Changes in the Serum Biochemical Indices and Intestinal Microbial Community of Rabbitfish (Siganus oramin) in Three Different Habitats
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Design and Feeding Trial
2.2. Sample Collection
2.3. Biochemical Analysis
2.4. 16S rDNA Sequencing of Intestinal Microbiota
2.5. Bioinformatics Analysis of Intestinal Microbiota
2.6. Statistical Analysis
3. Results
3.1. Changes in Serum Biochemical Indexes
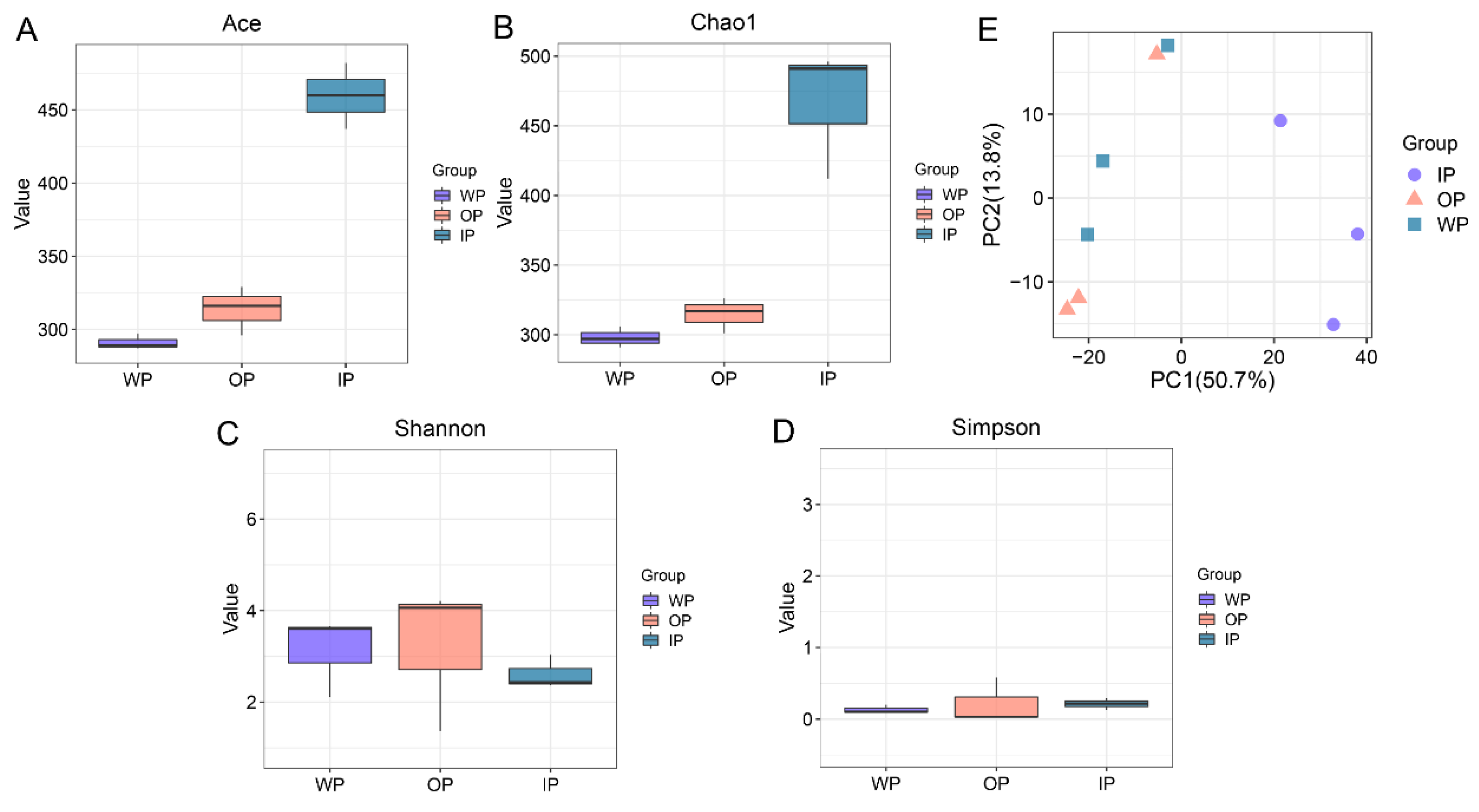
3.2. Changes in the Diversity of Intestinal Microbiota
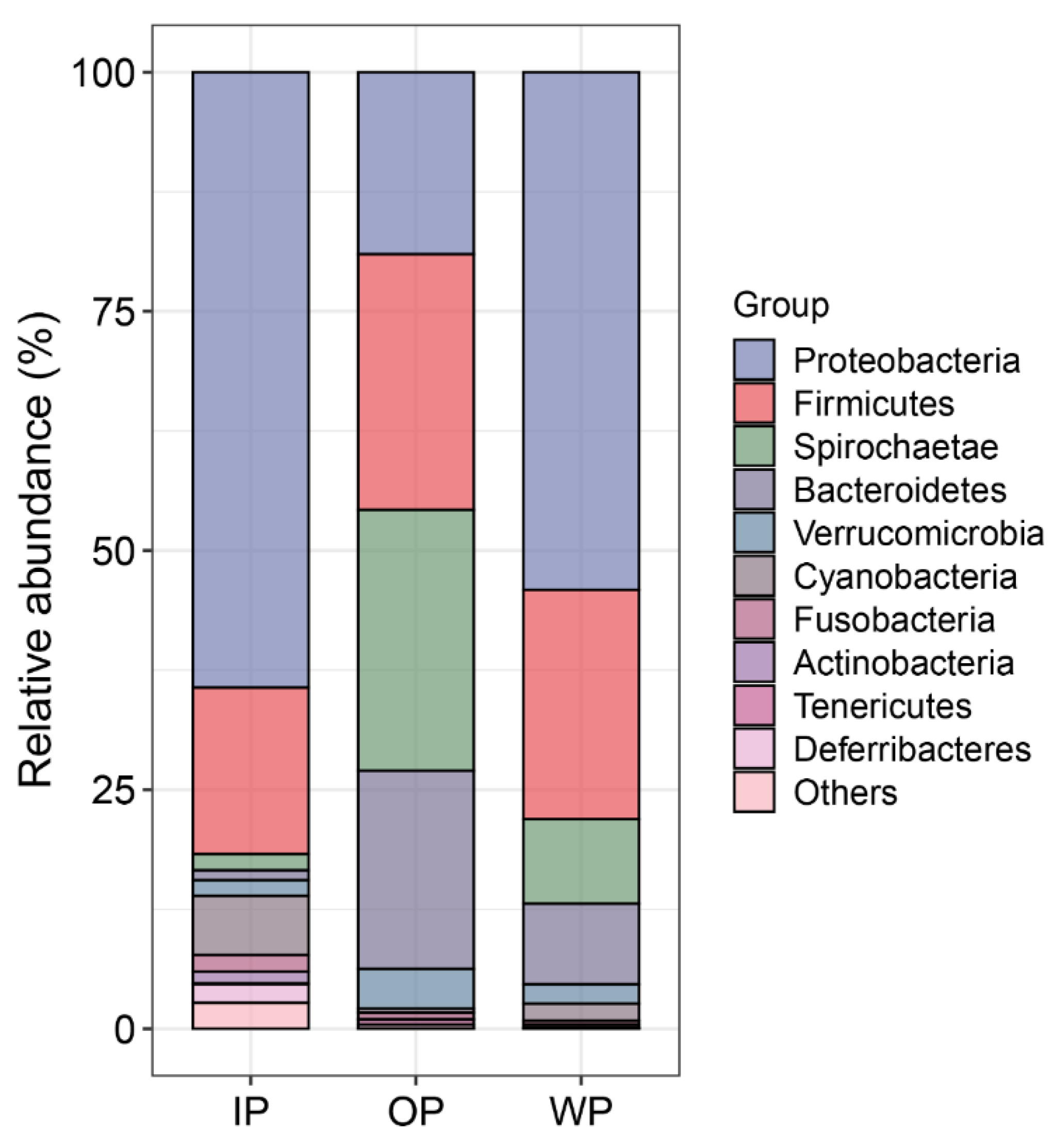
3.3. Changes in the Community Composition of Intestinal Microbiota
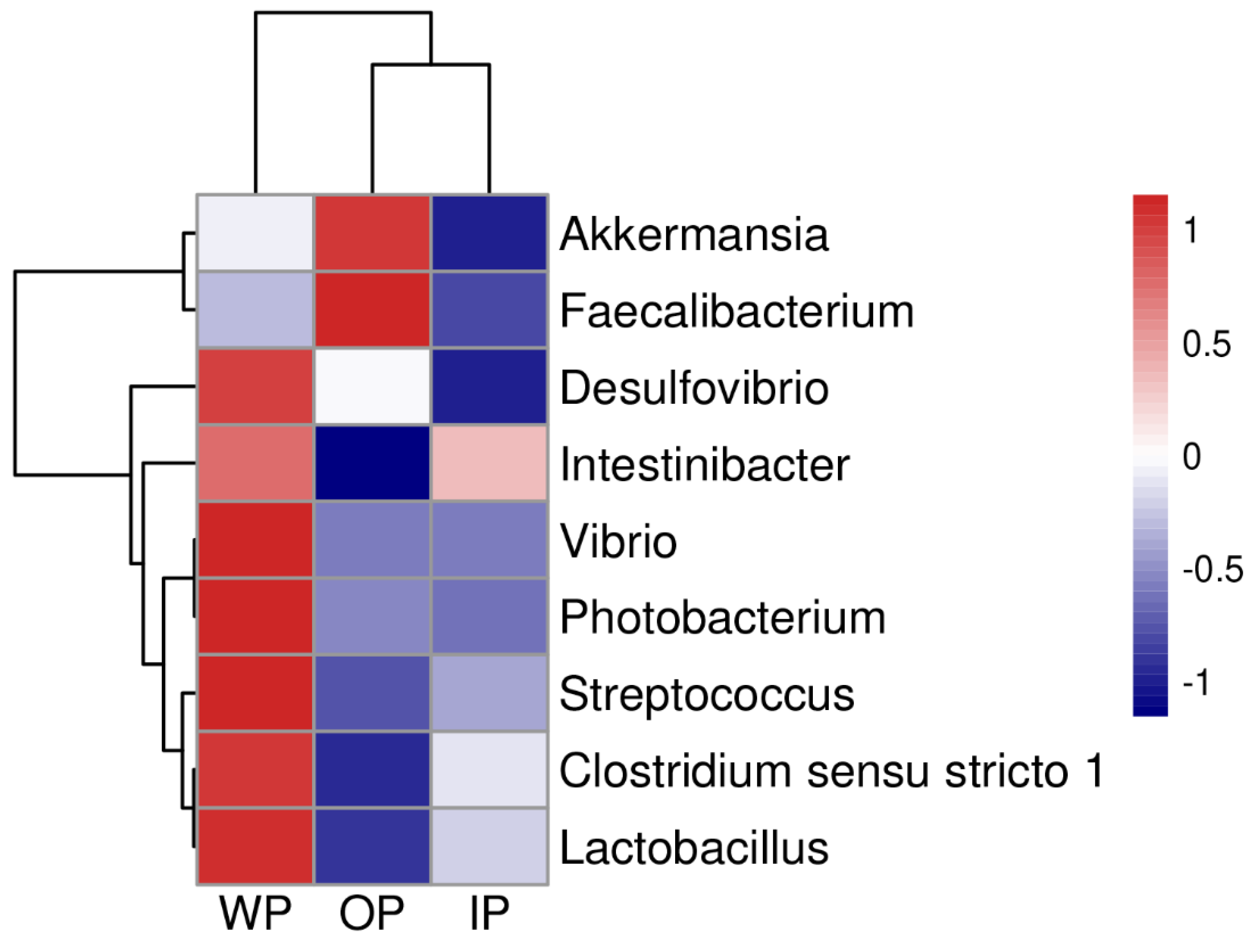
3.4. Differential Analysis of Intestinal Bacteria
3.5. Network Relationships of Intestinal Microbial Community
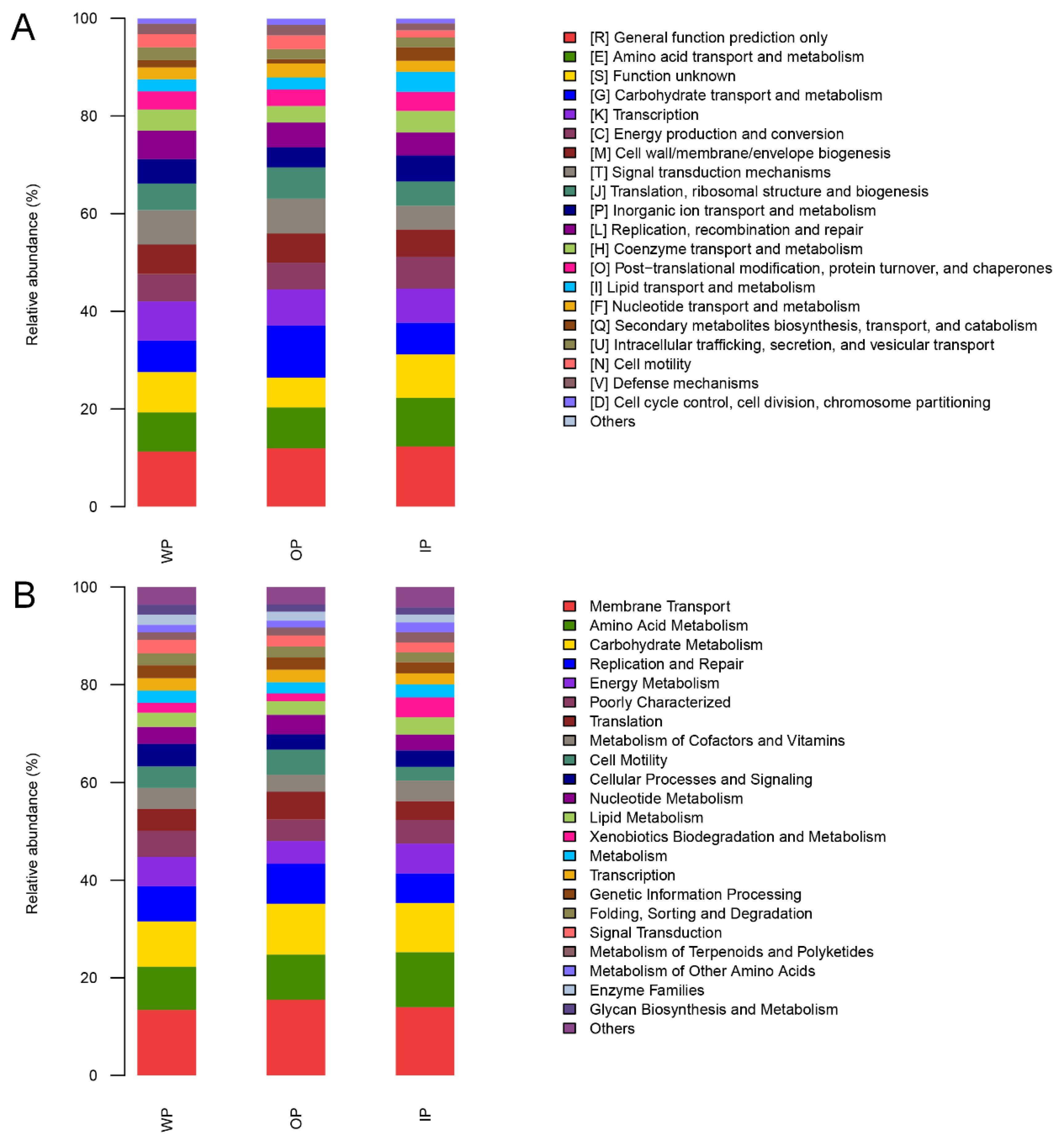
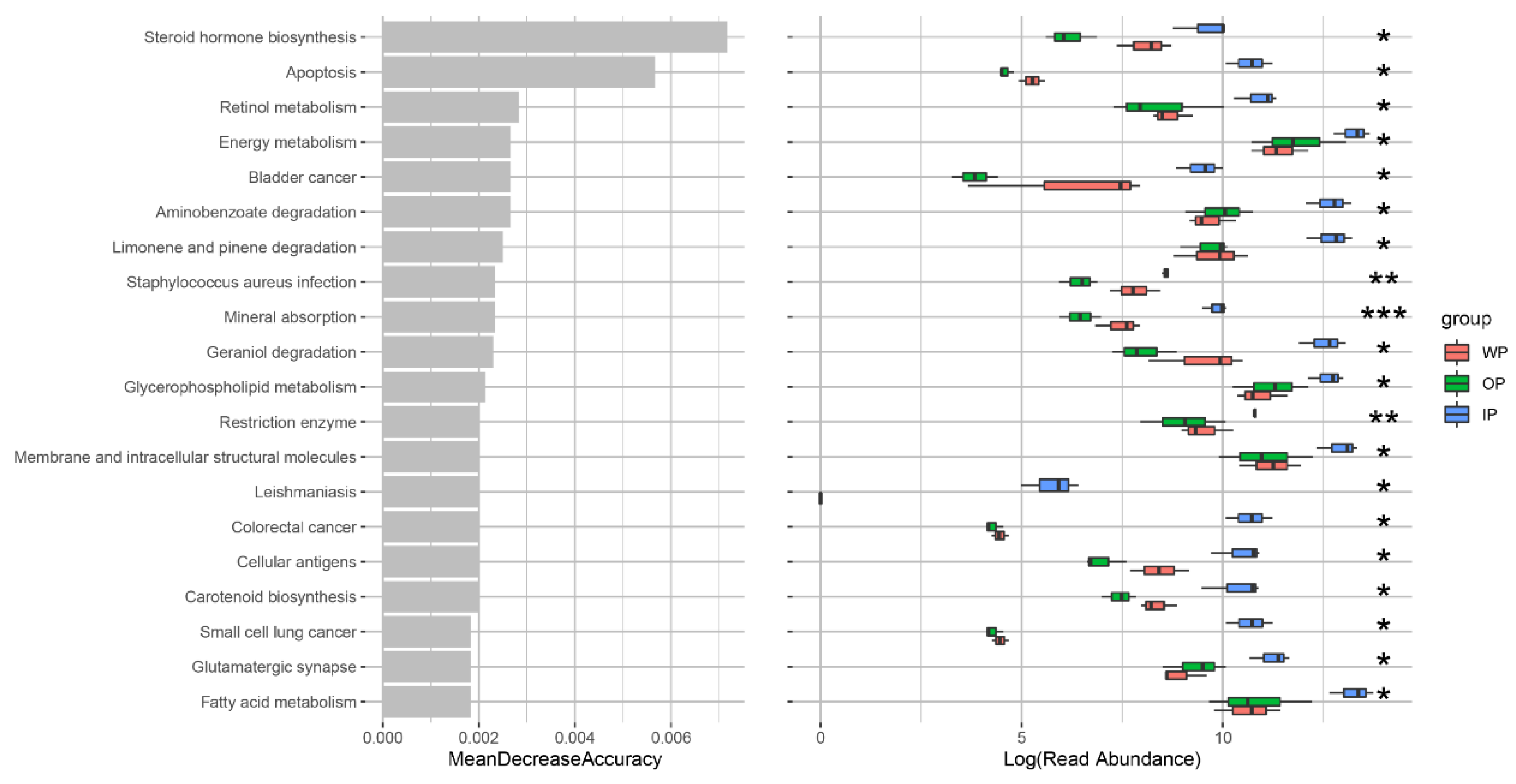
3.6. Changes in Intestinal Microbial Metabolic Function
4. Discussion
4.1. Response of Serum Biochemical Indicators to Three Different Habitats
4.2. Response of Intestinal Microbiota to Three Different Habitats
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Peng, M.; Xiao, S.; Hong, C.; Pan, C.; Feng, P.; Li, M.; Zeng, D.; Yang, C.; Jiang, W.; Chen, X. Genetic diversity analysis of different geographical populations of Siganus canaliculatus along the South China Coast. J. Hydroecol. 2022, 43, 127–133. [Google Scholar] [CrossRef]
- Yang, Y.; Huang, X.; Lin, H.; Yu, W.; Li, T.; Huang, Z.; Gou, P.; Yu, W. Optimal feeding frequency for juvenile Siganus oramin. Chin. J. Anim. Nutr. 2020, 32, 1809–1816. [Google Scholar] [CrossRef]
