Sonic Hedgehog and TDP-43 Participate in the Spontaneous Locomotor Recovery in a Mouse Model of Spinal Motoneuron Disease
Abstract
:1. Introduction
2. Materials and Methods
2.1. Neurotoxin-Induced Motoneuron Depletion
2.2. Grid Walk Test
2.3. Immunohistochemical Analyses and Microscopy
2.4. Western Blotting Quantification
2.5. Statistical Analysis
3. Results
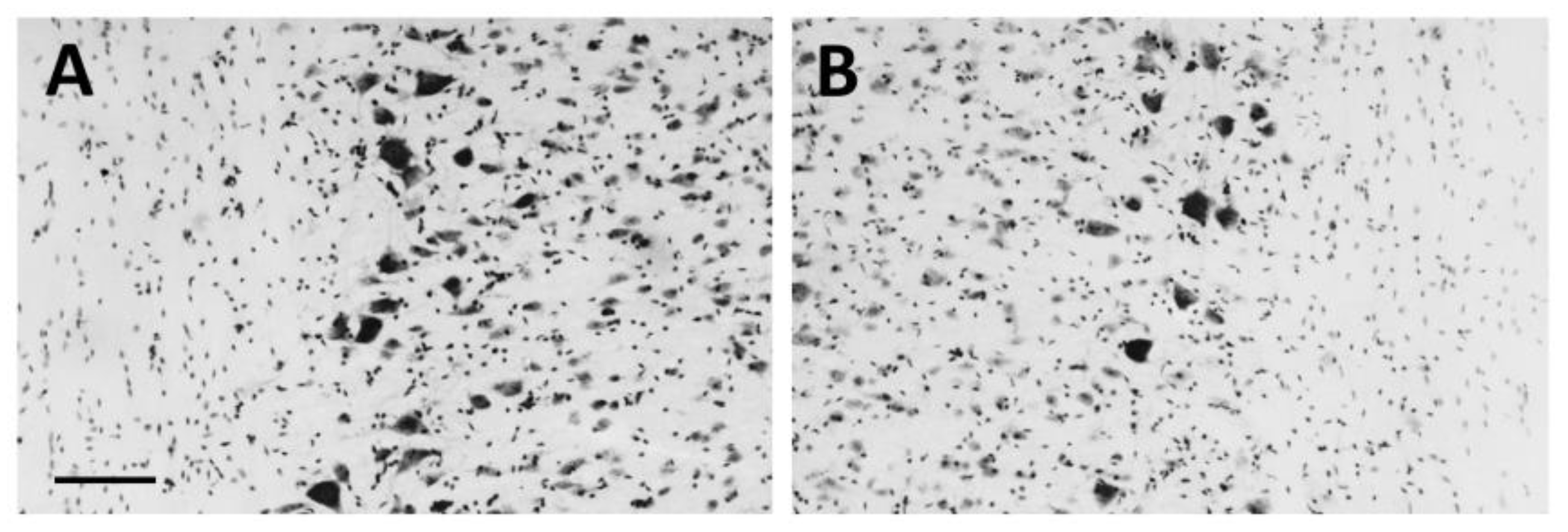
3.1. Neurotoxic Motoneuron Depletion
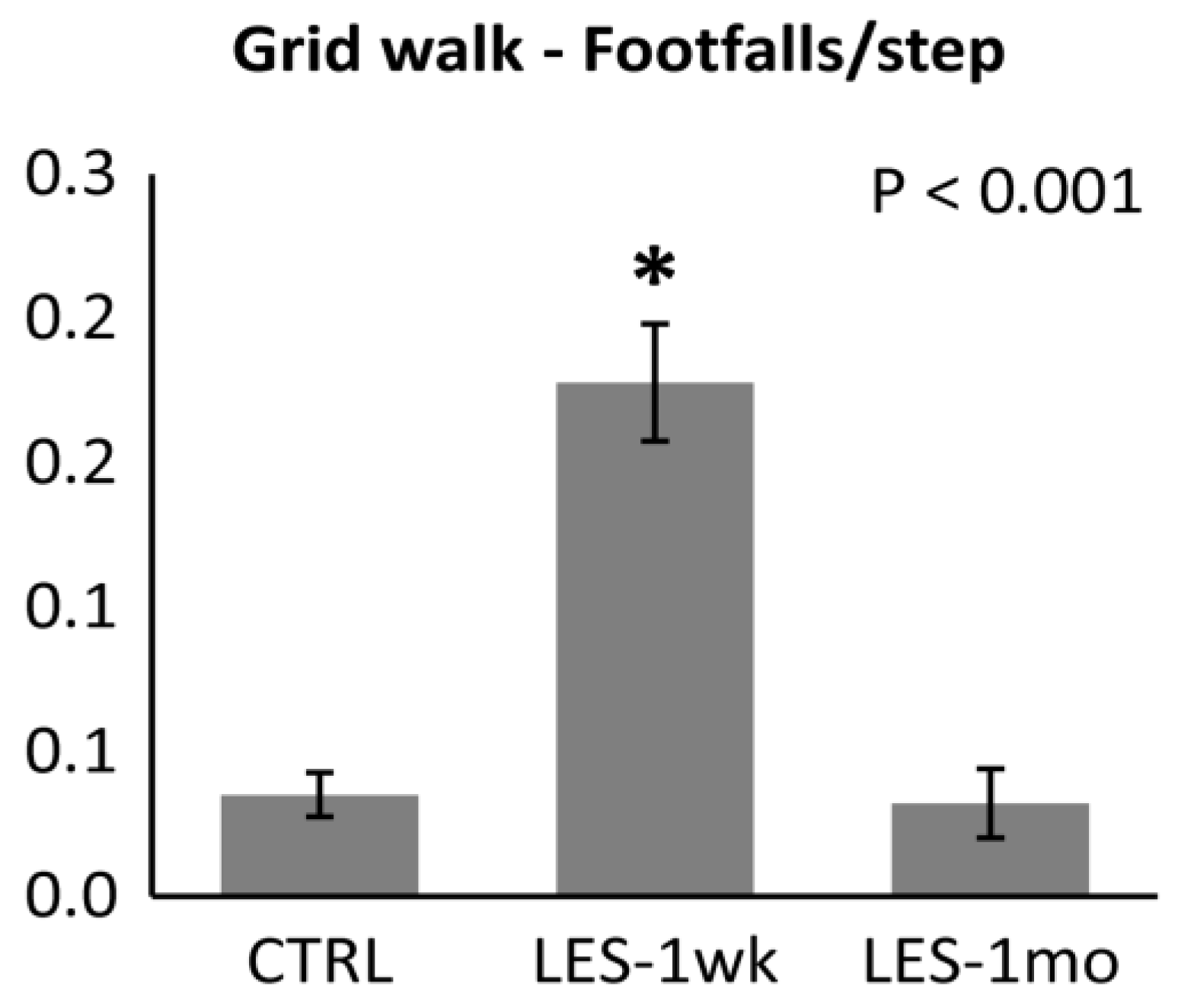
3.2. Locomotor Activity after CTB-Sap Lesion
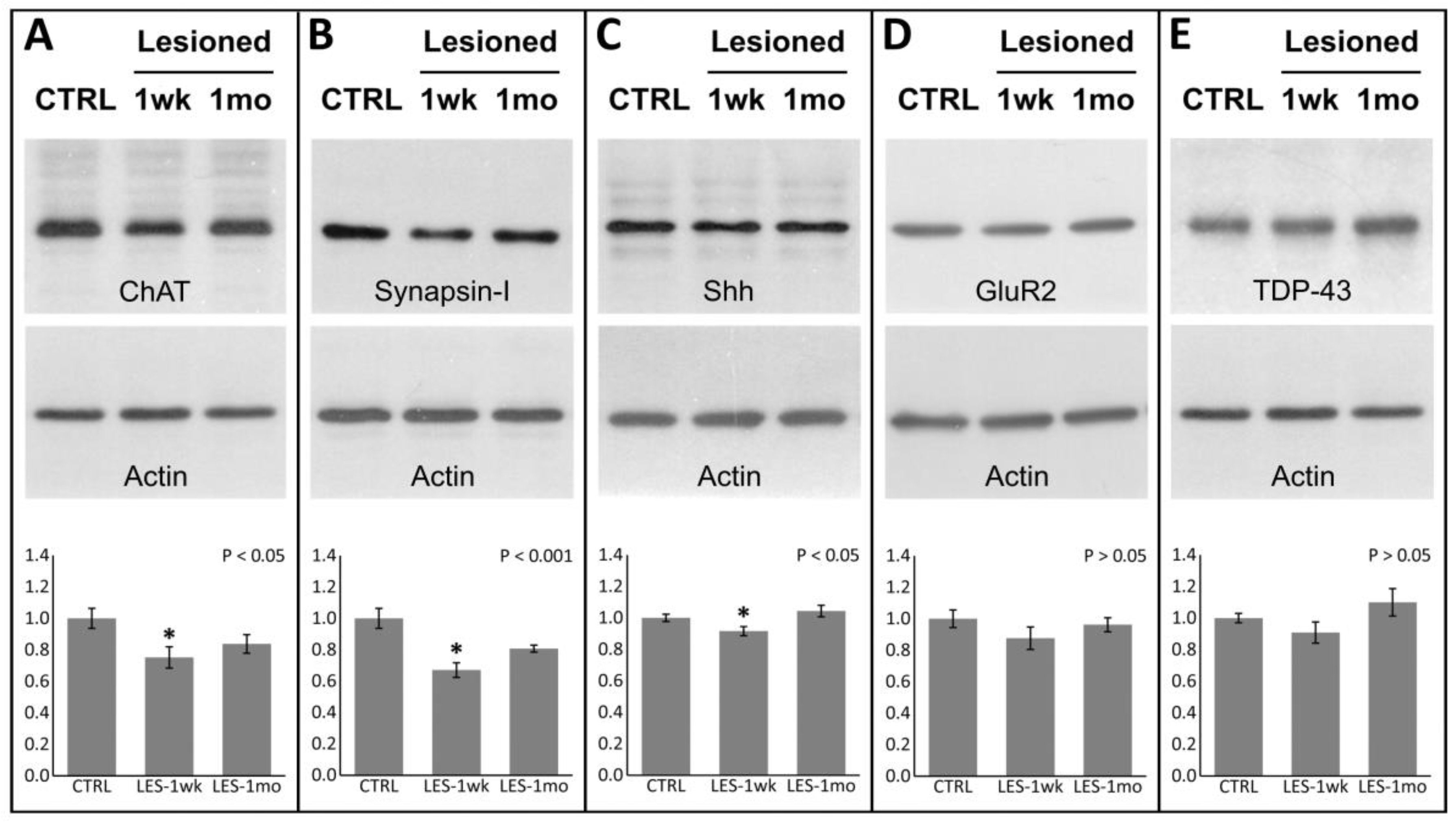
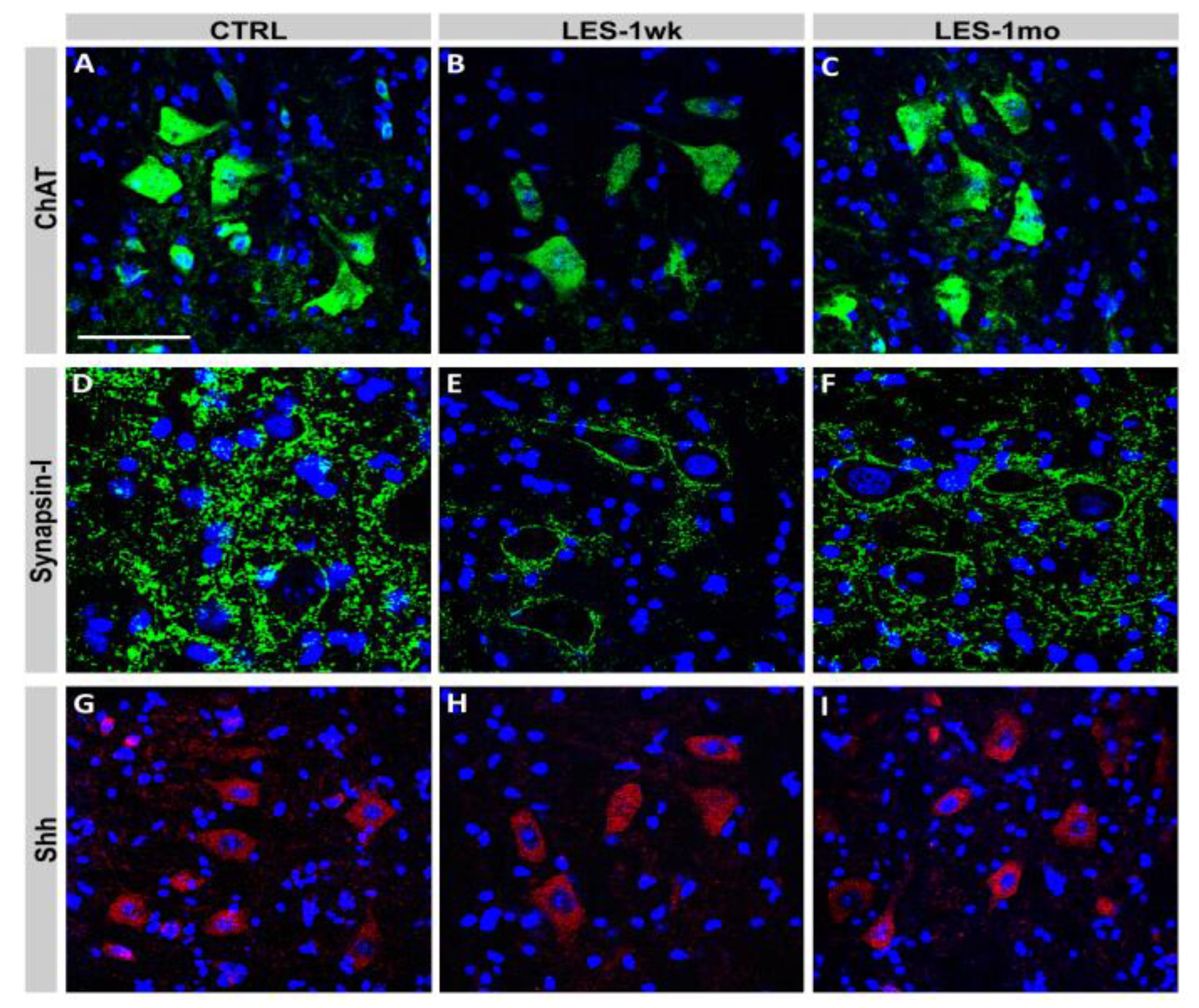
3.3. Protein Expression after CTB-Sap Lesion
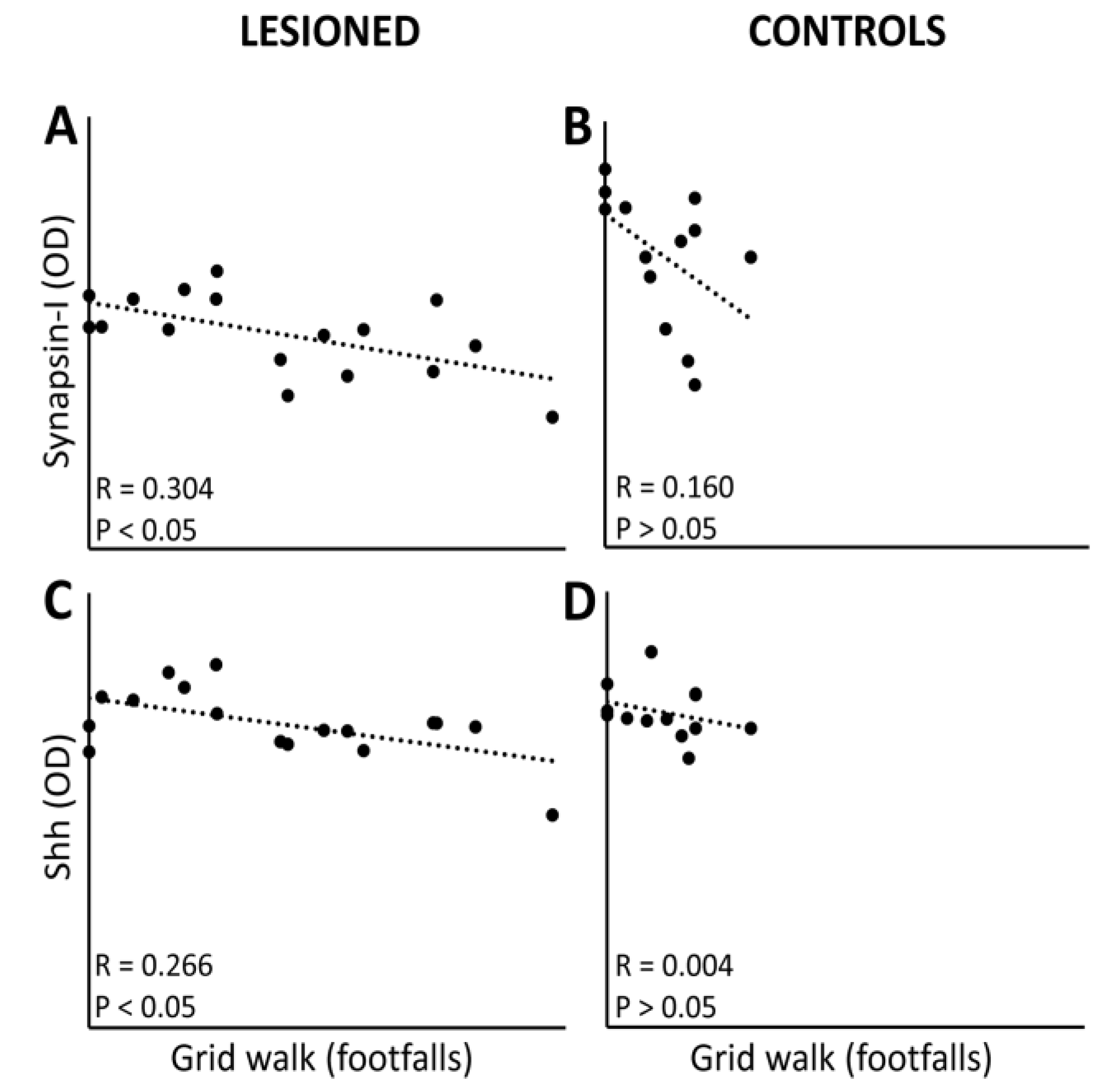
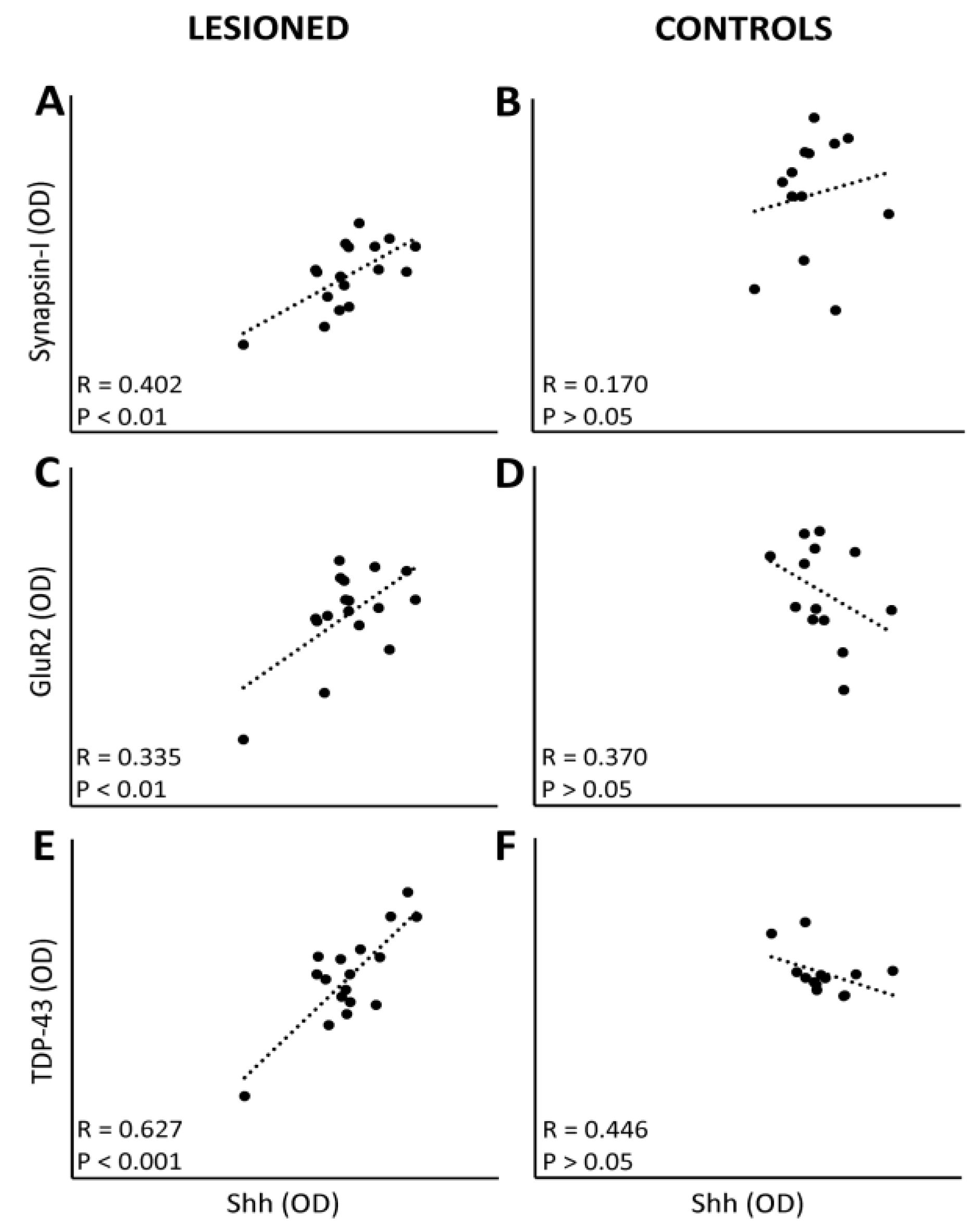
3.4. Linkage between Protein Expression Levels and Motor Performance
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Horner, P.J.; Power, A.E.; Kempermann, G.; Kuhn, H.G.; Palmer, T.D.; Winkler, J.; Thal, L.J.; Gage, F.H. Proliferation and differentiation of progenitor cells throughout the intact adult rat spinal cord. J. Neurosci. 2000, 20, 2218–2228. [Google Scholar] [PubMed]
- Weiss, S.; Dunne, C.; Hewson, J.; Wohl, C.; Wheatley, M.; Peterson, A.C.; Reynolds, B.A. Multipotent CNS stem cells are present in the adult mammalian spinal cord and ventricular neuroaxis. J. Neurosci. 1996, 16, 7599–7609. [Google Scholar] [PubMed]
- Mothe, A.J.; Tator, C.H. Proliferation, migration, and differentiation of endogenous ependymal region stem/progenitor cells following minimal spinal cord injury in the adult rat. Neuroscience 2005, 131, 177–187. