.; Baker, J. Measurement of Cellulase Activities; NREL/TP-510-42628; National Renewable Energy Laboratory: Golden, CO, USA, 2008. [Google Scholar]
- Sluiter, A.; Hames, B.; Hyman, D.; Payne, C.; Ruiz, R.; Scarlata, C.; Sluiter, J.; Templeton, D.; Wolfe, J. Determination of Total Solids in Biomass and Total Dissolved Solids in Liquid Process Samples; NREL/TP-510-42619; National Renewable Energy Laboratory: Golden, CO, USA, 2008; Volume 9, pp. 1–6. [Google Scholar]
- Zhang, Y.H.P.; Ding, S.Y.; Mielenz, J.R.; Cui, J.B.; Elander, R.T.; Laser, M.; Himmel, M.E.; McMillan, J.R.; Lynd, L.R. Fractionating recalcitrant lignocellulose at modest reaction conditions. Biotechnol Bioeng 2007, 97, 214–223. [Google Scholar] [CrossRef] [PubMed]
- Khaleghian, H.; Karimi, K.; Behzad, T. Ethanol production from rice straw by sodium carbonate pretreatment and Mucor hiemalis fermentation. Ind. Crops Prod. 2015, 76, 1079–1085. [Google Scholar] [CrossRef]
- Jeihanipour, A.; Karimi, K.; Niklasson, C.; Taherzadeh, M.J. A novel process for ethanol or biogas production from cellulose in blended-fibers waste textiles. Waste Manag. 2010, 30, 2504–2509. [Google Scholar] [CrossRef] [PubMed]
- Kootstra, A.M.J.; Beeftink, H.H.; Scott, E.L.; Sanders, J.P. Comparison of dilute mineral and organic acid pretreatment for enzymatic hydrolysis of wheat straw. Biochem. Eng. J. 2009, 46, 126–131. [Google Scholar] [CrossRef]
- Miller, G.L. Use of dinitrosalicylic acid reagent for determination of reducing sugar. Ana. Chem. 1959, 31, 426–428. [Google Scholar] [CrossRef]
- Karimi, K.; Emtiazi, G.; Taherzadeh, M.J. Ethanol production from dilute-acid pretreated rice straw by simultaneous saccharification and fermentation with Mucor indicus, Rhizopus oryzae, and Saccharomyces cerevisiae. Enzym. Microb. Technol. 2006, 40, 138–144. [Google Scholar] [CrossRef]
- Hashemi, S.S.; Mirmohamadsadeghi, S.; Karimi, K. Biorefinery development based on whole safflower plant. Renew. Energy 2020, 152, 399–408. [Google Scholar] [CrossRef]
- Sluiter, A.; Hames, B.; Ruiz, R.; Scarlata, C.; Sluiter, J.; Templeton, D.; Crocker, D.L.A.P. Determination of Structural Carbohydrates and Lignin in Biomass; NREL/TP-510-42618; National Renewable Energy Laboratory: Golden, CO, USA, 2012. [Google Scholar]
- Sluiter, A.; Ruiz, R.; Scarlata, C.; Sluiter, J.; Templeton, D. Determination of Extractives in Biomass; NREL/TP-510-42619; National Renewable Energy Laboratory: Golden, CO, USA, 2008. [Google Scholar]
- Cheng, Q.; Wang, J.; McNeel, J.; Jacobson, P. Water retention value measurements of cellulosic materials using a centrifuge technique. BioResources 2010, 5, 1945–1954. [Google Scholar]
