Pattern-Induced Visual Discomfort and Anxiety in Migraineurs: Their Relationship and the Effect of Colour
Abstract
:1. Introduction
1.1. Overview
1.2. Migraines, Anxiety and Fear
1.3. Colour, Pattern Senstivity and Mood
1.4. Unconscious Assessment of Pattern Anxiety—The Dot-Probe Task
1.5. Current Study
2. Materials and Methods
2.1. Participants and Screening
2.2. Material and Methods
2.2.1. Materials
- The VDS comprises 23 items for measuring visual discomfort. The items include questions on perceptual, somatic and performance problems when exposed to different light sources or when reading, as well as severe headache frequency, effective reading times and experiences of visual difficulties whilst reading. Respondents rated answers on a four-point Likert scale (0 = event never occurs, 3 = event occurs almost always). The scores are added. The VDS has good reliability and validity, with a reliable internal consistency estimate of 0.91 [40].
- The IHS uses self-report diagnostic criteria to obtain data on migraine and headache type, frequency, duration and symptoms, presence of aura and visual discomfort symptoms, along with dyslexia and epilepsy questions.
- State-Trait Anxiety Inventory—Form Y (STAI [42]). Form Y includes 20 items assessing state and 20 items assessing trait anxiety. Items are rated on a four-point Likert scale (1 = almost never to 4 = almost