- Gómez, G.D.; Balcázar, J.L. A review on the interactions between gut microbiota and innate immunity of fish. FEMS Immunol. Med. Microbiol. 2008, 52, 145–154. [Google Scholar] [CrossRef]
- Li, X.; Ringø, E.; Hoseinifar, S.H.; Lauzon, H.L.; Birkbeck, H.; Yang, D. The adherence and colonization of microorganisms in fish gastrointestinal tract. Rev. Aquac. 2019, 11, 603–618. [Google Scholar] [CrossRef]
- Yang, Y.; Lin, H.; Xing, Y.; Wen, J.; Fang, W.; Zhang, J.; Duan, Y. Response signatures of intestinal microbiota and gene transcription of the tiger grouper Epinephelus fuscoguttatus to nervous necrosis virus infection. Aquaculture 2022, 550, 737848. [Google Scholar] [CrossRef]
- Nayak, S.K. Role of gastrointestinal microbiota in fish. Aquac. Res. 2010, 41, 1553–1573. [Google Scholar] [CrossRef]
- Sun, Y.; Li, Q.; Liu, J.; Zhuang, P.; Yang, J.; Peng, B.; Qin, B.; Zhao, F.; Feng, G.; Huang, X. Comparison of digestive tract flora and enzyme activity of juvenile siganus guttatus under two feeding conditions. Acta Hydrob. Sin. 2023, 47, 495–504. [Google Scholar] [CrossRef]
- Zhang, Z.; Han, X.; Xu, Y.; Huang, T.; Hu, Z. Study on microbial diversity in Siganus oramin culture environment. In Proceedings of the Compilation of Papers and Abstracts of the 9th Congress and Symposium of Guangdong Provincial Genetic Society, Guangzhou, China, 19–20 December 2014; p. 223. [Google Scholar]
- Bahmani, M.; Kazemi, R.; Donskaya, P. A comparative study of some hematological features in young reared sturgeons (Acipenser persicus and Huso huso). Fish Physiol. Biochem. 2001, 24, 135–140. [Google Scholar] [CrossRef]