- Hashemi, S.S.; Karimi, K.; Nosratpour, M.J.; Sárvári Horváth, I. Efficient biogas and ethanol production from safflower straw using sodium carbonate pretreatment. Energ Fuel 2016, 30, 10592–10601. [Google Scholar] [CrossRef]
- Kumar, N.; Gautam, R.; Stallings, J.D.; Coty, G.G.; Lynam, J.G. Secondary agriculture residues pretreatment using deep eutectic solvents. Waste Biomass Valorization 2021, 12, 2259–2269. [Google Scholar] [CrossRef]
- Shafiei, M.; Karimi, K.; Zilouei, H.; Taherzadeh, M.J. Enhanced ethanol and biogas production from pinewood by NMMO pretreatment and detailed biomass analysis. Biomed. Res. Int. 2014, 2014, 469378. [Google Scholar] [CrossRef]
- Park, S.; Baker, J.O.; Himmel, M.E.; Parilla, P.A.; Johnson, D.K. Cellulose crystallinity index: Measurement techniques and their impact on interpreting cellulase performance. Biotechnol. Biofuels 2010, 3, 10. [Google Scholar] [CrossRef] [Green Version]
- Garvey, C.J.; Parker, I.H.; Simon, G.P. On the interpretation of X-ray diffraction powder patterns in terms of the nanostructure of cellulose I fibres. Macromol. Chem. Phys. 2005, 206, 1568–1575. [Google Scholar] [CrossRef]
- He, J.; Cui, S.; Wang, S.Y. Preparation and crystalline analysis of high-grade bamboo dissolving pulp for cellulose acetate. J. Appl. Polym. Sci. 2008, 107, 1029–1038. [Google Scholar] [CrossRef]
- Alvira, P.; Ballesteros, M.; Negro, M.J. Progress on enzymatic saccharification technologies for biofuels production. Biofuel Technol. 2013, 145–169. [Google Scholar] [CrossRef]
- Colom, X.; Carrillo, F.; Nogués, F.; Garriga, P. Structural analysis of photodegraded wood by means of FTIR spectroscopy. Polym. Degrad. Stab. 2003, 80, 543–549. [Google Scholar] [CrossRef]
- Motaung, T.; Anandjiwala, R. Effect of alkali and acid treatment on thermal degradation kinetics of sugar cane bagasse. Ind. Crops Prod. 2015, 74, 472–477. [Google Scholar] [CrossRef]
- Segal, L.; Creely, J.J.; Martin Jr, A.; Conrad, C. An empirical method for estimating the degree of crystallinity of native cellulose using the X-ray diffractometer. Text. Res. J. 1959, 29, 786–794. [Google Scholar] [CrossRef]
- Cao, Y.; Tan, H. Study on crystal structures of enzyme-hydrolyzed cellulosic materials by X-ray diffraction. Enzym. Microb. Technol. 2005, 36, 314–317. [Google Scholar] [CrossRef]