always) with some items reverse scored. For trait anxiety, participants were required to respond to how they feel generally, and for state anxiety, how they feel right now. The STAI has high internal consistency and reliability (Cronbach’s α between 0.85 and 0.95 [42]) and convergent validity, with high correlations greater than 0.82 at p < 0.001 with the Anxiety Sensitivity Index [45].
- Subjective Units of Discomfort Scale (SUDS [43]). This scale was used to measure moment-to-moment participant anxiety on a self-rating scale from 0 to 10 (0 = not anxious at all, to 10 = extremely anxious), where participants imagine having a ‘distress thermometer’ measuring their anxiety. The SUDS has been validated as global measures of physical and emotional discomfort (Tanner, 2012). The SUDS concurrent validity has been supported with empirical data finding moderate correlations of 0.69 of the SUDS with the STAI [46]. SUDS ratings were conducted at four times during the experiment.
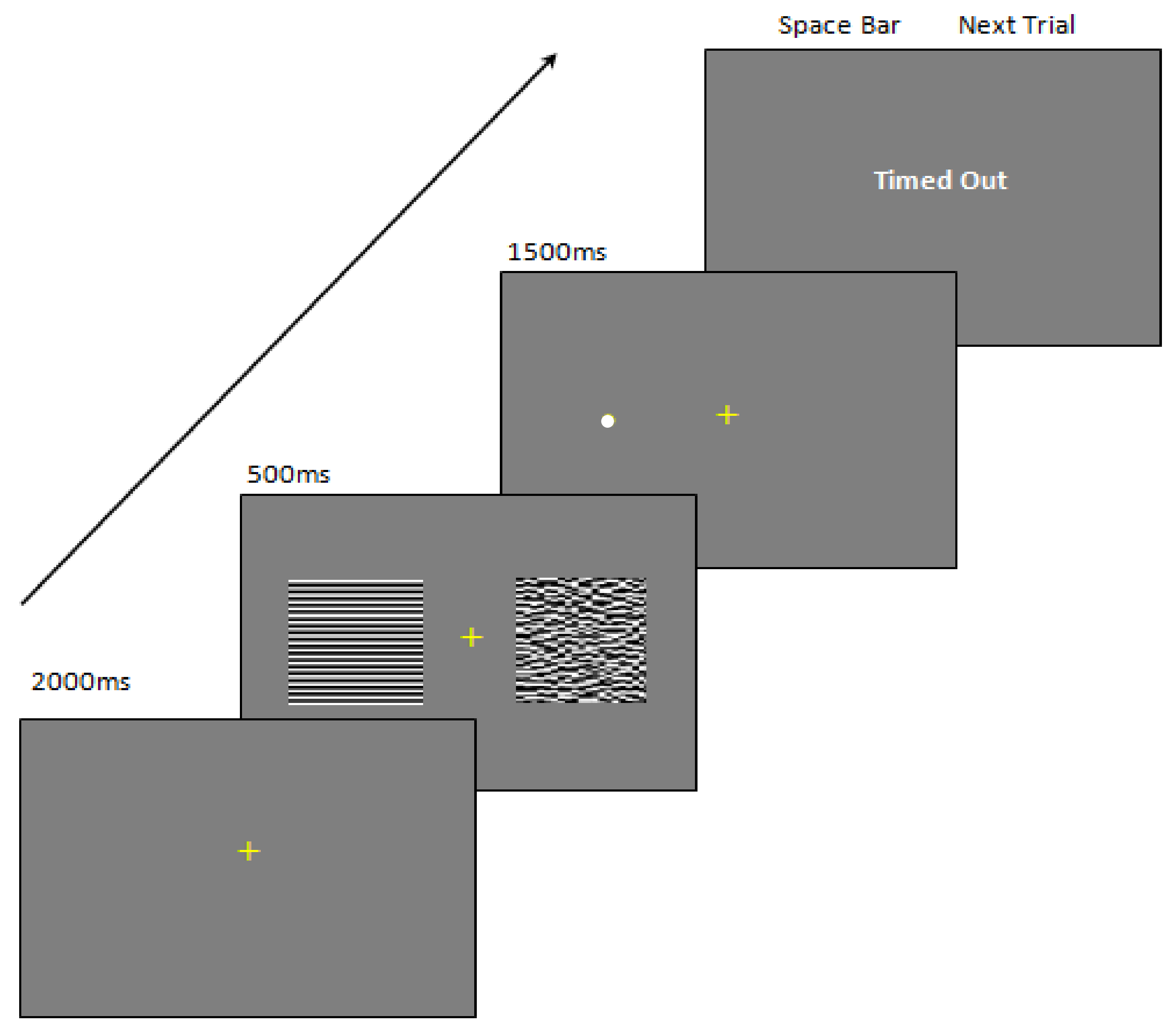
2.2.2. Experimental Apparatus
2.2.3. Experimental Procedure
3. Results
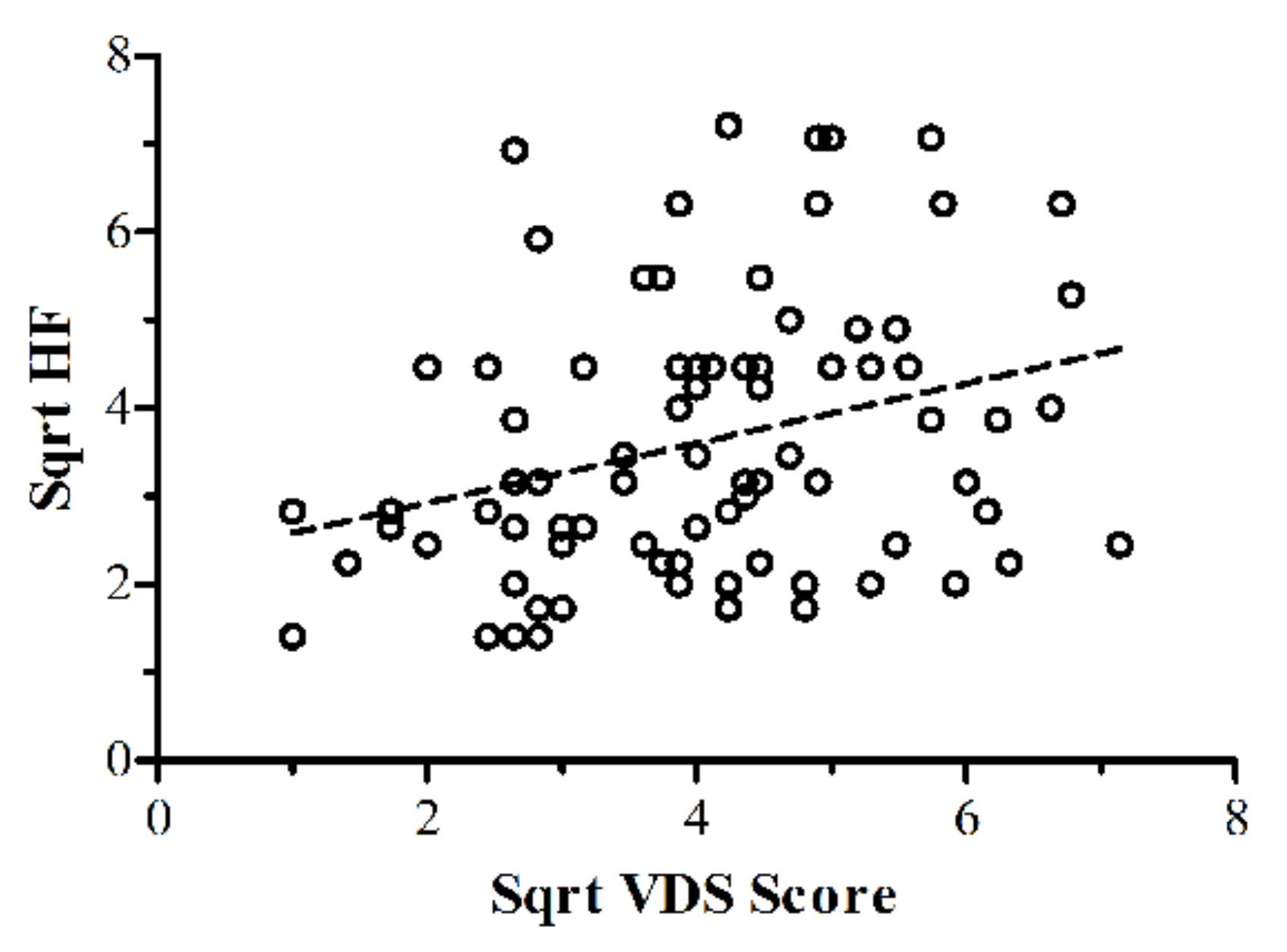
3.1. Analyses of Pool’s VDS and IHS Screening Data
3.2. Analyses of Experimental Results
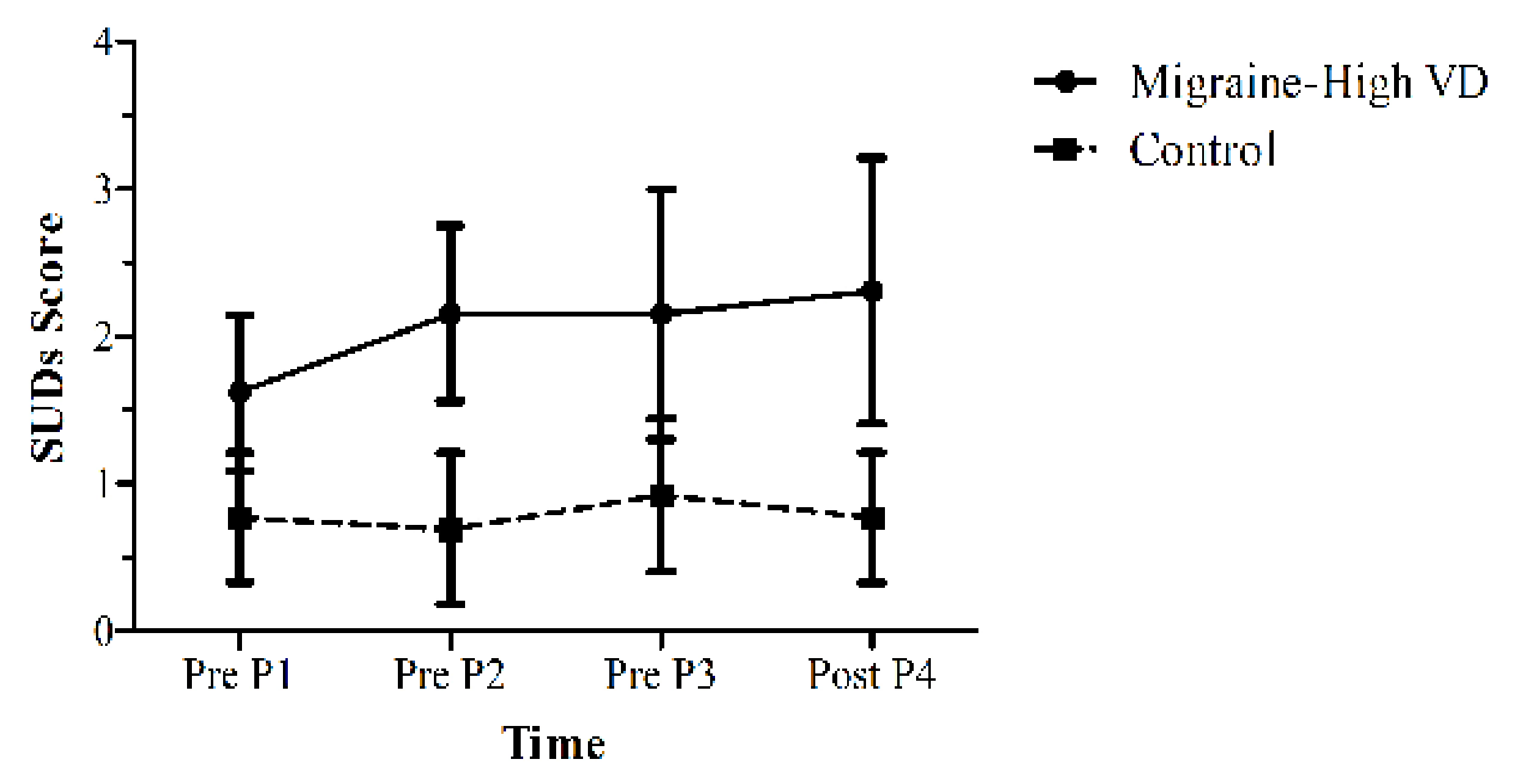
3.2.1. Anxiety
3.2.2. Spatial Frequency and Visual Discomfort
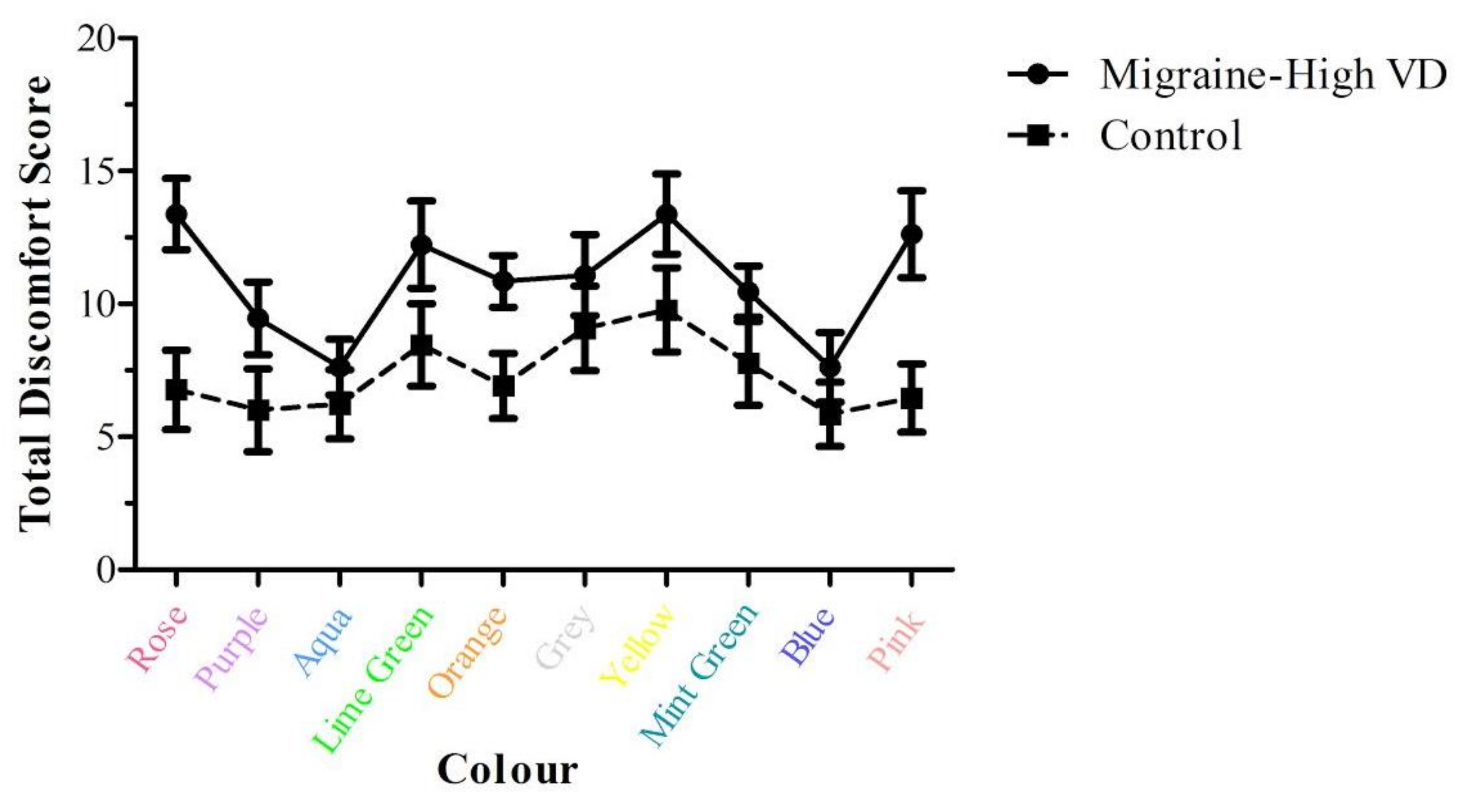
3.2.3. Colour and Visual Discomfort