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Lu, P.; McKay, H.M.; Bernot, T.; Keirstead, H.; Steward, O.; Gage, F.H.; Edgerton, V.R.; Tuszynski, M.H. Endogenous neurogenesis replaces oligodendrocytes and astrocytes after primate spinal cord injury. J. Neurosci. 2006, 26, 2157–2166. [Google Scholar] [CrossRef] [PubMed]
- Edgerton, V.R.; Tillakaratne, N.J.; Bigbee, A.J.; de Leon, R.D.; Roy, R.R. Plasticity of the spinal neural circuitry after injury. Ann. Rev. Neurosci. 2004, 27, 145–167. [Google Scholar] [CrossRef] [PubMed]
- Gulino, R.; Dimartino, M.; Casabona, A.; Lombardo, S.A.; Perciavalle, V. Synaptic plasticity modulates the spontaneous recovery of locomotion after spinal cord hemisection. Neurosci. Res. 2007, 57, 148–156. [Google Scholar] [CrossRef] [PubMed]
- Llewellyn-Smith, I.J.; Martin, C.L.; Arnolda, L.F.; Minson, J.B. Tracer-toxins: Cholera toxin B-saporin as a model. J. Neurosci. Methods 2000, 103, 83–90. [Google Scholar] [CrossRef]
- Wiley, R.G.; Kline, R.H., IV. Neuronal lesioning with axonally transported toxins. J. Neurosci. Methods 2000, 103, 73–82. [Google Scholar] [CrossRef]
- Fargo, K.N.; Sengelaub, D.R. Testosterone manipulation protects motoneurons from dendritic atrophy after contralateral motoneuron depletion. J. Compos. Neurol. 2004, 469, 96–106. [Google Scholar] [CrossRef] [PubMed]
- Gulino, R.; Perciavalle, V.; Gulisano, M. Expression of cell fate determinants and plastic changes after neurotoxic lesion of adult mice spinal cord by cholera toxin-B saporin. Eur. J. Neurosci. 2010, 31, 1423–1434. [Google Scholar] [CrossRef] [PubMed]