- Zhang, Y.H.P.; Lynd, L.R. Toward an aggregated understanding of enzymatic hydrolysis of cellulose: Noncomplexed cellulase systems. Biotechnol. Bioeng. 2004, 88, 797–824. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Zhang, L.; Liu, D. Biomass recalcitrance. Part I: The chemical compositions and physical structures affecting the enzymatic hydrolysis of lignocellulose. Biofuel Bioprod. Bior. 2012, 6, 465–482. [Google Scholar] [CrossRef]
- Taherzadeh, M.J.; Karimi, K. Pretreatment of lignocellulosic wastes to improve ethanol and biogas production: A review. Int. J. Mol. Sci. 2008, 9, 1621–1651. [Google Scholar] [CrossRef] [PubMed]


| Substrate | Pretreatment | Glucan 1 | Xylan | Galactan | Arabinan + Mannan | ASL 2 | AIL 3 | Total Lignin | Ash |
|---|---|---|---|---|---|---|---|---|---|
| Poplar | Untreated | 51.4 | 18.7 | 0.2 | 5.7 | 4.7 ± 0.1 | 20.4 ± 0.1 | 25.1 | 0.17 ± 0.10 |
| Autohydrolysis | 57.8 | 9.4 | ND4 | 3.5 | 2.7 ± 0.1 | 25.1 ± 0.1 | 27.8 | 0.93 ± 0.27 | |
| Sulfuric Acid | 64.1 | 4.7 | ND | 1.9 | 1.6 ± 0.1 | 31.3 ± 0.1 | 32.9 | 0.83 ± 0.17 | |
| Phosphoric Acid | 61.2 | 4.8 | ND | 3.2 | 2.7 ± 0.1 | 20.3 ± 0.1 | 23 | 0.34 ± 0.01 | |
| Hot Sodium Hydroxide | 65.3 | 9.2 | ND | 3.8 | 3.5 ± 0.2 | 19.7 ± 0.4 | 23.2 | 0.73 ± 0.05 | |
| Cold Sodium Hydroxide | 55.4 | 9.6 | ND | 4.1 | 3.9 ± 0.2 | 19.9 ± 0.2 | 23.8 | 0.36 ± 0.19 | |
| Sodium Carbonate | 52 | 9.7 | ND | 4.2 | 4.1 ± 0.1 | 19.2 ± 0.1 | 23.3 | 0.23 ± 0.49 | |
| Pine | Untreated | 44.2 | 6.8 | 1.3 | 11 | 2.9 ± 0.1 | 20.2 ± 0.1 | 23.1 | 0.55 ± 0.02 |
| Autohydrolysis | 51.8 | 4.1 | ND | 5.3 | 2.2 ± 0.1 | 19.7 ± 0.8 | 21.9 | 0.72 ± 0.14 | |
| Sulfuric Acid | 52.7 | 4 | ND | 3 | 2.7 ± 0.1 | 26.5 ± 0.1 | 29.2 | 0.89 ± 0.05 | |
| Phosphoric Acid | 47.4 | 3.1 | 1.6 | 3.2 | 2.7 ± 0.2 | 24.7 ± 0.4 | 27.4 | 0.72 ± 0.51 | |
| Hot Sodium Hydroxide | 47 | 8.6 | 0.7 | 3.2 | 3.1 ± 0.2 | 22.4 ± 0.7 | 25.5 | 0.33 ± 0.12 | |
| Cold Sodium Hydroxide | 45.9 | 5.9 | 1.3 | 5.4 | 2.9 ± 0.1 | 19.5 ± 0.4 | 22.4 | 0.43 ± 0.10 | |
| Sodium Carbonate | 48.6 | 6.6 | 1.1 | 3.7 | 3.2 ± 0.5 | 24.7 ± 0.5 | 27.9 | 0.56 ± 0.23 | |
| Rice Straw | Untreated | 41.8 | 18.7 | ND | 3.2 | 4.3 ± 0.1 | 15.7 ± 0.6 | 20 | 7.08 ± 0.25 |
| Autohydrolysis | 52.7 | 7.9 | ND | 5.4 | 2.5 ± 0.1 | 15.9 ± 0.7 | 18.4 | 7.91 ± 0.81 | |
| Sulfuric Acid | 59.8 | 5.6 | ND | 1.5 | 2.6 ± 0.1 | 16.8 ± 0.4 | 19.4 | 11.91 ± 0.16 | |
| Phosphoric Acid | 63 | 5.3 | ND | 3.2 | 2.6 ± 0.1 | 16.8 ± 0.2 | 19.4 | 11.44 ± 0.07 | |
| Hot Sodium Hydroxide | 71.2 | 8.9 | ND | 2.3 | 1.1 ± 0.1 | 8.7 ± 0.1 | 9.7 | 7.63 ± 0.05 | |
| Cold Sodium Hydroxide | 60.5 | 10.1 | ND | 2.7 | 2.0 ± 0.1 | 13.3 ± 0.3 | 15.3 | 8.45 ± 0.19 | |
| Sodium Carbonate | 57.8 | 12 | ND | 2.2 | 1.6 ± 0.1 | 9.2 ± 0.3 | 10.7 | 11.58 ± 0.32 |