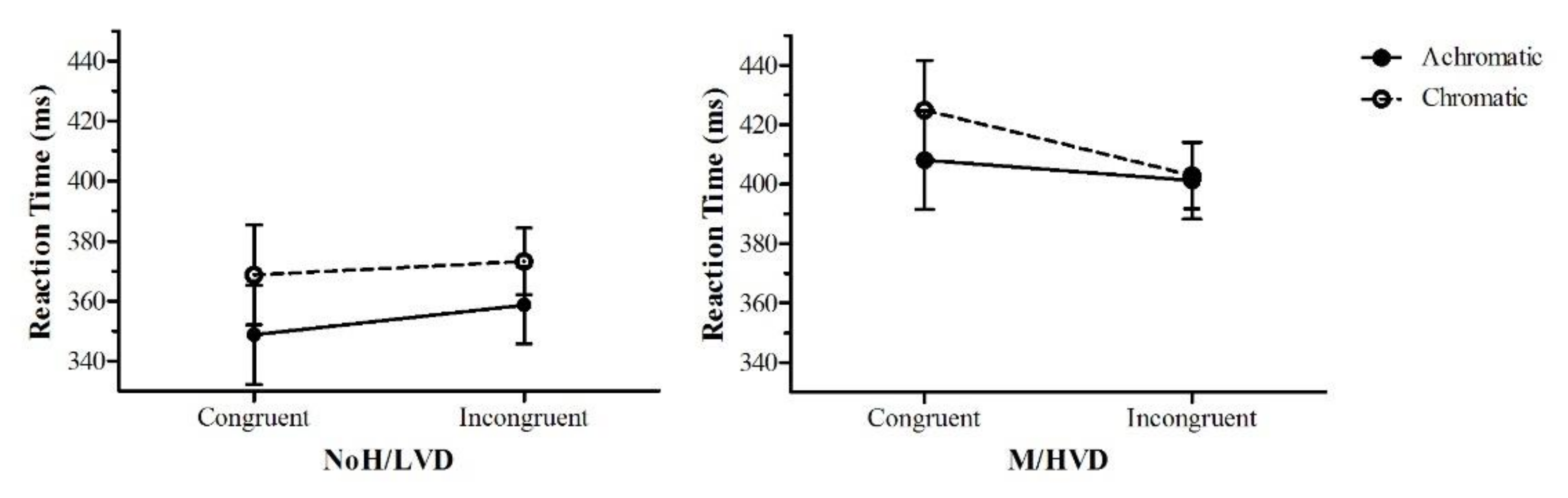
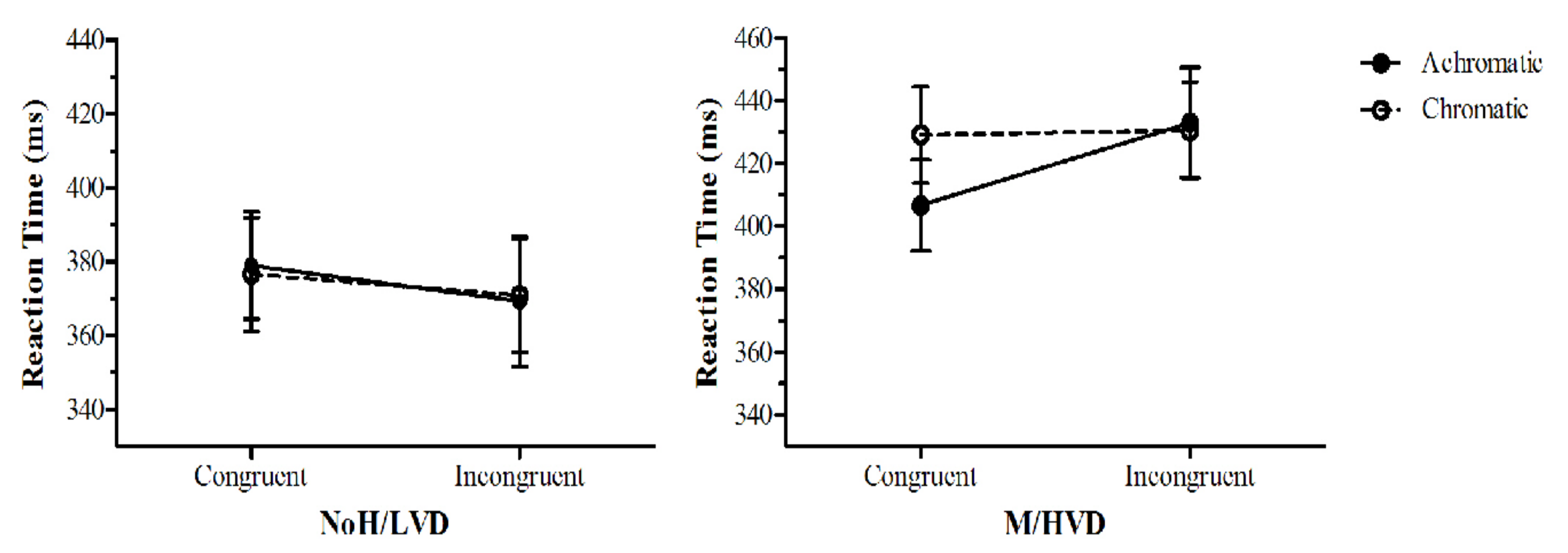
3.2.4. Dot-Probe Analyses
4. Discussion
4.1. Visual Discomfort and Migraines
4.2. Visual Discomfort and Anxiety and Colour
4.3. Unconscious Anxiety Effects and the Effect of Colour in the Dot-Probe Task
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wilkins, A.J.; Jeanes, R.J.; Pumprey, P.D.; Laskier, M. Rate of Reading Test: Its reliability, and its validity in the assessment of the effects of coloured overlays. Ophthal Physiol. Opt. 1996, 16, 491–497. [Google Scholar] [CrossRef]
- Huang, J.; Zong, X.; Wilkins, A.; Jenkins, B.; Bozoki, A.; Cao, Y. fMRI evidence that precision ophthalmic tints reduce cortical hyperactivation in migraine. Cephalalgia 2011, 31, 925–936. [Google Scholar] [CrossRef] [Green Version]
- Good, P.A.; Taylor, R.H.; Mortimer, M.J. The use of tinted glasses in childhood migraine. Headache J. Head Face Pain 1991, 31, 533–536. [Google Scholar] [CrossRef] [PubMed]
- Evans, B.J.W.; Patel, R.; Wilkins, A.J. Optometric function in visually sensitive migraine before and after treatment with tinted spectacles. Ophthal. Physiol. Opt. 2002, 22, 130–142. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martin, P.R.; Teoch, H. Effects of visual stimuli and a stressor on head pain. Headache 1999, 39, 705–715. [Google Scholar] [CrossRef]