- Chen, Y.X.; Xiao, L.Y.; Yan, T.M.; Zhao, H.T.; Shen, S.J.; Zhou, D.G. Hematological of wild and cultured Schizothoracin fishes. Acta Hydrob. Sin. 2009, 33, 905–910. [Google Scholar] [CrossRef]
- Coz-Rakovac, R.; Strunjak-Perovic, I.; Hacmanjek, M.; Topic Popovic, N.; Lipej, Z.; Sostaric, B. Blood chemistry and histological properties of wild and cultured sea bass (Dicentrarchus labrax) in the North Adriatic Sea. Vet. Res. Commun. 2005, 29, 677–687. [Google Scholar] [CrossRef]
- Zhou, X.; Li, M.; Abbas, K.; Wang, W. Comparison of haematology and serum biochemistry of cultured and wild Dojo loach Misgurnus anguillicaudatus. Fish Physiol. Biochem. 2009, 35, 435–441. [Google Scholar] [CrossRef]
- Djangmah, J.S. The effects of feeding and starvationon copper in the blood and hepatopancreas, and onblood proteins of Crangon vulgaris (Fabricius). Comp. Biochem. Phys. 1970, 32, 709–731. [Google Scholar] [CrossRef]
- Edgar, R.C. UPARSE: Highly accurate OTU sequences from microbial amplicon reads. Nat. Methods 2013, 10, 996–998. [Google Scholar] [CrossRef] [PubMed]
- Segata, N.; Izard, J.; Waldron, L.; Gevers, D.; Miropolsky, L.; Garrett, W.S.; Huttenhower, C. Metagenomic biomarker discovery and explanation. Genome Biol. 2011, 12, R60. [Google Scholar] [CrossRef]
- Wood, M.E.; Yasutake, W.T.; Halver, J.E.; Woodall, A.N. Chemical and histological studies of wild and hatchery salmon in fresh water. Trans. Am. Fish. Soc. 1960, 89, 301–307. [Google Scholar] [CrossRef]
- Christofilogiannis, P. The Veterinary Approach to Sea Bass and Sea Bream. In Aquaculture for Veterinarians; Brown, L., Ed.; Pergamon Press: Oxford, UK, 1993; pp. 379–395. [Google Scholar]