- Gulino, R. Neuroplasticity and repair in rodent neurotoxic models of spinal motoneuron disease. Neural Plast. 2016, 2016. [Google Scholar] [CrossRef] [PubMed]
- Gulino, R.; Litrico, L.; Leanza, G. Long-term survival and development of fetal ventral spinal grafts into the motoneuron-depleted rat spinal cord: Role of donor age. Brain Res. 2010, 1323, 41–47. [Google Scholar] [CrossRef] [PubMed]
- Abrous, D.N.; Koehl, M.; Le Moal, M. Adult neurogenesis: From precursors to network and physiology. Physiol. Rev. 2005, 85, 523–569. [Google Scholar] [CrossRef] [PubMed]
- Hagg, T. Molecular regulation of adult CNS neurogenesis: An integrated view. Trends Neurosci. 2005, 28, 589–595. [Google Scholar] [CrossRef] [PubMed]
- Lledo, P.M.; Alonso, M.; Grubb, M.S. Adult neurogenesis and functional plasticity in neuronal circuits. Nat. Rev. Neurosci. 2006, 7, 179–193. [Google Scholar] [CrossRef] [PubMed]
- Fuccillo, M.; Joyner, A.L.; Fishell, G. Morphogen to mitogen: The multiple role of hedgehog signaling in vertebrate neural development. Nat. Rev. Neurosci. 2006, 7, 772–783. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.G.; Spassky, N.; Romaguera-Ros, M.; Garcia-Verdugo, J.M.; Aguilar, A.; Schneider-Maunoury, S.; Alvarez-Buylla, A. Hedgehog signaling and primary cilia are required for the formation of adult neural stem cells. Nat. Neurosci. 2008, 11, 277–284. [Google Scholar] [CrossRef] [PubMed]
- Palma, V.; Lim, D.A.; Dahmane, N.; Sánchez, P.; Brionne, T.C.; Herzberg, C.D.; Gitton, Y.; Carleton, A.; Alvarez-Buylla, A.; Ruiz i Altaba, A. Sonic hedgehog controls stem cell behavior in the postnatal and adult brain. Development 2005, 132, 335–344. [Google Scholar] [CrossRef] [PubMed]