- Hamelsky, S.W.; Lipton, R.B. Psychiatric comorbidity of migraine. Headache 2006, 46, 1327–1333. [Google Scholar] [CrossRef] [PubMed]
- Hollis, J.; Allen, P.M.; Fleischmann, D.; Aulak, R. Personality dimensions of people who suffer from visual stress. Ophthal. Physiol. Opt. 2006, 27, 603–610. [Google Scholar] [CrossRef] [Green Version]
- Pelligrini, R.J.; Schauss, A.G.; Miller, M.E. Room colour and aggression in a criminal detention holding cell: A test of the “tranquilizing pink” hypothesis. Orthomol. Psychiatry 1981, 10, 174–181. [Google Scholar]
- Wilkins, A.J.; Patel, R.; Adjamian, R.; Evans, B.J.W. Tinted spectacles and visually sensitive migraine. Cephalalgia 2002, 22, 711–719. [Google Scholar] [CrossRef]
- MacLeod, C.; Mathews, A.; Tata, P. Attentional bias in emotional disorders. J. Abnorm. Psychology 1986, 95, 15–20. [Google Scholar] [CrossRef]
- Goadsby, P.J. Pathophysiology of migraine. Genet. Epidemiol. 2009, 27, 335–360. [Google Scholar] [CrossRef]
- International Classification of Headache Disorders. Headache Classification Subcommittee of the International Headache Society. Int. Classif. Headache Disorders Cephalalgia 2013, 33, 629–808. [Google Scholar]
- Borsook, D.; Maleki, N.; Becerra, L.; McEwan, B. Understanding migraine through the lens of maladaptive stress responses: A model disease of allostatic load. Neuron 2012, 73, 219–234. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Burstein, R. Deconstructing migraine into peripheral and central sensitization. Pain 2001, 89, 110–117. [Google Scholar] [CrossRef]