- Wu, F.; Wen, H.; Jiang, M.; Liu, W.; Zhong, W.W.; Tian, J. Effects of different dietary carbohydrate levels on growth performance, body composition and serum biochemical indices of juvenile Hybrid Tilapia (Oreochromis niloticus♀ × O. aureus♂). J. South China Agric. Univ. 2011, 32, 91–95. [Google Scholar] [CrossRef]
- Hu, D.; Ma, J.; Wang, C.; Qiao, H.; Wang, J.; Li, B.; Sun, Y. Effects of replacement of dietary fish meal by Nannochloropsis sp. mealon growth performance, body composition, and serum biochemical indices of juvenile turbot (Scophthalmus maximus L). Prog. Fish. Sci. 2019, 40, 21–30. [Google Scholar] [CrossRef]
- Wang, K.; Han, Y.; Dong, J.; Hao, Q.; Zhao, R.; Chen, X. Comparison of fatty acids and blood indexes between wild and cultured populations of Culter alburnus in Xingkai Lake. Freshwater Fish. 2012, 42, 14–18. [Google Scholar] [CrossRef]
- Liu, M.; Mi, G.; Guo, J.; Yuan, J. Effects of internal-circulation pond aquaculture model on growth performance, morphological indices, serum biochemical indices and muscle nutritional components of Pelteobagrus fulvidraco. Chin. J. Anim. Nutr. 2019, 31, 1704–1717. [Google Scholar] [CrossRef]
- Zhang, Y.; Sun, B.; Nie, P. Immune tissues and cells of fish: A review. Acta Hydrob. Sin. 2000, 24, 648–654. [Google Scholar] [CrossRef]
- Yu, L.; Li, X.; Yi, J.; Huang, Z.; Chen, D.; Wang, Z. Effects of different water velocities on the free radical metabolism of juvenile Spinibarbus sinensis. J. Fish. Sci. Chin. 2014, 21, 101–107. [Google Scholar]