- Gulino, R.; Gulisano, M. Involvement of brain-derived neurotrophic factor and sonic hedgehog in the spinal cord plasticity after neurotoxic partial removal of lumbar motoneurons. Neurosci. Res. 2012, 73, 238–247. [Google Scholar] [CrossRef] [PubMed]
- Gulino, R.; Gulisano, M. Noggin and sonic hedgehog are involved in compensatory changes within the motoneuron-depleted mouse spinal cord. J. Neurol. Sci. 2013, 332, 102–109. [Google Scholar] [CrossRef] [PubMed]
- Gulino, R.; Parenti, R.; Gulisano, M. Novel mechanisms of spinal cord plasticity in a mouse model of motoneuron disease. Biomed. Res. Int. 2015, 2015, 654637. [Google Scholar] [CrossRef] [PubMed]
- Buratti, E.; Baralle, F.E. Multiple roles of TDP-43 in gene expression, splicing regulation, and human disease. Front. Biosci. 2008, 13, 867–878. [Google Scholar] [CrossRef] [PubMed]
- Cohen, T.J.; Lee, V.M.Y.; Trojanowski, J.Q. TDP-43 functions and pathogenic mechanisms implicated in TDP-43 proteinopathies. Trends Mol. Med. 2011, 17, 659–667. [Google Scholar] [CrossRef] [PubMed]
- Strong, M.J.; Volkening, K.; Hammond, R.; Yang, W.; Strong, W.; Leystra-Lantz, C.; Shoesmith, C. TDP-43 is a human low molecular weight neurofilament (hNFL) mRNA-binding protein. Mol. Cell. Neurosci. 2007, 35, 320–327. [Google Scholar] [CrossRef] [PubMed]
- Arai, T.; Hasegawa, M.; Akiyama, H.; Ikeda, K.; Nonaka, T.; Mori, H.; Mann, D.; Tsuchiya, K.; Yoshida, M.; Hashizume, Y.; et al. TDP-43 is a component of ubiquitin-positive tau-negative inclusions in frontotemporal lobar degeneration and amyotrophic lateral sclerosis. Biochem. Biophys. Res. Commun. 2006, 351, 602–611. [Google Scholar] [CrossRef] [PubMed]