- Wilkins, A.J. Visual Stress; Oxford University Press: Oxford, UK, 1995. [Google Scholar]
- Fisher, R.S.; Harding, G.; Erba, G.; Barkley, G.L.; Wilkins, A. Photic-and pattern-induced seizures: A review for the Epilepsy Foundation of America Working Group. Epilepsia 2005, 46, 1426–1441. [Google Scholar] [CrossRef]
- Hedborg, K.; Anderberg, U.M.; Muhr, C. Stress in migraine: Personality-dependent vulnerability, life events, and gender are of significance. Upsala J. Med. Sci. 2011, 116, 187–199. [Google Scholar] [CrossRef]
- Annau, Z.; Kamin, L.J. The conditioned emotional response as a function of intensity of the US. J. Comp. Physiol. Psychol. 1961, 54, 428–432. [Google Scholar] [CrossRef]
- LeDoux, J.E. The Emotional Brain: The Mysterious Underpinnings of Emotional Life; Simon & Schuster: New York, NY, USA, 1996. [Google Scholar]
- Ohman, A.; Mineka, S. Fears, phobias, and preparedness: Toward an evolved module of fear and fear learning. Psychol. Rev. 2001, 108, 483–522. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Öhman, A.; Nordby, H.; d’Elia, G. Orienting and schizophrenia: Stimulus significance, attention, and distraction in a signaled reaction time task. J. Abnorm. Psychol. 1986, 95, 326–334. [Google Scholar] [CrossRef]