- Jing, Q. The Growth Performance, Hematological Physiology and Stress Resistance of Tiger Pufferfish (Takifugu Rubripes) under Different Culture Systems. Master’s Thesis, Shandong Agricultural University, Taian, China, 2018. [Google Scholar]
- Lu, Y. Comparison of Morphological, Musclequality, Blood Physiological and Biochemical Indexes between Wild and Farm Bostrychus sinensis and Research on Related Factors. Master’s Thesis, Guangxi University, Nanning, China, 2017. [Google Scholar]
- Cottrell, M.T.; Kirchman, D.L. Natural assemblages of marine proteobacteria and members of the Cytophaga-Flavobacter cluster consuming low- and high-molecular weight dissolved organic matter. Appl. Environ. Microbiol. 2000, 66, 1692–1697. [Google Scholar] [CrossRef] [PubMed]
- Klase, G.; Lee, S.; Liang, S.; Kim, J.; Zo, Y.G.; Lee, J. The microbiome and antibiotic resistance in integrated fishfarm water: Implications of environmental public health. Sci. Total Environ. 2019, 649, 1491–1501. [Google Scholar] [CrossRef]
- Söllinger, A.; Tveit, A.T.; Poulsen, M.; Noel, S.J.; Bengtsson, M.; Bernhardt, J.; Hellwing, A.L.F.; Lund, P.; Riedel, K.; Schleper, C.; et al. Holistic assessment of rumen microbiome dynamics through quantitative metatranscriptomics reveals multifunctional redundancy during key steps of anaerobic feed degradation. mSystems 2018, 3, e00038-18. [Google Scholar] [CrossRef]
- Paster, B.J.; Canaleparola, E. Physiological diversity of rumen spirochetes. Appl. Environ. Microbiol. 1982, 43, 686–693. [Google Scholar] [CrossRef] [PubMed]
- Riviere, D.; Desvignes, V.; Pelletier, E.; Chaussonnerie, S.; Guermazi, S.; Weissenbach, J.; Li, T.; Camacho, P.; Sghir, A. Towards the definition of a core of microorganisms involved in anaerobic digestion of sludge. ISME J. 2009, 3, 700. [Google Scholar] [CrossRef] [PubMed]