- Bigio, E.H. TAR DNA-binding protein-43 in amyotrophic lateral sclerosis, frontotemporal lobar degeneration, and Alzheimer disease. Acta Neuropathol. 2008, 116, 135–140. [Google Scholar] [CrossRef] [PubMed]
- Aizawa, H.; Sawada, J.; Hideyama, T.; Yamashita, T.; Katayama, T.; Hasebe, N.; Kimura, T.; Yahara, O.; Kwak, S. TDP-43 pathology in sporadic ALS occurs in motor neurons lacking the RNA editing enzyme ADAR2. Acta Neuropathol. 2010, 120, 75–84. [Google Scholar] [CrossRef] [PubMed]
- Feiguin, F.; Godena, V.K.; Romano, G.; D’Ambrogio, A.; Klima, R.; Baralle, F.E. Depletion of TDP-43 affects Drosophila motoneurons terminal synapsis and locomotor behavior. FEBS Lett. 2009, 583, 1586–1592. [Google Scholar] [CrossRef] [PubMed]
- Hideyama, T.; Kwak, S. When does ALS start? ADAR2-GluA2 hypothesis for the etiology of sporadic ALS. Front. Mol. Neurosci. 2011, 4, 33. [Google Scholar] [CrossRef] [PubMed]
- Wang, I.F.; Wu, L.S.; Chang, H.Y.; Shen, C.K. TDP-43, the signature protein of FTLD-U, is a neuronal activity-responsive factor. J. Neurochem. 2008, 105, 797–806. [Google Scholar] [CrossRef] [PubMed]
- Liu-Yesucevitz, L.; Bassell, G.J.; Gitler, A.D.; Hart, A.C.; Klann, E.; Richter, J.D.; Warren, S.T.; Wolozin, B. Local RNA translation at the synapse and in disease. J. Neurosci. 2011, 31, 16086–16093. [Google Scholar] [CrossRef] [PubMed]
- Godena, V.K.; Romano, G.; Romano, M.; Appocher, C.; Klima, R.; Buratti, E.; Baralle, F.E.; Feiguin, F. TDP-43 regulates Drosophila neuromuscular junctions growth by modulating Futsch/MAP1B levels and synaptic microtubules organization. PLoS ONE 2011, 6, e17808. [Google Scholar] [CrossRef] [PubMed]