- Nulty, D.D.; Wilkins, A.J.; Williams, J.M.G. Mood, pattern sensitivity and headache: A longitudinal study. Psychol. Med. 1987, 17, 705–713. [Google Scholar] [CrossRef]
- Harle, D.E.; Evans, B.J.W. The optometric correlates of migraine. Ophthal. Physiol. Opt. 2004, 24, 369–383. [Google Scholar] [CrossRef]
- Aldrich, A.; Hibbard, P.; Wilkins, A. Vision and hyper-responsiveness in migraine. Vision 2019, 3, 62. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shepherd, A.J.; Hine, T.J.; Beaumont, H.M. Colour and spatial frequency are related to visual pattern sensitivity in migraine. Headache 2013, 1087–1103. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hamedani, Z.; Solgi, E.; Skates, H.; Hine, T.; Fernando, R.; Lyons, J.; Dupre, K. Visual discomfort and glare assessment in office environments: A review of light-induced physiological and perceptual responses. Build. Environ. 2019, 153, 267–280. [Google Scholar] [CrossRef]
- Harle, D.E.; Shepherd, A.J.; Evans, B.J. Visual stimuli are common triggers of migraine and are associated with pattern glare. Headache 2006, 46, 1431–1440. [Google Scholar] [CrossRef]
- Jacobs, K.W.; Suess, J.F. Effects of four psychological primary colors on anxiety state. Percept. Mot. Ski. 1975, 41, 207–210. [Google Scholar] [CrossRef]
- Wilson, Arousal properties of red versus green. Percept. Mot. Ski. 1996, 23, 947–949.