- Ge, L.; Liu, Q.; Wang, Y.; Li, Y.; Yan, X.; Kang, L. Research on the pathogenicity of Staphylococcus sp. from fish to Carassius auratus and Danio rerio. Heilongjiang Agric. Sci. 2014, 8, 69–70. [Google Scholar] [CrossRef]
- Liu, F.; Liu, G.; Li, F. Characterization of two pathogenic Photobacterium strains isolated from Exopalaemon carinicauda causing mortality of shrimp. Aquaculture 2016, 464, 129–135. [Google Scholar] [CrossRef]
- Ji, Q.; Wang, S.; Ma, J.; Liu, Q. A review: Progress in the development of fish Vibrio spp. vaccines. Immunol. Lett. 2020, 226, 46–54. [Google Scholar] [CrossRef] [PubMed]
- Gibson, G.R.; Macfarlane, G.T.; Cummings, J.H. Occurrence of sulphate-reducing bacteria in human faeces and the relationship of dissimilatory sulphate reduction to methanogenesis in the large gut. J. Appl. Bacteriol. 1988, 65, 103–111. [Google Scholar] [CrossRef]
- Ichiishi, S.; Tanaka, K.; Nakao, K.; Izumi, K.; Mikamo, H.; Watanabe, K. First isolation of Desulfovibrio from the human vaginal flora. Anaerobe 2010, 16, 229–233. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.W.; Augusto, L.A.; Zhao, L.P.; Caroff, M. Desulfovibrio desulfuricans isolates from the gut of a single individual: Structural and biological lipid A characterization. FEBS Lett. 2015, 589, 165–171. [Google Scholar] [CrossRef] [PubMed]
- Mayo, B.; BeĺenFlórez, A.B. Lactic Acid Bacteria: Lactobacillus spp.: Lactobacillus plantarum. In Encyclopedia of Dairy Sciences, 3rd ed.; Elsevier: Amsterdam, The Netherlands, 2022; pp. 206–217. [Google Scholar]