| Substrate | Pretreatment Type | LOI | TCI | α1508/898 | CrI (%) | Crystallite Size |
|---|---|---|---|---|---|---|
| Poplar | Untreated | 1.58 | 1.53 | 1.14 | 67.3 | 2.37 nm |
| Autohydrolysis | 1.62 | 1.47 | 1.37 | 60.03 | 2.38 nm | |
| Sulfuric Acid | 2.08 | 1.43 | 1.78 | 60.69 | 1.88 nm | |
| Phosphoric Acid | 1.5 | 1.37 | 1.09 | 31.38 | 0.93 nm | |
| Hot Sodium Hydroxide | 1.58 | 1.28 | 1.18 | 66.16 | 2.64 nm | |
| Cold Sodium Hydroxide | 1.46 | 1.26 | 1.11 | 56.14 | 2.09 nm | |
| Sodium Carbonate | 1.62 | 1.3 | 1.16 | 67.13 | 2.62 nm | |
| Pine | Untreated | 1.53 | 1.42 | 1.38 | 53.98 | 1.67 nm |
| Autohydrolysis | 1.41 | 1.33 | 1.45 | 59.1 | 2.47 nm | |
| Sulfuric Acid | 1.92 | 1.3 | 2.13 | 64.83 | 1.72 nm | |
| Phosphoric Acid | 1.3 | 1.25 | 1.23 | 23.92 | 0.86 nm | |
| Hot Sodium Hydroxide | 1.5 | 1.32 | 1.39 | 55.51 | 2.02 nm | |
| Cold Sodium Hydroxide | 1.45 | 1.33 | 1.33 | 45.2 | 1.56 nm | |
| Sodium Carbonate | 1.7 | 1.3 | 1.49 | 57.68 | 1.94 nm | |
| Rice Straw | Untreated | 1.83 | 1.31 | 1.27 | 66.25 | 1.78 nm |
| Autohydrolysis | 1.69 | 1.35 | 1.08 | 70.77 | 2.00 nm | |
| Sulfuric Acid | 1.86 | 1.25 | 1.26 | 68.25 | 1.98 nm | |
| Phosphoric Acid | 1.33 | 1.35 | 0.91 | 35.32 | 1.07 nm | |
| Hot Sodium Hydroxide | 1.36 | 1.17 | 0.6 | 73.56 | 3.27 nm | |
| Cold Sodium Hydroxide | 1.5 | 1.13 | 0.89 | 62.68 | 2.05 nm | |
| Sodium Carbonate | 1.61 | 1.29 | 0.84 | 60.35 | 2.14 nm |
| Substrate | Pretreatment | Initial pH | Acid Buffering Capacity (mL) | Base Buffering Capacity (mL) |
|---|---|---|---|---|
| Poplar | Untreated | 4.78 | 0.22 | 0.12 |
| Autohydrolysis | 6.33 | 0.2 | 0.01 | |
| Sulfuric Acid | 5.75 | 0.17 | 0.02 | |
| Phosphoric Acid | 7.14 | 0.27 | 0 | |
| Hot Sodium Hydroxide | 8.6 | 0.5 | 0 | |
| Cold Sodium Hydroxide | 9.49 | 0.42 | 0 | |
| Sodium Carbonate | 9.16 | 0.57 | 0 | |
| Pine | Untreated | 5.4 | 0.2 | 0.03 |
| Autohydrolysis | 7.3 | 0.24 | 0 | |
| Sulfuric Acid | 6.33 | 0.25 | 0.02 | |
| Phosphoric Acid | 6.02 | 0.4 | 0.04 | |
| Hot Sodium Hydroxide | 9.36 | 0.67 | 0 | |
| Cold Sodium Hydroxide | 8.77 | 0.54 | 0 | |
| Sodium Carbonate | 9.21 | 0.57 | 0 | |
| Rice Straw | Untreated | 6.24 | 0.3 | 0.01 |
| Autohydrolysis | 7.03 | 0.3 | 0 | |
| Sulfuric Acid | 6.5 | 0.3 | 0.01 | |
| Phosphoric Acid | 6.95 | 0.35 | 0 | |
| Hot Sodium Hydroxide | 10.5 | 0.63 | 0 | |
| Cold Sodium Hydroxide | 9.33 | 0.57 | 0 | |
| Sodium Carbonate | 8.88 | 0.97 | 0 |
| Substrate | Pretreatment Type | Recovery (g/kg Substrate) | Glucose Production (g/kg Substrate) | Ethanol Production (g/kg Substrate) | Glycerol Yield (%) | Ethanol Yield (%) |