- Valdez, P.; Mehrabian, A. Effects of colour on emotions. J. Exp. Psychol. 1994, 123, 394–409. [Google Scholar] [CrossRef]
- Profusek, P.J.; Rainey, D.W. Effects of baker-miller pink and red on state anxiety, grip strength, and motor precision. Percept. Mot. Ski. 1987, 65, 941–942. [Google Scholar] [CrossRef]
- Todd, J.; van Ryckeghem, D.M.; Sharpe, L.; Crombez, G. Attentional bias to pain-related information: A meta-analysis of dot-probe studies. Health Psychol. Rev. 2018, 12, 419–436. [Google Scholar] [CrossRef]
- Eysenck, M.W. Anxiety and Cognition: A Unified Theory; Psychology Press: Hove, UK, 1997. [Google Scholar]
- Van Bockstaele, B.; Verschuere, B.; Tibboel, H.; De Houwer, J.; Crombbez, G.; Koster, H.W. A review of current evidence for the causal impact of attentional bias on Fear and Anxiety. Psychol. Bull. 2014, 140, 682–721. [Google Scholar] [CrossRef]
- Rosen, J.B.; Schulkin, J. From normal fear to pathological anxiety. Psychol. Rev. 1998, 105, 325–350. [Google Scholar] [CrossRef]
- Bar-Haim, Y.; Lamy, D.; Pergamin, L.; Bakermans-Kranenburg, M.J.; van IJzendoorn, M.H. Threat-related attentional bias in anxious and nonanxious individuals: A meta-analytic study. Psychol. Bull. 2007, 133, 1–24. [Google Scholar] [CrossRef] [Green Version]
- Mogg, K.; Bradley, B.P. Time course of attentional bias for fear-relevant pictures in spider-fearful individuals. Behav. Res. Ther. 2006, 44, 1241–1250. [Google Scholar] [CrossRef]
- Harvey, A.G.; Watkins, E.; Mansell, W.; Shafran, R. Cognitive Behavioural Processes Across Psychological Disorders: A Transdiagnostic Approach to Research and Treatment. Oxford University Press: Oxford, UK, 2004. [Google Scholar]
- Salemink, E.; van den Hout, M.A.; Kindt, M. Selective attention and threat: Quick orienting versus slow disengagement and two versions of the dot-probe task. Behav. Res. Ther. 2007, 45, 607–615. [Google Scholar] [CrossRef]
- Conlon, E.G.; Lovegrove, W.J.; Chekaluk, E.; Pattison, P.E. Measuring visual discomfort. Vis. Cogn. 1999, 6, 637–663. [Google Scholar] [CrossRef]
- Marcus, D.A.; Soso, J.J. Migraine and stripe-induced visual discomfort. Arch. Neurol. 1989, 1129–1132. [Google Scholar] [CrossRef] [PubMed]
- Spielberger, C.D.; Gorsuch, R.L.; Lushene, R.E.; Vagg, P.R.; Jacobs, G.A. The State-Trait Anxiety Inventory for Adults Manual; Mind Garden: Menlo Park, CA, USA, 1983. [Google Scholar]
- Wolpe, J. The Practice of Behaviour Therapy, 4th ed.; Pergamon: Oxford, UK, 1990. [Google Scholar]
- Fricke, T.R.; Siderov, J. Stereopsis, stereotests, and their relation to vision screening and clinical practice. Clin. Exp. Optom. 1997, 80, 165–172. [Google Scholar] [CrossRef]
- Smeets, G.; Merckelbach, H.; Griez., E. Panic disorder and right-hemisphere reliance. Anxiety Stress Coping 1997, 10, 245–255. [Google Scholar] [CrossRef]
- Kaplan, D.M.; Smith, T.; Coons, J. A validity study of the subjective unit of discomfort (SUD) score. Meas. Eval. Couns. Dev. 1995, 27, 195–199. [Google Scholar]
- Wilkins, A.J.; Evans, B.J. Visual stress, its treatment with spectral filters, and its relationship to visually induced motion sickness. Appl. Ergon. 2010, 41, 509–515. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wilkins, A.J.; Nimmo-Smith, J.I.; Tait, A.; McManus, C.; Della Sala, S.; Tilley, A.; Arnold, K.; Barrie, M.; Scott, S. A neurological basis for visual discomfort. Brain 1984, 107, 989–1017. [Google Scholar] [CrossRef]
- Penaccio, O.; Wilkins, A. Visual discomfort and the spatial distribution of Fourier energy. Vis. Res. 2015, 108, 1–7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fang, F.; He, S. Cortical responses to invisible objects in the human dorsal and ventral pathways. Nat. Neurosci. 2005, 8, 1380–1385. [Google Scholar] [CrossRef]