- Heinken, A.; Khan, M.T.; Paglia, G.; Rodionov, D.A.; Harmsen, H.J.M.; Thiele, I. Functional metabolic map of Faecalibacterium prausnitzii, a beneficial human gut microbe. J. Bacteriol. 2014, 196, 3289–3302. [Google Scholar] [CrossRef]
- Duan, Y.; Wang, Y.; Dong, H.; Ding, X.; Liu, Q.; Li, H.; Zhang, J.; Xiong, D. Changes in the intestine microbial, digestive, and immune-related genes of Litopenaeus vannamei in response to dietary probiotic Clostridium butyricum supplementation. Front. Microbiol. 2018, 9, 2191. [Google Scholar] [CrossRef]
- Song, Y.L.; Liu, C.X.; McTeague, M.; Summanen, P.; Finegold, S.M. Clostridium bartlettii sp. nov., isolated from human faeces. Anaerobe 2004, 10, 179–184. [Google Scholar] [CrossRef]
- Rodriguez, J.; Hiel, S.; Delzenne, N.M. Metformin: Old friend, new ways of action-implication of the gut microbiome? Curr. Opin. Clin. Nutr. 2018, 21, 294–301. [Google Scholar] [CrossRef]
- Yang, W.Y.; Chou, C.H.; Wang, C. The effects of feed supplementing Akkemansia muciniphila on incidence, severity, and gut microbiota of necrotic enteritis in chickens. Poultry Sci. 2022, 101, 101751. [Google Scholar] [CrossRef]
- Xiao, F.; Liao, L.; Xu, Q.; He, Z.; Xiao, T.; Wang, J.; Huang, J.; Yu, Y.; Wu, B.; Yan, Q. Hostmicrobiota interactions and responses to grass carp reovirus infection in Ctenopharyngodon idellus. Environ. Microbiol. 2021, 23, 431–447. [Google Scholar] [CrossRef]
- Xiao, F.; Zhu, W.; Yu, Y.; He, Z.; Wu, B.; Wang, C.; Shu, L.; Li, X.; Yin, H.; Wang, J.; et al. Host development overwhelms environmental dispersal in governing the ecological succession of zebrafish gut microbiota. NPJ Biofilms Microbiol. 2021, 7, 5. [Google Scholar] [CrossRef]