- Estes, P.S.; Daniel, S.G.; McCallum, A.P.; Boehringer, A.V.; Sukhina, A.S.; Zwick, R.A.; Zarnescu, D.C. Motor neurons and glia exhibit specific individualized responses to TDP-43 expression in a Drosophila model of amyotrophic lateral sclerosis. Dis. Models Mech. 2013, 6, 721–733. [Google Scholar] [CrossRef] [PubMed]
- Gulino, R.; Forte, S.; Parenti, R.; Gulisano, M. TDP-43 as a modulator of synaptic plasticity in a mouse model of spinal motoneuron degeneration. CNS Neurol. Disord. Drug Targets 2015, 14, 55–60. [Google Scholar] [CrossRef] [PubMed]
- Phelps, P.E.; Barber, R.P.; Houser, C.R.; Crawford, G.D.; Salvaterra, P.M.; Vaughn, J.E. Postnatal development of neurons containing choline acetyltransferase in rat spinal cord: An immunocytochemical study. J. Compos. Neurol. 1984, 229, 347–361. [Google Scholar] [CrossRef] [PubMed]
- Gulino, R.; Cataudella, T.; Casamenti, F.; Pepeu, G.; Stanzani, S.; Leanza, G. Acetylcholine release from fetal tissue homotopically grafted to the motoneuron-depleted lumbar spinal cord. An in vivo microdialysis study in the awake rat. Exp. Neurol. 2007, 204, 326–338. [Google Scholar] [CrossRef] [PubMed]
- Greengard, P.; Valtorta, F.; Czernik, A.J.; Benfenati, F. Synaptic vesicle phosphoproteins and regulation of synaptic function. Science 1993, 780–785. [Google Scholar] [CrossRef]
- Hilfiker, S.; Pieribone, V.A.; Czernik, A.J.; Kao, H.; Augustine, G.J.; Greengard, P. Synapsins as regulators of neurotransmitter release. Philos. Trans. R. Soc. Lond. B 1999, 354, 269–279. [Google Scholar] [CrossRef] [PubMed]