- Gray, K.L.; Adams, W.J.; Hedger, N.; Newton, K.E.; Garner, M. Faces and awareness: Low-level, not emotional factors determine perceptual dominace. Emotion 2013, 13, 537–544. [Google Scholar] [CrossRef]
- Cohen, J. A power primer. Psychol. Bull. 1992, 112, 155–159. [Google Scholar] [CrossRef]
- Steiner, T.J.; Scher, A.I.; Stewart, W.F.; Kolodner, K.; Liberman, J.; Lipton, R.B. The prevalence and disability burden of adult migraine in England and their relationships to age, gender and ethnicity. Cephalalgia 2003, 23, 519–527. [Google Scholar] [CrossRef] [PubMed]
- Hayne, D.P.; Martin, P.R. Relating photophobia, visual aura, and visual triggers of headache and migraine. Headache 2019, 59, 430–442. [Google Scholar] [CrossRef]
- Zanker, J.M.; Hermens, F.; Walker, R. Quantifying and modeling the strength of motion illusions perceived in static patterns. J. Vis. 2010, 10, 13. [Google Scholar] [CrossRef] [PubMed]
- Shepherd, A.J. Colour vision in migraine: Selective deficits for S-cone discriminations. Cephalalgia 2005, 25, 412–423. [Google Scholar] [CrossRef]
- Vieira, A.; van der Linde, I.; Bright, P.L.; Wilkins, A. Preference for lighting chromaticity in migraine with aura. Headache J. Head Face Pain 2020, 60, 1124–1131. [Google Scholar] [CrossRef] [PubMed]
- Siniatchkin, M.; Groppa, S.; Jerosch, B.; Muhle, H.; Kurth, C.; Shepherd, A.J.; Siebner, H.; Stephani, U. Spreading photoparoxysmal EEG response is associated with an abnormal cortical excitability pattern. Brain 2007, 130, 78–87. [Google Scholar] [CrossRef] [Green Version]
- Ferrari, M.D. Migraine. Lancet 1997, 9108, 1043–1105. [Google Scholar]
- Mollon, J.D.; Krauskopf, J. Reaction time as a measure of the temporal response properties of individual colour mechanisms. Vis. Res. 1973, 13, 27–40. [Google Scholar] [CrossRef] [Green Version]
- Cosentino, G.; Fierro, B.; Vigneri, S.; Talamanca, S.; Paladino, P.; Baschi, R.; Indovino, S.; Maccora, S.; Valentino, F.; Fileccia, E.; et al. Cyclical changes of cortical excitability and metaplasticity in migraine: Evidence from a repetitive transcranial magnetic stimulation study. Pain 2014, 155, 1070–1078. [Google Scholar] [CrossRef]
- Wentura, D.; Rothermund, K.; Bak, P. Automatic vigilance: The attention-grabbing power of approach- and avoidance-related social information. J. Personal Soc. Psychol. 2000, 78, 1024–1037. [Google Scholar] [CrossRef]


| Group | Selection Basis |
|---|---|
| No Headache | Participants claiming to not experience headaches. |
| Headache | Participants claiming to experience headaches but not migraines. |
| Migraine Undiagnosed (U) | Participants claiming to experience migraines (self-diagnosed) and meeting classic migraine criteria (with aura) according to the IHS. |
| Migraine Diagnosed (D) | Participants claiming to have been diagnosed with migraines (with aura) by a General Medical Practitioner or Neurologist. |
| Condition | Position 1 * | Position 2 | Colour |
| 1 | 0.5 cpd, scrambled | 3 cpd | achromatic |
| 2 | 0.5 cpd, scrambled | 3 cpd, scrambled | achromatic |
| 3 | 3 cpd, scrambled | 3 cpd | achromatic |
| 4 | 0.5 cpd, scrambled | 3 cpd | preferred colour |
| 5 | 0.5 cpd, scrambled | 3 cpd, scrambled | preferred colour |
| 6 | 3 cpd, scrambled | 3 cpd | preferred colour |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hine, T.J.; White, Y.B.Z. Pattern-Induced Visual Discomfort and Anxiety in Migraineurs: Their Relationship and the Effect of Colour. Vision 2022, 6, 1. https://doi.org/10.3390/vision6010001
Hine TJ, White YBZ. Pattern-Induced Visual Discomfort and Anxiety in Migraineurs: Their Relationship and the Effect of Colour. Vision. 2022; 6(1):1. https://doi.org/10.3390/vision6010001
Chicago/Turabian StyleHine, Trevor J., and Yolande B. Z. White. 2022. "Pattern-Induced Visual Discomfort and Anxiety in Migraineurs: Their Relationship and the Effect of Colour" Vision 6, no. 1: 1. https://doi.org/10.3390/vision6010001