| Index | The WP Group | The OP Group | The IP Group |
|---|---|---|---|
| TP (g/L) | 33.20 ± 1.60 a | 76.40 ± 1.78 b | 34.33 ± 2.34 a |
| ALB (g/L) | 5.20 ± 1.41 a | 5.30 ± 0.71 a | 3.23 ± 1.08 a |
| GLU (mmol/L) | 20.12 ± 2.52 a | 14.21 ± 1.87 a | 21.72 ± 2.86 a |
| TG (mmol/L) | 12.35 ± 0.51 ab | 10.68 ± 1.26 a | 14.95 ± 1.29 b |
| TC (mmol/L) | 7.54 ± 0.43 ab | 5.63 ± 0.85 a | 13.20 ± 2.93 b |
| HDL-C (mmol/L) | 0.56 ± 0.05 a | 1.14 ± 0.34 a | 0.46 ± 0.07 a |
| LDL-C (mmol/L) | 1.56 ± 0.43 a | 0.70 ± 0.07 a | 1.74 ± 0.79 a |
| ALP (U/L) | 71.17 ± 5.24 a | 91.50 ± 9.61 a | 95.17 ± 25.54 a |
| ALT (U/L) | 7.48 ± 0.47 a | 8.29 ± 0.23 a | 8.72 ± 0.30 a |
| AST (U/L) | 63.17 ± 11.20 a | 57.00 ± 2.93 a | 84.50 ± 9.96 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, Y.; Lin, H.; Huang, X.; Dong, H.; Duan, Y. Changes in the Serum Biochemical Indices and Intestinal Microbial Community of Rabbitfish (Siganus oramin) in Three Different Habitats. Fishes 2024, 9, 96. https://doi.org/10.3390/fishes9030096
Yang Y, Lin H, Huang X, Dong H, Duan Y. Changes in the Serum Biochemical Indices and Intestinal Microbial Community of Rabbitfish (Siganus oramin) in Three Different Habitats. Fishes. 2024; 9(3):96. https://doi.org/10.3390/fishes9030096
Chicago/Turabian StyleYang, Yukai, Heizhao Lin, Xiaolin Huang, Hongbiao Dong, and Yafei Duan. 2024. "Changes in the Serum Biochemical Indices and Intestinal Microbial Community of Rabbitfish (Siganus oramin) in Three Different Habitats" Fishes 9, no. 3: 96. https://doi.org/10.3390/fishes9030096