- Turner, K.M.; Burgoyne, R.D.; Morgan, A. Protein phosphorylation and the regulation of synaptic membrane traffic. Trends Neurosci. 1999, 22, 459–464. [Google Scholar] [CrossRef]
- Ying, Z.; Roy, R.R.; Edgerton, V.R.; Gómez-Pinilla, F. Exercise restores levels of neurotrophins and synaptic plasticity following spinal cord injury. Exp. Neurol. 2005, 193, 411–419. [Google Scholar] [CrossRef] [PubMed]
- Bassani, S.; Valnegri, P.; Beretta, F.; Passafaro, M. The GLUR2 subunit of AMPA receptors: Synaptic role. Neuroscience 2009, 158, 55–61. [Google Scholar] [CrossRef] [PubMed]
- Isaac, J.T.R.; Ashby, M.; McBain, C.J. The role of the GluR2 subunit in AMPA receptor function and synaptic plasticity. Neuron 2007, 54, 859–871. [Google Scholar] [CrossRef] [PubMed]
- Kawahara, Y.; Ito, K.; Sun, H.; Aizawa, H.; Kanazawa, I.; Kwak, S. Glutamate receptors: RNA editing and death of motor neurons. Nature 2004, 427, 801. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, N.; Petralia, R.S.; Currier, D.G.; Wang, Y.X.; Kim, A.; Mattson, M.P.; Yao, P.J. Sonic hedgehog regulates presynaptic terminal size, ultrastructural and function in hippocampal neurons. J. Cell Sci. 2012, 125, 4207–4213. [Google Scholar] [CrossRef] [PubMed]
- Fawcett, J.W.; Asher, R.A. The glial scar and central nervous system repair. Brain Res. Bull. 1999, 49, 377–391. [Google Scholar] [CrossRef]
- Raivich, G.; Bohatschek, M.; Kloss, C.U.; Werner, A.; Jones, L.L.; Kreutzberg, G.W. Neuroglial activation repertoire in the injured brain: Graded response, molecular machanisms and cues to physiological function. Brain Res. Rev. 1999, 30, 77–105. [Google Scholar] [CrossRef]