|---|---|---|---|---|---|---|
| Poplar | Untreated | - | 162.5 ± 0.2 | 60.2 ± 0.1 | 0.99 ± 0.2 | 20.87 ± 0.48 |
| Autohydrolysis | 677.9 | 292.8 ± 0.7 | 112.2 ± 0.6 | 1.64 ± 0.1 | 51.02 ± 2.54 | |
| Sulfuric Acid | 622.4 | 356.6 ± 1.3 | 119.6 ± 0.2 | 1.94 ± 0.1 | 53.45 ± 0.77 | |
| Phosphoric Acid | 763 | 493.3 ± 0.7 | 221.2 ± 0.1 | 3.24 ± 0.1 | 84.44 ± 0.42 | |
| Hot Sodium Hydroxide | 565 | 342.1 ± 2.1 | 130.7 ± 0.1 | 1.80 ± 0.2 | 63.19 ± 0.39 | |
| Cold Sodium Hydroxide | 773.5 | 459.2 ± 3.4 | 136.8 ± 0.1 | 1.54 ± 0.3 | 56.91 ± 0.24 | |
| Sodium Carbonate | 760.1 | 344.2 ± 0.6 | 128.9 ± 0.1 | 2.45 ± 0.2 | 58.15 ± 0.50 | |
| Pine | Untreated | - | 125.0 ± 3.2 | 55.9 ± 0.1 | 1.08 ± 0.1 | 22.55 ± 0.12 |
| Autohydrolysis | 714.2 | 177.6 ± 1.3 | 63.9 ± 0.1 | 1.07 ± 0.1 | 30.78 ± 0.29 | |
| Sulfuric Acid | 617.8 | 151.3 ± 0.1 | 56.2 ± 0.1 | 1.08 ± 0.1 | 30.80 ± 0.05 | |
| Phosphoric Acid | 792.8 | 446.0 ± 0.6 | 177.6 ± 0.1 | 2.80 ± 0.1 | 84.25 ± 0.16 | |
| Hot Sodium Hydroxide | 761.8 | 306.3 ± 0.4 | 90.2 ± 0.1 | 1.49 ± 0.4 | 44.97 ± 0.15 | |
| Cold Sodium Hydroxide | 936 | 340.5 ± 0.3 | 97.3 ± 0.3 | 1.35 ± 0.1 | 40.38 ± 1.30 | |
| Sodium Carbonate | 847.5 | 233.2 ± 0.2 | 71.1 ± 0.1 | 1.11 ± 0.2 | 30.76 ± 0.06 | |
| Rice Straw | Untreated | - | 262.5 ± 0.3 | 115.5 ± 0.2 | 1.94 ± 0.2 | 53.13 ± 0.58 |
| Autohydrolysis | 574.5 | 305.4 ± 0.2 | 142.7 ± 0.1 | 2.57 ± 0.2 | 84.12 ± 0.36 | |
| Sulfuric Acid | 510.8 | 322.6 ± 0.6 | 145.8 ± 0.1 | 3.16 ± 0.1 | 85.10 ± 0.17 | |
| Phosphoric Acid | 665.8 | 493.2 ± 0.6 | 206.2 ± 0.1 | 3.08 ± 0.4 | 87.64 ± 0.27 | |
| Hot Sodium Hydroxide | 332.3 | 267.8 ± 0.4 | 123.5 ± 0.1 | 4.22 ± 0.4 | 92.95 ± 0.01 | |
| Cold Sodium Hydroxide | 665.5 | 513.7 ± 0.3 | 195.1 ± 0.2 | 3.17 ± 0.1 | 86.41 ± 0.85 | |
| Sodium Carbonate | 593 | 352.9 ± 0.1 | 166.3 ± 0.2 | 4.40 ± 0.4 | 86.36 ± 1.11 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bay, M.S.; Eslami, F.; Karimi, K. The Relationship between Structural Features of Lignocellulosic Materials and Ethanol Production Yield. Designs 2022, 6, 119. https://doi.org/10.3390/designs6060119
Bay MS, Eslami F, Karimi K. The Relationship between Structural Features of Lignocellulosic Materials and Ethanol Production Yield. Designs. 2022; 6(6):119. https://doi.org/10.3390/designs6060119
Chicago/Turabian StyleBay, Mohammad Saber, Fatemeh Eslami, and Keikhosro Karimi. 2022. "The Relationship between Structural Features of Lignocellulosic Materials and Ethanol Production Yield" Designs 6, no. 6: 119. https://doi.org/10.3390/designs6060119