- McGoldrick, P.; Joyce, P.I.; Fisher, E.M.; Greensmith, L. Rodent models of amyotrophic lateral sclerosis. Biochim. Biophys. Acta 2013, 1832, 1421–1436. [Google Scholar] [CrossRef] [PubMed]
- Tsao, W.; Jeong, Y.H.; Lin, S.; Ling, J.; Price, D.L.; Chiang, P.M.; Wong, P.C. Rodent models of TDP-43: Recent advances. Brain Res. 2012, 1462, 26–39. [Google Scholar] [CrossRef] [PubMed]

© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gulino, R.; Parenti, R.; Gulisano, M. Sonic Hedgehog and TDP-43 Participate in the Spontaneous Locomotor Recovery in a Mouse Model of Spinal Motoneuron Disease. J. Funct. Morphol. Kinesiol. 2017, 2, 11. https://doi.org/10.3390/jfmk2020011
Gulino R, Parenti R, Gulisano M. Sonic Hedgehog and TDP-43 Participate in the Spontaneous Locomotor Recovery in a Mouse Model of Spinal Motoneuron Disease. Journal of Functional Morphology and Kinesiology. 2017; 2(2):11. https://doi.org/10.3390/jfmk2020011
Chicago/Turabian StyleGulino, Rosario, Rosalba Parenti, and Massimo Gulisano. 2017. "Sonic Hedgehog and TDP-43 Participate in the Spontaneous Locomotor Recovery in a Mouse Model of Spinal Motoneuron Disease" Journal of Functional Morphology and Kinesiology 2, no. 2: 11. https://doi.org/10.3390/jfmk2020011
APA StyleGulino, R., Parenti, R., & Gulisano, M. (2017). Sonic Hedgehog and TDP-43 Participate in the Spontaneous Locomotor Recovery in a Mouse Model of Spinal Motoneuron Disease. Journal of Functional Morphology and Kinesiology, 2(2), 11. https://doi.org/10.3390/jfmk2020011







